Abstract
Flowering in many orchids is determined by the resource status of plants, which in turn is influenced by habitat management. Most European orchids require high light intensities for photosynthesis, failing to flower and fruit at low light levels. Agricultural practices, especially fertilisation and mowing/grazing regime, can therefore influence the fitness and reproductive success of orchids. The studied species, autumn lady’s-tresses (Spiranthes spiralis), is a small, long-lived and late-flowering perennial orchid and one of the most sensitive to low light availability. The general species’ fitness, measured as a set of robust morphological traits in relation to vegetation height, which directly reflect the time of the last seasonal mowing and so the light availability, was the main scope of this research. A total of 2442 flowering exemplars (of which 427 were morphologically evaluated) were recorded on 26 grassland patches applying the systematic scanning of the potential growing sites in the Goričko Natural Park (NE Slovenia). We revealed that earlier mowing negatively affects the density of flowering individuals and plant fitness in general. The number of the rosette leaves was found to be the most important trait that could be used as a proxy for the general plant fitness of this orchid species because the significant positive correlation with the measured morphological traits was confirmed. The number of the rosette leaves shows a significant negative relation to vegetation height. Late seasonal mowing, which has a significant positive impact on the plant fitness, enhances Spiranthes spiralis population viability and density.
Introduction
Grasslands classified according to the European Union Habitats interpretation manual as semi-natural dry grasslands and scrubland facies, include important orchid sites, as well as being of great interest due to the high species richness and the number of rare or threatened entities found on them (Kull and Hutchings Citation2006; Poschlod and Wallis DeVries Citation2002). Understanding and measuring how the distribution of biodiversity, particularly for rare and endangered taxa, shifts in space and over time as habitats change are central issues in conservation biology (Vogt-Schilb et al. Citation2016).
Orchids are a particularly interesting group for studying changes in the distribution of species; they are particularly vulnerable to climate and land-cover changes (Pfeifer et al. Citation2006; Wotavová, Balounová, and Kindlmann Citation2004) due to their narrow ecological preferences. Orchids are sensitive to habitat changes produced by ecological succession or disturbances (Vogt-Schilb et al. Citation2015; Wotavová, Balounová, and Kindlmann Citation2004). Also, orchids are poor competitors when conditions permit high densities of other herbaceous plants (Landi et al. Citation2009; Wotavová, Balounová, and Kindlmann Citation2004). Most European orchids require high light intensities for photosynthesis, failing to flower and fruit at low light levels (Dorland and Willems Citation2006; Jacquemyn et al. Citation2009). Orchid species may be particularly vulnerable to the effects of habitat management for at least two reasons (Meekers and Honnay Citation2011). First, most orchids are highly site-specific and often require particular environmental conditions, for example, the presence of mycorrhizal fungi, to complete their life cycle (Rasmussen Citation1995). Second, some orchid species rely on specialised pollinators, making them more vulnerable to pollinator limitation (Meekers and Honnay Citation2011). Life history characteristics of terrestrial orchids are generally well known, but detailed ecological data are available for relatively few species (Gill Citation1996; Hutchings Citation1987; Sieg and King Citation1995). Also, only a few studies have evaluated the influence of changes in vegetation cover on orchids (Schrautzer et al. Citation2011; Wotavová, Balounová, and Kindlmann Citation2004).
Flowering and the survival of populations of many European orchid species are determined by the resource status of plants, which in turn is influenced by appropriate habitat management (mowing), climatic conditions, or patterns of herbivory (Kull Citation2002; Waite and Hutchings Citation1991). Agricultural and grazing practices can therefore influence the fitness of orchids by limiting competitors (dominant plants) and permitting the creation of “regeneration gaps” (Grubb Citation1986).
Even if mowing is generally considered beneficial to the fitness of meadow orchids (Kull Citation2002), its occurrence does not automatically ensure persistence of orchid populations (Tamm Citation1991). There are two mechanisms by which mowing can affect orchid performance (Janečková et al. Citation2006). Early mowing can suppress their competitors, dominant grasses, whereas late mowing (August, September) removes the old plant biomass, thus reducing shading of orchids in the subsequent year and increasing light available for photosynthesis (Lepš Citation1999).
