Abstract
The importance of heathlands as habitats for plants and thus for nature conservation is recognized by European Directive 92/43 (Habitats Directive). However, heathlands are threatened by habitat loss and quality degradation due to several drivers. Temperate Calluna vulgaris communities in the Po basin and in the Southern Alps (NW Italy) are disjunct from the core distribution area in Western Europe and occur at their climatic limits. This study aimed to analyze floristic patterns of heather communities in NW Italy in order to provide detailed recommendations for local conservation needs. Data on plant species composition (phytosociological relevés) and abiotic (environmental and geographical) factors were jointly analyzed using multivariate statistical analyses, to provide a quantitative and statistical interpretation of variation among heathland communities. We show that diversity in species composition was associated with variation in abiotic factors, and we sorted an initial list of “typical species” of the heather’s habitat among indicator species. Several subtypes of heathlands were also recognized and related to habitats “élémentaires”, which require specific conservation measures to preserve their floristic diversity. Finally, we proposed a revised syntaxonomy of heathlands for NW Italy.
Introduction
Heathlands include temperate shrubby vegetation dominated by heather (Calluna vulgaris (L.) Hull) growing on nutrient-poor soils. In Europe, temperate heathlands are largely distributed in western regions under oceanic influence (Gimingham Citation1972), where they also exhibit the highest species diversity (González Citation1998). In some lowland areas of north-western Italy (Lombardy and Piedmont) heathlands were historically so widely distributed that they had a strong influence on toponyms and on socio-cultural heritage for centuries (Brusa and Piazza Citation2015), similarly as in many other Western Europe countries (Webb Citation1998). Temperate heathlands are mainly regressive communities that replace woodlands (Ellenberg Citation1988) and they are mostly anthropogenic in the Po basin and the Southern Alps (Pavari Citation1927; Tinner et al. Citation2005; Valese et al. Citation2014).
Heathlands are part of the landscape and support a large number of species belonging to different systematic groups. The role of heathlands in nature conservation is also recognized by the European Union: “European dry heaths” (cod. Nature 2000: 4030) are listed among habitats of Annex I of European Directive 92/43 (Habitats Directive), which includes all natural habitat types of community value for which conservation special areas of conservation are required to designate in each Member State.
In recent decades the importance of heathland conservation has been stressed as they are threatened by habitat loss and quality degradation due to several drivers (Fagúndez Citation2012): habitat fragmentation and destruction, pollution and eutrophication, increasing atmospheric CO2 and climate change, secondary succession and management, and the invasion by alien species. At varying intensities, all these drivers negatively affect heathlands in Southern Alps and in the Po basin (Ascoli and Bovio Citation2010; Brusa and Piazza Citation2015; Cerabolini, Ceriani, and De Andreis Citation1998; Lonati et al. Citation2009). Moreover, these heathlands are disjunct from the main distribution area of Western Europe heathlands and occur at the climatic limits of Calluna vulgaris.
The Habitats Directive prescribes the protection of habitats against potentially damaging activities. Member States are asked to take appropriate measures to maintain or restore habitat quality and report their conservation state every six years (Evans and Arvela Citation2011); surveys and monitoring of habitats are necessary to satisfy this requirement. Roughly, most habitats of Annex I can be considered as equivalent to a phytosociological alliance. Some are more narrowly defined while others represent landscape units rather than habitats (Evans Citation2006). However, the majority of habitat definitions is based on phytosociological syntaxa, often resulting in different interpretations among countries and phytosociological schools (Evans Citation2010). In addition, monitoring and surveillance of biodiversity in the European Union must include habitats that are not included in the Natura 2000 network. It has been concluded that more supportive information could be developed from existing sources such as the phytosociological literature (Bunce et al. Citation2013).
The aim of this study was to analyse plant species patterns in heather communities in the Southern Alps and in the Po basin, and to relate these to abiotic (environmental and geographical) factors in order to draw conclusions for conservation. More precisely, we analyzed phytosociological data and tested the possible floristic dependence from previously supposed abiotic gradients (Cerabolini, Ceriani, and De Andreis Citation1998), with the objective of determining the typical species of “European dry heaths”.
Material and methods
Study area
The study area is located in the Po basin and the Southern Alps and covers two Italian administrative regions (Piedmont, Lombardy) close to the Swiss border (Figure ). The study area encompasses a latitudinal range of approximately 200 km from the Dora Baltea River (western limit) to the Camonica Valley (eastern limit), and a longitudinal range of approximately 100 km from the alpine Ossola Valley (northward) to the hilly belt near the Po Plain (southward).
Figure 1. The study area. Groups of relevés emerging from the cluster analysis are identified by average values of latitude/longitude.
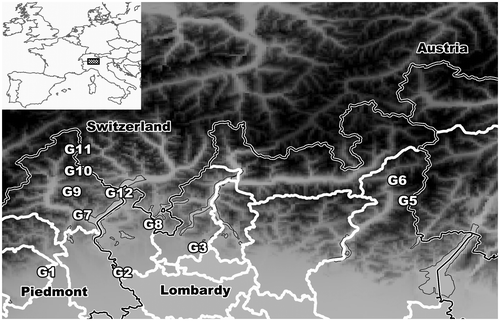
From the geological point of view, recent deposits of fluvial or fluvial–glacial origin (Holocene) are situated southernmost and at lower altitudes (150–250 m a.s.l.), where gravelly sediments mixed with siliceous sands occur mainly in the valley of the Ticino River (marking the boundary between Piedmont and Lombardy). Older deposits of glacial origin (Pleistocene) are positioned on terraces (200–500 m a.s.l.) on both sides of the Ticino River. Heathlands are located on the most ancient deposits (Mindel) that underwent severe leaching from which clayey soils originated. The hills (400–600 m a.s.l.), where heathlands are limited to the steepest slopes, mainly consist of a conglomerate with siliceous rocks (Pleistocene). The mountains of the northernmost part of the study area cover a wide altitudinal range (300–1200 m a.s.l.). Bedrocks include several types of igneous (e.g. granite) and metamorphic rocks (e.g. gneiss, schist), sharing a common siliceous composition.