Tall (and dense) herbaceous vegetation competes with orchids (Janečková et al. Citation2006) and represents a factor of disturbance, as it reduces the amount of available light (Maccherini Citation2006). The majority of orchid species may be tolerant to reduced light availability at the beginning of vegetation closure (Vogt-Schilb et al. Citation2016). Vogt-Schilb et al. (Citation2016) recorded the following orchid species: Anacamptis laxiflora (Lam.) R. M. Bateman, Pridgeon & M. W. Chase, Spiranthes aestivalis (Poir.) Rich and Spiranthes spiralis as most sensitive to low light availability. Spiranthes spiralis flowers in August–September, and the number of flowering plants is largely determined by the plant’s performance during the previous year. Plant performance is mostly determined by site management (Whigham and Willems Citation2003).
Spiranthes spiralis (L.) Chevall., a small, long-lived perennial orchid, was studied. This species is well adapted to survive in open habitats, and it can become abundant when the vegetation is kept short by rabbits or domesticated grazing animals (Jacquemyn and Hutchings Citation2010). Due to the remarkable phenology of Spiranthes spiralis, late mowing, just before the species starts to flower, should be of great importance. Its rosettes remain active until the next spring, and so late mowing at the appropriate time increases available light for photosynthesis during winter and spring months, and thereby determines the amount of stored carbohydrates in the underground storage organs for the next year for this particular orchid species. The direct effects of late mowing on Spiranthes spiralis plant fitness and population viability are poorly understood.
We have chosen Spiranthes spiralis as a study species not only due to its interesting biology and phenology, but because it is a good flagship species for further conservation guidelines, as already stated by Böhnert, Hecht, and Stapperfenne (Citation1986). Orchids in general are considered to be important indicators of the habitat quality and high biodiversity of the European semi-natural grasslands in general. Since the proportion of flowering plants within natural populations varies substantially from one year to the next, we focused on a single season sampling per location. Late seasonal mowing time on grassland patches also varies substantially from one year to the next according to climatic conditions.
We aimed to reveal the effect of the vegetation height, directly influenced by the last mowing date on spatial density and fitness of flowering individuals of Spiranthes spiralis. Our research questions were as follows:
| (1) | Is there any relation between vegetation height (which directly reflects the time length since the last mowing) and species’ frequency and density? | ||||
| (2) | How are the robust morphological traits correlated? Is there one proxy parameter for the plant fitness of the flowering individuals? | ||||
| (3) | How is the plant fitness related to the time length since the last mowing? | ||||
Methods
The species
Spiranthes spiralis (L.) Chevall. is a small, long-lived perennial orchid that is widely distributed in Southern Europe, in the Mediterranean region, and in coastal areas of North Africa (Tutin et al. Citation1980; Ziegenspeck Citation1936). Spiranthes spiralis can be found in suitable open habitats throughout much of Europe (Willems Citation2006). The species is fairly common in eastern (Greece, Asia Minor, Turkey, Cyprus, Lebanon and Israel), central (Italy) and western (the Balearics) (Jacquemyn and Hutchings Citation2010) areas, and reaches its north-western distribution limit in the southern part of the British Isles and the Netherlands (Jacquemyn and Hutchings Citation2010). Further north, it has been recorded in southernmost Sweden, east to the Baltic States and in western Ukraine (Jacquemyn and Hutchings Citation2010). Preston and Hill (Citation1997) include Spiranthes spiralis as a member of the European Southern-temperate element, and Summerhayes (1951) refers to it as belonging to the southern Eurasian element (Jacquemyn and Hutchings Citation2010). In central Europe, the species is less common; a few locations are known in Slovakia (Králik Citation1998), the Czech Republic (Jatinova and Schmitak Citation1996), Switzerland (Reinhard et al. Citation1991), southern Germany (AHO Bayern Citation2015) and Austria (Bohner, Kerschbaumsteiner, and Starlinger Citation2010). The species is found on a wide range of substrates, ranging from shallow chalk and limestone substrates to sand and gravel in dunes and slightly acidic heathlands (Jacquemyn and Hutchings Citation2010).
Spiranthes spiralis is a non-bulbous geophyte that is capable of multiplying and spreading through vegetative reproduction. However, the capacity for vegetative reproduction seems to be quite limited (Jacquemyn and Hutchings Citation2010). Vegetative reproduction occurs (Fig. D) by the growth of a lateral bud on the underground stem, the new plant forming its own tubers, and eventually the connection between the mother plant and vegetative younglings withers away (Wells Citation1967). However, all rosettes that grow adjacent to each other are not necessarily the product of vegetative reproduction, as assumed by Wells (Citation1967), but can also arise from the seedlings, since Spiranthes spiralis has a limited seed dispersion range due to the relative heaviness of its seeds (Jacquemyn and Hutchings Citation2010). In this way, small groups of plants are formed, which are easily distinguished from plants that have arisen from seeds (Jacquemyn et al. Citation2007).