In the study area, temperature decreases with altitude of approximately 0.5°C every 100 m (Belloni Citation1975). Mean annual precipitation ranges from 1000 to 2500 mm, with the highest values found in the mountains near Lake Maggiore (Biancotti et al. Citation1998; Ceriani and Carelli Citation1999). According to Gimingham (Citation1972), European lowland heathlands occur only in a temperate oceanic climate (type “Cfb” of Köppen’s climate classification system). A summer arid period is lacking even at low altitudes in the study area; nevertheless, the mean temperature is above 22°C in July; therefore a warm climate (Köppen’s class of “Cfa”) is evident over a large part of lowland heathlands, i.e. outside Gimingham’s range.
Data sampling and analysis
Heathlands of the study area were investigated through extensive field surveys over the past 20 years. Heather communities related to coniferous forests (Vaccinio-Piceetea) have been excluded. The analysis was thus restricted to 75 heathlands of the deciduous forest belt (Querco-Fagetea). At least one phytosociological relevé was recorded in each of the 75 sites, whenever a heather community was subjectively recognized as developed. Plot size ranged between 15 and 100 m2. The following abiotic (environmental and geographical) factors were assigned to each relevé:
| • | latitude (LAT) and longitude (LONG) coordinates of the administrative municipality where the site is located; | ||||
| • | altitude (ALT), in meters above sea level; | ||||
| • | geological substrate: sandy and gravelly soils on recent deposits (REC_DEP); clayey soils on old deposits (OLD_DEP); igneous or metamorphic rocks (ROCK); conglomerate rock. | ||||
A presence/absence matrix of 187 species × 392 relevés and a quantitative matrix of six abiotic variables × 392 relevés were jointly analyzed using Canonical Correspondence Analysis (CCA; Ter Braak Citation1987) in order to explore relationships between species composition and abiotic factors (Monte Carlo test, 9999 permutations). Prior to the analysis, species with less than five presences were removed from the floristic data-set, and geological substrates were converted in dummy variables.
Hierarchical clustering (I) of the six abiotic variables rescaled on 0–1 was performed using Euclidean distance and Ward’s method (Murtagh and Legendre Citation2014). The best cut level in the dendrogram was determined using the “silhouette” method (Rousseeuw Citation1987). A subsequent multivariate analysis of variance (MANOVA) tested the consistency of cluster groups on the first two CCA axes. Floristic composition in each cluster group of relevés was derived from the matrix species × relevés. After that, Pearson’s phi coefficient of association (Chytrý et al. Citation2002) was calculated to statistically analyse the association between a species and cluster groups; in this way, indicator species were detected for each group or cluster of groups (Cáceres and Legendre Citation2009).
Our own groups of relevés (hereafter denoted by letter G and a number between 1 and 12) were then compared with previously published data sets on heathlands from the same study area, namely:
| • | Antonietti (Citation1970): seven variants of a phytosociological “association” from the Swiss mountains (Cantone Ticino) and, to a lesser extent, from Italy, between Lake Maggiore and Lake Como; | ||||
| • | Giacomini (Citation1958): a table of phytosociological relevés on recent deposits near the Ticino River; | ||||
| • | Guglielmetto Mugion (Citation1996): a phytosociological table of a heathland on clayey soils from Vauda, Piedmont (west of the study area); | ||||
| • | Hofer (Citation1967): a data-set from the same area investigated by Antonietti (Citation1970); | ||||
| • | Lonati and Siniscalco (Citation2010): two groups of relevés from the Southern Alps in the province of Biella (Piedmont); | ||||
| • | Oberdorfer (Citation1964): a table of phytosociological relevés from the Swiss Alps (Cantone Ticino). | ||||
In addition, data sets from north-eastern Italy (Karst: Feoli, Pignatti, and Pignatti Citation1981; other sites in Friuli: Poldini, Oriolo, and Francescato Citation2004) were included in the analysis to verify possible penetration of Illyrian (Western Balkan) species in the south central and western Alps, as supposed by Cerabolini et al. (Citation2005).
The final data-set consisted of a matrix of 413 species × 27 data sets (including our 12 groups of relevés and 15 published data sets), in which species values were estimated from the midpoint value of percentage class (V = 90, IV = 70, etc.) shown in each data-set. The matrix was submitted to hierarchical clustering using Bray Curtis’ distance and Ward’s method (cluster analysis II), and “silhouette” method to cut the dendrogram. The association between a species and a group of data sets (i.e. checking for indicator species) was performed through the point biserial correlation coefficient, the abundance-based counterpart of the phi coefficient (Cáceres and Legendre Citation2009). Habitat preference of each species was inferred from the main phytosociological literature as follows: wetlands (Alnetea glutinosae, Isoeto–Nanojuncetea, etc.), mesophilous grasslands (Molinio–Arrhenatheretea), dry grasslands (Festuco–Brometea), acidophilous grasslands (Nardetea strictae), heathlands (Calluno–Ulicetea), woodland herbaceous edges (Trifolio–Geranietea) and forests (Erico–Pinetea and Querco–Fagetea).
Data on Calluna vulgaris were removed from all data sets. Species nomenclature and syntaxonomic names follow respectively Conti et al. (Citation2005) and Biondi et al. (Citation2014). Statistical analyses were computed using some packages in the R software distribution (R Core Team Citation2015).
Results
In the CCA, 14.9% of the total variance was explained by all the abiotic variables. Monte Carlo test of the overall model, single abiotic variables and axes consistently gave p-values <0.001, providing a statistical validation of the coupling of the floristic data-set and abiotic factors. The proportion of the constrained variance explained by the first two CCA axes (Figure ) was, respectively, 34.3% and 21.1% (18.4% for CCA axis 3). Latitude, altitude and rocky substrate, and, conversely, old deposits, accounted for most of the variation along the first axis; the two remaining abiotic variables, longitude and recent deposits, explained most of the variation along the second axis.
Figure 2. CCA ordination. Only factors and groups of rlevés (G1–G12) from cluster analysis are shown in the biplot.
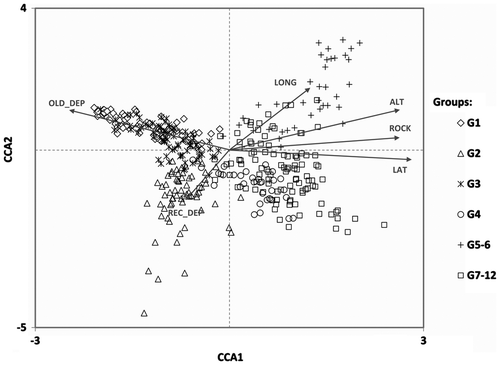
Twelve groups were retained after the cluster analysis (I) on abiotic variables (Figure ) that were well separated (MANOVA, Pillai’s trace, F22,760 = 151.32, p < 0.001) along the first two CCA axes (Figure ):
| • | G1: on old deposits in the western part of the study area (Piedmont); | ||||
| • | G2: on recent deposits in central study area (along the Ticino River); | ||||
| • | G3: on old deposits, like G1, but in the eastern part of the study area (Lombardy); | ||||
| • | G4: on conglomerate rock; | ||||
| • | G5–G6: on siliceous rocks in the eastern part of the study area (Lombardy), at low (G6) or high (G5) altitude; | ||||
| • | G7–G12: on siliceous rocks, like G5–G6, but in the western part of the study area (Piedmont and Lombardy); G7–G12 groups are separated from each other by geographic coordinates and altitude. | ||||
Figure 3. Cluster analysis (I) on the abiotic factor data of the relevés. Twelve groups (G1–G12) were recognized in the dendrogram.
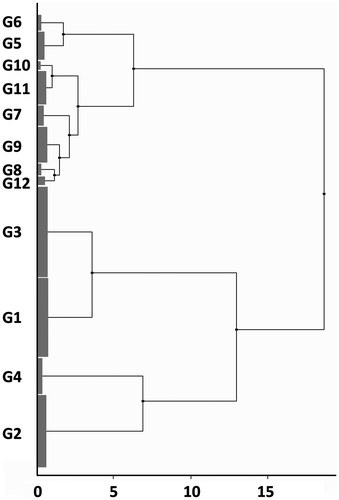
Single groups or clusters of groups recognized by cluster analysis (I) on the basis of geological and geographical variables were denoted by one or more indicator species (Table ). The cluster groups G5–G6 and G7–G12 were split after node 6 (i.e. following the separation from other groups); thus, in checking indicator species, it was far more convenient and practical to consider them as two main groups: they shared several species (Juniperus communis, Polygala chamaebuxus, Solidago virgaurea, etc.) and exhibited more exclusive species (G5–G6: Erica carnea, Phyteuma scheuchzeri, Brachypodium rupestre, etc.; G7–G12: Festuca acuminata, Allium lusitanicum, Euphorbia cyparissias, etc.). Similarly, G1 and G3 shared several species (Frangula alnus, Populus tremula, Gentiana pneumonanthe, etc.) but also exhibited exclusive species (G1: Salix rosmarinifolia, Carex panicea, Serratula tinctoria, etc.; G3: Salix caprea, Juncus tenuis, Lotus pedunculatus, etc.). On the other hand, G2 and G4 had only two species in common (Hypericum perforatum and Aira caryophyllea) and differed by the presence of alien species (G2; Dichanthelium acuminatum, Pinus rigida, Prunus serotina, etc.) or trees (G4: Castanea sativa, Quercus pubescens, Ostrya carpinifolia).
Table 1. Indicator species of groups G1–G12 (cluster analysis I) recognized on the base of the phi coefficient of association. Only the first 12 species are listed for each group or cluster of groups in the dendrogram, if statistically significant. Figures are the percentage of relevés occupied by a given species (those that are statistically significant are shown in bold).
The floristic relationships among data sets are revealed in the dendrogram produced by the cluster analysis (II), which returned seven groups (A–G), as shown in Figure . Floristic features of groups were well supported by indicator species (Table ). Wetland species were strict indicators of group A. Forest species were shared by all groups, although single species were indicators of one (e.g. group C: Pinus nigra and Sesleria autumnalis), two (groups C and D: Erica carnea; groups A and B: Quercus robur), or three groups (e.g. Fraxinus ornus in C, D, and F). Species of mesophilous grasslands were also shared by all groups; Molinia caerulea subsp. arundinacea occurred in all groups, except in group C. Groups C and F were poor in species of acidophilous grassland. Groups C and E exhibited many species of dry grasslands as indicators, but they shared only Scabiosa columbaria s.l. Heathland species were under-represented in Group A, unlike the other groups. Species of woodland fringes occurred mainly in group C and were rare in groups B and D.