The height of the flower stalks varies between 5 and 28 cm and it may bear up to 37 small, white flowers (<0.5 cm), which are arranged in a tight left- or right-handed spiral on the upper half of the stalk. Rarely, there is no perceptible twist in the insertion of flowers upon the stem. Seed capsules become ripe in October or November, and the seeds are wind-dispersed. Few insect species have been observed pollinating Spiranthes spiralis (Jacquemyn and Hutchings Citation2010). The species appears to be specifically adapted to bee pollination (Van der Cingel Citation1995). Darwin (Citation1877) mentions three bumblebees belonging to two different species. The flowers with nectar and a honey scent attract hymenopterans, bumblebee species, mainly Bombus lapidarius (Linnaeus 1758) and Bombus pascuorum (Scopoli 1763) (Willems and Lahtinen Citation1997). In the study of Claessens and Kleynen (Citation2016) various bumblebees, Bombus terrestris (Linnaeus 1758), Bombus lapidarius and Bombus sylvarum (Linnaeus 1761) honeybees and also small solitary bees Halictus simplex (Blüthgen 1923) were observed pollinating the species. Autogamy was not observed in this species (Jacquemyn et al. Citation2007).
Spiranthes spiralis has a remarkable phenology in central Europe, where it is by far the latest flowering of all native orchids. Its late flowering may have restricted its distribution, both northwards and above moderate altitudes, where the occurrence of early snow is likely to limit the seed-formation success (Jacquemyn and Hutchings Citation2010). Ziegenspeck (Citation1936) mentions that Spiranthes spiralis can be found in a range of meadow and grassland communities throughout the central Europe, but it was also observed near the field borders and along forest edges. The species is well adapted to survive in non-forest habitats where it can become abundant when the vegetation is kept short by grazing animals. Since the rosette is closely appressed to the ground, damage to the leaves by trampling, grazing or frost appears to be very limited (Tyteca Citation2000).
The factors that determine whether a plant will flower or not are complex and still poorly understood (Jacquemyn and Hutchings Citation2010). In a population monitored in the Netherlands between 1981 and 2004, the proportion of the emergent population that flowered ranged from 0% to 100% (mean 37.6%) in-between seasons (Jacquemyn and Hutchings Citation2010).
In Slovenia, the species occurs rather dispersed through the whole area with higher densities in regions on limestone and flysch substrates. However, the data about its occurrence in the eastern and north-eastern parts of the country are very scarce (Jogan et al. Citation2001; Kocjan Citation2014). In the last decade, new finds of Spiranthes spiralis were recorded in several different parts of NE Slovenia (Bakan Citation2006; Dolinar Citation2015; Kocjan Citation2014; Ravnik Citation2002), many of those unpublished. However, no data have been published for Goričko Natural Park prior to this study. All locations presented in the manuscript were newly discovered by the authors. We consider the population, located inside the borders of Goričko Natural Park to be one of the richest in the country even though the appropriate habitats – extensively used dry and semi-dry secondary grasslands – have deteriorated considerably.
In Slovenia, this species is protected by law and included in the Red List (Anonymus Citation2002) as a vulnerable taxon (VU). For that reason, the investigation of proper management, mowing practices (since grazing is absent in many areas) are of great importance in order to ensure the long-term survival of this species. Also, preservation of semi-dry grasslands of Goričko is of great importance for ensuring the existence of the populations within the Goričko Natural Park.
Study area
The study area lies within the borders of the Goričko Natural Park, which is part of northeast Slovenia, bordering Austria and Hungary. Goričko Natural Park (Figure ) was established in 2003 in order to protect the well-preserved traditional, central European agricultural landscape (Figure A). The mosaic structure of the landscape and the variety of secondary habitats within the park were formed by the simultaneous effects of both natural and anthropogenic in the sense of geopolitical, economic and social drivers (Kaligarič, Sedonja, and Šajna Citation2008). In the last 30 years, traditional land management has drastically changed. The grassland areas severely decreased, mainly due to abandonment of livestock farming and intensification of crop production, hence transforming grasslands into arable land. One of the most important and disappearing grassland habitats in Goričko Natural Park is the semi-dry acid grassland of the Festuco-Brometea class (Škornik Citation2003). The area of Goričko Natural Park (Figure ) has a central European climate with relatively dry and mild winters. Mean annual precipitation values range from 750 mm to 850 mm. The driest months are considered to be January and February, while most of the rain falls between July and August. The mean annual temperature ranges between 9°C and 10°C (ARSO Citation2015). The colline belt of Goričko Natural Park consists mainly of Tertiary sandstone and clay sediments, making the soils acidic and forming a soft hilly landscape with relatively small inclination differences (Činč Juhant and Planjšek Citation2002).