Figure 4. Cluster analysis (II) on data sets from our unpublished relevés (G1–G12) and from publications regarding heathlands in the study area. Seven groups (A–G) were recognized in the dendrogram, correspondingly assigned to five associations: A, Salici rosmarinifoliae–Callunetum; B, Jasiono montanae–Callunetum; C, a nameless Illyrian association; D, Cytiso supini–Antennarietum; E–F, Chamaecytiso hirsuti–Callunetum.

Table 2. Indicator species of cluster analysis (II) recognized on the base of the point biserial correlation coefficient. Species are arranged according to habitat preference. Figures correspond to classes of presence (those that are statistically significant are shown in bold).
Discussion
Plant species composition and indicator species
In the present study, we detected variation in floristic composition even if the groups of relevés were not primarily based on species but on abiotic factors. The identification of diagnostic species (e.g. indicator species) by means of statistical analysis is an important step in vegetation science, because these species can be used to characterize and indicate plant community types (Chytrý and Tichý Citation2003). If relevés are independently classified from floristic data, as in the present study, the significance of statistical tests performed on the indicator species will be meaningful, i.e. the indicator species can thus be fully considered as indicators, in the true sense of the word (Borcard, Gillet, and Legendre Citation2011).
Instead of describing plant communities using concepts based on subjectivity, e.g. the assertion of character and differential species (Chytrý and Tichý Citation2003), phytosociologists should refer to statistical methods in data analysis, even for the most simple studies (Kent Citation2012) and where possible include more rigorous sampling designs (Chiarucci Citation2007). Preferential relevés made using the phytosociological approach (i.e. a preferential sampling) can be analyzed by statistical tests (Botta-Dukát et al. Citation2007), although results must be interpreted with caution (Michalcová et al. Citation2011). In order to avoid any possible bias, our floristic-independent groups of relevés were compared with each other and then with published data sets by means of statistical tests. In this way, differences in species composition (i.e. the indicator species in Tables and ) can be considered robust as it is based on the “modern” concept of fidelity (Chytrý et al. Citation2002).
Our results have practical consequences for the implementation of the Habitats Directive. A habitat is considered to have a favorable conservation status when the “typical species” they support themselves grow under favorable conditions. The term “typical species” is not defined by the Habitats Directive although conceivably typical species should be selected to reflect favorable structure and functioning of the habitat type. Within a given country, different “typical species” may be needed for different parts of the habitat range or for different subtypes of this habitat (Evans and Arvela Citation2011). The selection of “typical species” and habitat subtypes are primarily based on floristic data (Maciejewski Citation2010). In the present study, indicator species of each cluster group reflect particular ecological conditions or even biogeographic patterns in heathland habitat. The loss of indicator species could reveal change in habitat quality (e.g. decrease of wetland species in response to drainage or decreased rainfall) and therefore deterioration of its conservation status. An initial list of “typical species” could be derived from indicator species but, it seems unreasonable to consider that indicator species can include all typical species. For example, Gentiana pneumonanthe was found to be more abundant at sites with the endangered butterfly Maculinea (Phengaris) alcon (Maes and Dyck Citation2005), therefore it could be considered a “typical species” for wet heathlands. Indeed, G. pneumonanthe was selected as an indicator species by cluster analysis of relevés (Table , G1 and G3), but our analysis failed to find it when other data sets were evaluated (in Table , G. pneumonanthe was not reported, as the point biserial correlation coefficient equaled 0.826 for group A but was at limit of the statistical significance, p = 0.063). Finally, additional criteria could be considered for the determination of “typical species”, but indicator species should be the best choice on a local scale.
Syntaxonomic outcomes
The diversity in floristic composition recognized in the present study allows the syntaxonomy of heathlands in the Po basin and the Southern Alps to be revised. We can propose two improvements: a division of the heathland habitat into different subtypes and the assessment of the corresponding “typical species”. The subtypes correspond to “habitats élémentaires” (elementary habitats; Bensettiti Citation2001–05), which require specific conservation measures to preserve their floristic diversity. However, as pointed out (Table ), most species emerging as indicator species are not strictly heathland species. The reasons are probably the intrinsic nature of heathlands, i.e. a transient stage in the ecological succession of plant communities, and mostly to the high impact of traditional use in the study area (Brusa and Piazza Citation2015). For the latter reason, several “typical species” are vanishing, especially at low altitude.
Group A. Three data sets are included: Guglielmetto Mugion (Citation1996) and two groups of our relevés (G1 and G3). All characterize heathlands on clayey soils from old deposits, where waterlogging occurs in the topsoil layers. “Typical species” are wetland species such as Viburnum opulus, Hypericum humifusum, Agrostis canina, Salix rosmarinifolia (from Table ) and Lotus pedunculatus, Gentiana pneumonanthe, Lysimachia vulgaris, Juncus conglomeratus, Lythrum salicaria, Carex demissa, Drosera intermedia, Rhynchospora fusca, and Carex panicea (from Table ). Wet heathlands can thus be recognized as a separate community. Salix rosmarinifolia, a bush willow that grows scattered throughout the same layer of heather, appears to be the best candidate species for naming the new association: Salici rosmarinifoliae–Callunetum vulgaris (Genisto–Vaccinion alliance).