Figure 1. Geographic position of Slovenia in Europe (top left) and the research area of Goričko Natural Park in NE Slovenia (bottom left). The 26 sampled Spiranthes spiralis localities are within the Goričko Natural Park (right).
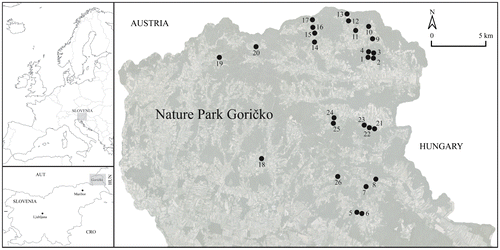
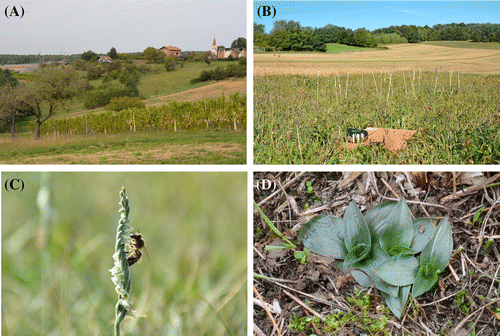
Figure 2. Traditional Central European agricultural landscape, typical for the Goričko Natural Park (A). Regularly mowed semi-dry grassland with Spiranthes spiralis specimens marked with sticks in order not to damage the individual plants (B). Apis mellifera observed while visiting the flowers of Spiranthes spiralis (C). Developed Spiranthes spiralis leaf rosettes (D).
Field methods
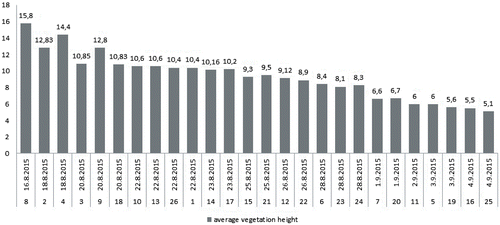
The study area of Goričko Natural Park was systematically scanned during early September 2015 in order to locate grassland patches with Spiranthes spiralis. Suitable patches with expected occurrences of Spiranthes spiralis were selected throughout the whole region, based on previous field experiences. We searched for regularly managed (mown) dry and semi-dry grassland patches. We also visited abandoned grasslands (of different stages), but no Spiranthes spiralis specimens were detected on them. Late mown grasslands are mown before the first Spiranthes spiralis individuals begin to flower; on the other hand, early mown are those fragments where the last mowing phase was performed in July or August, so that the vegetation is higher at the beginning of Spiranthes spiralis flowering. Observed dry and semi-dry grasslands are nutrient-poor and with low annual biomass production. Even when observing early mown fragments, the vegetation was no higher than 25–35 cm, and flowering Spiranthes specimens were clearly visible within the vegetation. All grassland patches were sampled within a 5-day period (13 September 2015 to 17 September 2015) in order to reduce the effect of vegetation growth in the sampling period after the last mowing phase. The time of the last mowing was obtained according to the interviews with land owners. Only the flowering orchid specimens were counted in the field. Immature plants produce rosettes for the first 4–6 years before producing stalks. Rosettes of non-flowering, immature specimens were not analysed. These rosettes are smaller and tend to produce fewer leaves, and the plants are not yet capable of sexual reproduction. We dismissed these, since they do not represent the “reproducing pool” of the investigated populations. Our goal was to evaluate mature plants only and those capable of reproducing, two reasons why we selected flowering plants only, to receive the full data set from each specimen. We have also measured some of the morphological traits on 20 randomly selected flowering specimens per patch. On patches where fewer than 20 flowering specimens were found, all flowering specimens were morphologically analysed. We measured (a) height of the inflorescence stalks (cm), (b) number of flowers per inflorescence, (c) inflorescence length (cm) and (d) the rosette-leaf number. The mean distance between flowers per inflorescence was calculated. The mean distance between the flowers is the ratio of the inflorescence length (cm), divided by the number of the flowers per inflorescence. This ratio was found to be important in the genus Spiranthes in terms of pollination success. The mean Spiranthes spiralis density per grassland patch was calculated as the number of flowering specimens/grassland area. The vegetation height was randomly measured 20 times per patch in the close vicinity of the flowering Spiranthes spiralis specimens.