Group B. Two data sets are included: Giacomini (Citation1958) and one group of our relevés G2, from gravelly deposits mixed with sands near the Ticino River. In contrast with the lowland heathlands of group A, they exhibit porous soils. Short lived species, flowering in spring before summer drought, can be retained as “typical species”: Filago arvensis, Filago minima, Micropyrum tenellum, Teesdalia nudicaulis, Aira caryophyllea (Table ), and Jasione montana (Table ); the endemic Euphrasia cisalpina, recently retrieved in the study area (Martignoni Citation2014), should be considered as another typical species of group B. Oberdorfer (Citation1964) reported these heathlands as Cytiso hirsuti–Callunetum vulgaris. However, his original description clearly referred to a heathland on bedrock (Montorfano, i.e. our G7 groups of relevés) and has been questioned (Cerabolini, Ceriani, and De Andreis Citation1998; Hofer Citation1967). Our analysis supports the distinctiveness of our group from Oberdorfer’s association, so that we propose a new association: Jasiono montanae–Callunetum vulgaris. In addition, the lack of mountain species (Arctostaphylos uva-ursi, Festuca acuminata, Genista pilosa, Phyteuma scheuchzeri, Polygala chamaebuxus, etc.) establishes its inclusion in the alliance Genistion tinctorio-germanicae
Group C. This group includes only one data-set from north-eastern Italy and was assigned to the Genisto–Callunetum illyricum (Feoli, Pignatti and Pignatti Citation1981). Due to the lack of typical Balkan species, Poldini, Oriolo, and Francescato (Citation2004) considered it as Chamaecytiso hirsuti–Callunetum vulgaris (Oberdorfer Citation1964), the south-eastern variant of the central European Cytiso supini–Callunetum vulgaris O. Bolós 1956 (=Cytiso supini–Antennarietum Preising 1953, according to Ellmauer Citation1993). However, the heathland includes exclusive Illyrian species (Centaurea jacea subsp. weldeniana, Eryngium amethystinum, Scorzonera villosa, Sesleria autumnalis) and many species of dry grasslands. The group is confirmed as an outlier in our analysis and its attribution to Oberdorfer’s associations is very unreliable. Its classification should be amended in an analysis involving Illyrian scrublands.
Group D. Three data sets are included in this group. Our two groups of relevés (G5 and G6) are from the eastern part of the study area, thus providing a floristic link to Friuli heathlands (Poldini, Oriolo and Francescato Citation2004) classified as Chamaecytiso hirsuti–Callunetum vulgaris. However, Group D is clearly separated from Group C (Karst heathlands) as reported above; additionally, it is different from western heathlands (groups E–F, including the true Chamaecytiso hirsuti–Callunetum vulgaris). The “typical species” are Cytisus nigricans, Erica carnea, Fraxinus ornus (Table ) and Phyteuma scheuchzeri, Brachypodium rupestre, Viola canina, Arctostaphylos uva-ursi, Lathyrus linifolius, Campanula scheuchzeri (Table ). Group D occurs on dry, sunny and warm mountains, and it presents floristic affinities with northern Apennine heathlands (Angiolini et al. Citation2007; Nowak Citation1987) which are assigned to the Erico herbaceae–Genistetum pilosae Oberdorfer and Hofmann Citation1967 ex Nowak Citation1987;. However, Deschampsia flexuosa, Genista pilosa, and Vaccinium myrtillus are infrequent in group D and in the original table of the association (Oberdorfer and Hofmann Citation1967); in addition, the Mediterranean Erica arborea is lacking. Surprisingly, there is a strong similarity with the floristic list of the “Calluno–Ericetum” described from the Austrian Southern Alps (Onno Citation1933) and currently included p.p. within the Cytiso supini–Antennarietum dioicae (Ellmauer Citation1993). Pending a complete phytosociological review of the Alpine heathlands, it seems more reasonable to provisionally assign group D to the latter association.
Groups E and G. These two groups are treated together due to the strong similarities in their species composition. Many species of dry grasslands are present in group E, in contrast with species of acidophilous grasslands occurring mainly in group G. Accordingly, “typical species” of group E include Dianthus carthusianorum, Hylotelephium maximum, Artemisia campestris, Phleum phleoides, Galium lucidum, Scabiosa columbaria s.l., Silene otites; and for group G, Danthonia decumbens, Potentilla erecta, and Carex pilulifera. However, Festuca acuminata, an endemic species mainly from the western Alps (Wallossek Citation1999) is the most prominent “typical species” of both groups. The groups include the provisional association “Gryllo–Callunetum” in all its variants (Antonietti Citation1970) and data sets reported in Hofer (Citation1967) and in Oberdorfer (Citation1964). There is a continuum in the floristic composition of the western heathlands on bedrock, largely due to anthropogenic factors, rendering irrelevant any attempt to subdivide the groups into new associations. We thus assign these groups to the association Chamaecytiso hirsuti–Callunetum vulgaris described by Oberdorfer (Citation1964); however, a new subassociation could be recognized where F. acuminata occurs. Nevertheless, a group of our relevés (G4) lacks F. acuminata because it includes heathlands on sedimentary rocks at low altitude; it could simply correspond to a floristic impoverishment due to local ecological factors.
Group F. This group includes three data sets from the western area of the Southern Alps. Two of them represent relevés including the western European Erica cinerea reported by Lonati and Siniscalco (Citation2010), who referred to the heathlands as floristic races within the Chamaecytiso hirsuti–Callunetum vulgaris. Our analysis confirmed these authors’ interpretation: group F includes our group G8 where Erica cinerea is missing (the species was not detected as a typical species for group F). In conclusion, group F is included in a broader definition of the western alpine association Chamaecytiso hirsuti–Callunetum vulgaris (including groups E and G), in which many floristic races of biogeographical significance (respectively with Erica cinerea and Festuca acuminata) could be detected hitherto.
Syntaxonomic scheme
The syntaxonomic proposal is in accordance with the guidelines of the International Code of Phytosociological Nomenclature (Weber, Moravec, and Theurillat Citation2000).
Calluno vulgaris–Ulicetea minoris Braun-Blanq. & Tüxen ex Klika in Klika & Hadač 1944
Vaccinio myrtilli–Genistetalia pilosae R.Schub. 1960
Genisto pilosae–Vaccinion uliginosi Br.-Bl. 1926
Cytiso supini–Antennarietum dioicae Preising 1953
Chamaecytiso hirsuti–Callunetum vulgaris Oberdorfer Citation1964
ericetosum cinereae Lonati and Siniscalco Citation2010
festucetosum acuminatae subass. nova, holotypus (Montecrestese, VB, Piedmont; 13/08/1998): Calluna vulgaris 4, Festuca acuminata 2, Quercus pubescens 2, Agrostis capillaris +, Allium lusitanicum +, Anthericum liliago +, Carex pilulifera +, Cytisus scoparius +, Danthonia decumbens +, Festuca stricta subsp. trachyphylla +, Fraxinus ornus +, Genista germanica +, Juniperus communis +, Molinia caerulea subsp. arundinacea +, Quercus robur +, Sempervivum arachnoideum +, Silene rupestris +, Vincetoxicum hirundinaria +, Stachys officinalis r
Genistion tinctorio-germanicae de Foucault 2008
Jasiono montanae–Callunetum vulgaris ass. nova hoc loco, holotypus (Cameri, NO, Piedmont; 04/07/1996): Calluna vulgaris 4, Molinia caerulea subsp. arundinacea 2, Rubus fruticosus agg. 2, Betula pendula 1, Cytisus scoparius 1, Dichanthelium acuminatum 1, Pinus sylvestris 1, Teucrium scorodonia 1, Agrostis capillaris +, Frangula alnus +, Hypericum perforatum +, Jasione montana +, Luzula multiflora +, Micropyrum tenellum +, Quercus pubescens +, Quercus robur +, Rumex acetosella +, Solidago gigantea +, Teesdalia nudicaulis +, Filago arvensis r, Hypochoeris radicata r
Salici rosmarinifoliae–Callunetum vulgaris ass. nova hoc loco, holotypus (Candelo, BI, Piedmont; 17/06/1996): Calluna vulgaris 5, Molinia caerulea subsp. arundinacea 3, Carex panicea 1, Frangula alnus 1, Populus tremula 1, Salix rosmarinifolia 1, Agrostis canina +, Carex pilulifera +, Danthonia decumbens +, Festuca filiformis +, Gentiana pneumonanthe +, Potentilla erecta +, Serratula tinctoria +, Carex demissa r, Carpinus betulus r, Drosera intermedia r, Genista tinctoria r, Succisa pratensis r
Notes on contributors
Bruno E. L. Cerabolini, PhD, Associate Professor of Environmental and Applied Botany (University of Insubria). Contributions: field surveys for data collection, data analysis, writing text.
Guido Brusa, PhD, Freelance biologist and institutional collaborator (University of Insubria). Contributions: field surveys for data collection, data analysis, writing text.
Roberta M. Ceriani, PhD, Biologist at an institutional body of nature conservation (Centro Flora Autoctona). Contributions: field surveys for data collection, refining the manuscript.
Stefano Armiraglio, PhD, Naturalist and curator of Botany (Brescia Civic Museum of Natural Sciences). Contributions: field surveys for data collection.
Cristina De Molli, PhD student, Master of Science (University of Insubria). Contributions: field surveys for data collection.
Simon Pierce, PhD, Assistant Professor of Environmental and Applied Botany Botany (University of Milan). Contributions: refining the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Angiolini, C., B. Foggi, D. Viciani, and A. Gabellini. 2007. “Acidophytic Shrublands in the North-West of the Italian Peninsula: Ecology, Chorology and Syntaxonomy.” Plant Biosystems 141: 134–163.10.1080/11263500701401315
- Antonietti, A. 1970. “Su un’associazione di brughiera del piede meridionale delle Alpi [On A Heathland Association in the Southern Foot of the Alps].” Berichte des Geobotanischen Institutes der ETH, Stiftung Rübel 40: 9–27.
- Ascoli, D., and G. Bovio. 2010. “Tree Encroachment Dynamics in Heathlands of Northwest Italy: The Fire Regime Hypothesis.” Forest 3: 137–143.
- Belloni, S. 1975. “Il clima delle province di Como e di Varese in relazione allo studio dei dissesti idrogeologici [The Climate of the Provinces of Como and Varese in Relation to the Study of Hydrogeological Disasters].” C.N.R. Fondazione per i Problemi Montani dell’Arco Alpino 99: 1–95.
- Bensettiti, F., ed. 2001‒05. Cahiers d’habitats Natura 2000. Connaissance et gestion des habitats et des espèces d’intérêt communautaire [Natura 2000 Habitat booklets. Knowledge and Management of Habitats and Species of Community Interest]. Paris: La Documentation française.
- Biancotti, A., G. Bellardone, S. Bovo, B. Cagnazzi, L. Giacomelli, and C. Marchisio. 1998. Distribuzione regionale di piogge e temperature [Regional distribution of precipitations and temperatures] Collana Studi Climatici in Piemonte –Volume 1. Torino: Regione Piemonte-Università di Torino.
- Biondi, E., C. BLASI, M. Allegrezza, I. Anzellotti, M. M. Azzella, E. Carli, S. Casavecchia, et al. 2014. “Plant communities of Italy: The Vegetation Prodrome.” Plant Biosystems 148: 728–814. 10.1080/11263504.2014.948527
- Borcard, D., F. Gillet, and P. Legendre. 2011. Numerical Ecology with R. New York: Springer Science & Business Media. 10.1007/978-1-4419-7976-6
- Botta-Dukát, Z., E. Kovács-Láng, T. Rédei, M., Kertész, and J. Garadnai. 2007. “Statistical and Biological Consequences of Preferential Sampling in Phytosociology: Theoretical Considerations and A Case Study.” Folia Geobotanica 42: 141–152. 10.1007/BF02893880
- Brusa, G., and D. Piazza. 2015. La brughiera pedemontana lombarda: aspetti storici, fattori ecologici e indicazioni gestionali per la sua conservazione [The Lombardy Piedmont Heather: Historical Aspects, Ecological Factors and Management Guidelines for its Conservation]. Lentate sul Seveso: Consorzio del Parco Brughiera Briantea.
- Bunce, R. G. H., M. M. B. Bogers, D. Evans, and R. H. G. Jongman. 2013. “Field Identification of Habitats Directive Annex I Habitats as a Major European Biodiversity Indicator.” Ecological Indicators 33: 105–110. 10.1016/j.ecolind.2012.10.004
- Cáceres, M., and P. Legendre. 2009. “Associations Between Species and Groups of Sites: Indices and Statistical Inference.” Ecology 90: 3566–3574. 10.1890/08-1823.1
- Cerabolini, B., R. Ceriani, and R. De Andreis. 1998. “Biogeographical, Synecological and Syntaxonomical Outlines of Lombardy and Piedmont Lowland Heathlands (NW Italy).” Colloques Phytosociologiques 28: 629–640.
- Cerabolini, B., S. Armiraglio, S. Assini, S. Verde, M. Caccianiga, C. Andreis, and F. Sartori. 2005. “Problematiche fitogeografiche e sintassonomiche del territorio lombardo: alcuni esempi [Phytogeographic and Syntaxonomic Problems of the Lombard Territory: Some Examples].” Informatore Botanico Italiano 37: 482–483.
- Ceriani, M., and M. Carelli. 1999. Carta delle precipitazioni medie, minime e massime annue del territorio alpino lombardo (registrate nel periodo 1891–1990) [Map of the Average, Minimum and Maximum Annual Precipitations in Lombardy Alpine region (recorded in the period 1891–1990)]. Milano: Regione Lombardia.
- Chiarucci, A. 2007. “To Sample or not to Sample? That is the Question.. For the Vegetation Scientist.” Folia Geobotanica 42: 209–216.10.1007/BF02893887
- Chytrý, M., and L. Tichý. 2003. “Diagnostic, Constant and Dominant Species of Vegetation Classes and Alliances of the Czech Republic: A Statistical Revision.” Folia Fac Sci Nat Univ Masaryk Brun Biol 108: 1–231.
- Chytrý, M., L. Tichý, J. Holt, and Z. Botta-Dukát. 2002. “Determination of Diagnostic Species with Statistical Fidelity Measures.” Journal of Vegetation Science 13: 79–90. 10.1111/j.1654-1103.2002.tb02025.x
- Conti, F., S. Abbate, A. Alessandrini, and C. Blasi. 2005. An annotated Check-List of the Italian Flora. Roma: Ministero per l’Ambiente.
- Ellenberg, H. 1988. Vegetation Ecology of Central Europe. Cambridge: Cambridge University Press.
- Ellmauer, T. 1993. “Calluno-Ulicetea.” In Die Pflanzengesellschaften Oesterreichs, edited by L. Mucina, G. Grabherr and T. Ellmauer, I. Teil, 402–419. Jena: Fischer.
- Evans, D. 2006. “The Habitats of the European Union Habitats Directive.” Biology and Environment: Proceedings of the Royal Irish Academy 106B: 167–173.
- Evans, D. 2010. “Interpreting the Habitats of Annex I: Past, Present and Future.” Acta Botanica Gallica 157: 677–686. 10.1080/12538078.2010.10516241
- Evans, D., and M. Arvela. 2011. Assessment and Reporting under Article 17 of the Habitats Directive. Explanatory Notes & Guidelines for the period 2007-2012. Paris: European Topic Centre on Biological Diversity.
- Fagúndez, J. 2012. “Heathlands Confronting Global Change: Drivers of Biodiversity Loss From Past to Future Scenarios.” Annals of Botany 111: 151–172.
- Feoli, E., E. Pignatti, and S. Pignatti. 1981. “Successione indotta dal fuoco nel Genisto-Callunetum del Carso Triestino [Fire-induced succession in the Genisto-Callunetum of the Trieste Karst].” Studi trentini di scienze naturali: Acta biologica 58: 231–240.
- Giacomini, V. 1958. “Sulla vegetazione della Brughiera di Gallarate [About the Vegetation of Heath Gallarate.]” Archivio Botanico e Biogeografico Italiano 34: 63–68.
- Gimingham, C. H. 1972. Ecology of Heathlands. London: Chapman Hall.
- González, T. E. D. 1998. “Síntesis de la vegetación arbustiva de Europa occidental. I: Brezales (Calluno-Ulicetea) [Synthesis of shrub Vegetation in Western Europe. I: Heathlands (Calluno-Ulicetea)].” Itinera geobotanica 11: 7–31.
- Guglielmetto Mugion, L. 1996. “Vegetational aspects of Calluna heathlands in the western Po plain (Turin, NW Piedmont, Italy).” Allionia 34: 343–348.
- Hofer, H. R. 1967. “Die wärmeliebenden Felsheiden Insubriens [Thermophilous Heathlands on rocks in Insubria].” Botanische Jahrbücher 87: 176–251.
- Kent, M. 2012. Vegetation Description and Data Analysis: A Practical Approach. Chichester: Wiley-Blackwell.
- Lonati, M., A. Gorlier, D. Ascoli, R. Marzano, and G. Lombardi. 2009. “Response of the alien species Panicum acuminatum to Disturbance in an Italian lowland heathland.” Botanica Helvetica 119: 105–111.10.1007/s00035-009-0063-3
- Lonati, M., and C. Siniscalco. 2010. “Syntaxonomy and synecology of Erica cinerea L. communities in the Alps (North-Western Italy).” Acta Botanica Gallica 157: 493–504. 10.1080/12538078.2010.10516225
- Maciejewski, L. 2010. Méthodologie d’élaboration des listes d’«espèces typiques» pour des habitats forestiers d’intérêt communautaire en vue de l’évaluation de leur état de conservation [Methodology to Elaborate Lists of ‘Typical Species’ For Forest Habitats of Community Interest in Order to Assess Their Conservation Status]. Paris: Rapport SPN 2010-12 / MNHN-SPN.
- Maes, D., and H. Dyck. 2005. “Habitat Quality and Biodiversity Indicator Performances of A Threatened Butterfly Versus A Multispecies Group For Wet Heathlands In Belgium.” Biological Conservation 123: 177–187. 10.1016/j.biocon.2004.11.005
- Martignoni, M. 2014. “Euphrasia cisalpina Pugsley (Orobanchaceae) nella Brughiera di Gallarate (Lombardia, Italia): dati storici e conferma della stazione nelle aree verdi dell’Aeroporto di Milano Malpensa [Euphrasia cisalpina Pugsley (Orobanchaceae) in the Heathland of Gallarate (Lombardy, Italy): Historical Data and Evidence of its Occurrence in the Green Areas of the Milano Malpensa Airport].” Atti della Società italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano 1: 19–24.
- Michalcová, D., S. Lvončík, M. Chytrý, and O. Hájek. 2011. “Bias in Vegetation Databases? A Comparison of Stratified-Random and Preferential Sampling.” Journal of Vegetation Science 22: 281–291.10.1111/jvs.2011.22.issue-2
- Murtagh, F., and P. Legendre. 2014. “Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion?” Journal of Classification 31: 274–295. 10.1007/s00357-014-9161-z
- Nowak, B. 1987. “Untersuchungen zur Vegetation Ostliguriens (Italien) [Studies on the Vegetation of Eastern Liguria].” Dissertationes Botanicae 111: 1–259.
- Oberdorfer, E. 1964. “Der insubrische Vegetationskomplex, seine Struktur und Abgrenzung gegen die submediterrane Vegetation in Oberitalien und in der Südschweiz [The Insubrian Vegetation Complex, its Structure and Delimitation Against Sub-Mediterranean Vegetation in Northern Italy and Southern Switzerland].” Beiträge Naturkundlicher Forschung SW-Deutschland 23: 141–187.
- Oberdorfer, E., and A. Hofmann. 1967. “Beitrag zur Kenntnis der Vegetation des nordapennin [Contribution to the Knowledge of the Vegetation in Northern Apennine].” Beitr Naturk Forsch SW Deutsch 26: 83–139.
- Onno, M. 1933. “Über das ‘Calluno-Ericetum’ in den südlichen Ostalpen [About the ‘Calluno-Ericetum’ in the Southern-Eastern Alps].” Österreichische botanische Zeitschrift 82: 235–244. 10.1007/BF01246319
- Pavari, A. 1927. “Boschi e brughiere [Forests and Heathlands].” In Le brughiere, edited by F. Luzzatto, E. Artini, U. Brizi, L. Fenaroli, U. Pratolongo, P. Parisi, V. Alpe and A. Pavari, 234–241. Milano: Federazione Italiana dei Consorzi Agrari.
- Poldini, L., G. Oriolo, and C. Francescato. 2004. “Mountain Pine Scrubs and Heaths with Ericaceae in the South-Eastern Alps.” Plant Biosystems 138: 53–85. 10.1080/11263500410001684125
- R Core Team. 2015. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/.
- Rousseeuw, P. J. 1987. “Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis.” Journal of Computational and Applied Mathematics 20: 53–65. 10.1016/0377-0427(87)90125-7
- Ter Braak, C. J. F. 1987. “The Analysis Of Vegetation–Environment Relationships by Canonical Correspondence Analysis.” Vegetatio 69: 69–77. 10.1007/BF00038688
- Tinner, W., M. Conedera, B. Ammann, and A. F. Lotter. 2005. “Fire Ecology North and South of the Alps since the Last Ice Age.” The Holocene 15: 1214–1226. 10.1191/0959683605hl892rp
- Valese, E., M. Conedera, A. C. Held, and D. Ascoli. 2014. “Fire, Humans and Landscape in the European Alpine Region During the Holocene.” Anthropocene 6: 63–74. 10.1016/j.ancene.2014.06.006
- Wallossek, C. 1999. “The Acidophilous Taxa of the Festuca Varia Group in the Alps: New Studies on Taxonomy and Phytosociology.” Folia Geobotanica 34: 47–75. 10.1007/BF02803076
- Webb, N. R. 1998. “The Traditional Management of European Heathlands.” Journal of Applied Ecology 35: 987–990.
- Weber, H. E., J. Moravec, and J. P. Theurillat. 2000. “International Code of Phytosociological Nomenclature.” Journal of Vegetation Science 11: 739–768. 10.2307/3236580