Data analysis
The spatial geometry data on patch areas were obtained using ArcGIS 9.3 Spatial analyst tools (ESRI Citation2010) by vectorising the plots, which were first drawn on printed orthophoto images (pixel size = 0.5 m) (GURS Citation2010). Spearman’s rank correlation coefficient for the non-normally distributed data was used to identify and test the strength of the relationship between (1) orchid population size regarding the number of flowering specimens per patch, (2) population density regarding the number of flowering specimens per m2 per patch, (3) grassland patch area, (4) vegetation height and (5) the observed morphological traits of the species (SPSS Inc. Citation2006).
Spearman’s rank correlation coefficient for the non-normally distributed data was used to identify and test the strength of the relationship between the observed morphological traits of the species (SPSS Inc. Citation2006) by analysing 427 flowering individuals.
Results
Semi-natural grasslands with Spiranthes spiralis
During the field research, 30 grassland patches with the presence of Spiranthes spiralis detected after thorough scanning of entire territory of the Natura Park Goričko were examined. We dismissed a closer evaluation and specimen measurement on four patches due to their inaccessibility. All the analysed data were collected from the remaining 26 patches (Tables and ). Flowering specimens of Spiranthes spiralis were more or less randomly dispersed within each patch area (no aggregation observed). The mean area of studied patches is 0.32 ha. All investigated patches are relatively well dispersed throughout the central and eastern part of the Goričko Natural Park (Figure ). No patches containing Spiranthes spiralis were found in the western part of study area, close to the border with Austria. In the western part of the Goričko Natural Park, near the border with Austria, fields dominate in the landscape structure in contrast to the central and eastern part of the Nature Park, where the traditional central European agricultural landscape consists of arable land, human infrastructure, road verges and especially grasslands. In the western part of the study area, the proportion of lowland is greater, and the elevation differences are lower compared with the central and eastern part of the Park. Grasslands present in the western part are more mesic rather than semi-dry; some are even flooded grasslands, not a suitable habitat for Spiranthes spiralis. From all the above, the majority of grassland fragments with Spiranthes spiralis were located in the central and eastern part of the study area.
Table 1. Researched grassland patches (N = 26) in Goričko Natural Park according to the community type, their patch area, elevation, number of flowering Spiranthes spiralis specimens per patch, the mean vegetation height and patch density of specimens of Spiranthes spiralis per 1 m2. Community type: Rb/A = Ranunculo bulbosi–Arrhenatheretum elatioris; H/Fr = Hypochoerido–Festucetum rupicolae.
Table 2. Mean values and standard deviations (SD) of the measured morphological traits of Spiranthes spiralis specimens on researched grassland patches (N = 26) in Goričko Natural Park.
The sampled, dry and semi-dry grassland patches belong to the Festuco–Brometea and Molinio–Arrhenatheretea classes (Table ), with the dominant associations Hypochoerido–Festucetum rupicolae Steinbuch 1980 and Ranunculo bulbosi–Arrhenatheretum elatioris Ellmauer and Mucina et al. 1993. The Molinio–Arrhenatheretea grasslands that we are discussing are not lowland, mesic, nutrient rich grasslands with a high biomass production.
All sampled patches have a similar inclination (0–4°), small elevation differences (Table ), southern exposition and a sandy soil structure. Sampled patches were all regularly mowed with no signs of abandonment or degradation phases. Such grasslands in the Goričko Natural Park are mown twice per season (Figure B). The second mowing period lasts for 14–20 days, and it takes place at the end of August and at the beginning of September using a hand-pushed sickle bar mower only, at a height of 4–7 cm (some even lower). However, in some seasons, especially when spring and summer receive less rainfall, the last mowing phase is performed in July or early August. Although none of the grassland patches showed clear signs of abandonment in the way of ruderalisation or afforestation, the differences in the vegetation height on all patches were clearly evident, solely due to the time that had passed since the last mowing.
Altogether, 2442 flowering individuals of Spiranthes spiralis were recorded during the field research. On average, 94 flowering individuals per patch were found (Table ). Flowering specimens observed in the field were in the same flowering phenophase: the majority of the flowers were fully opened with about 5–10% closed flower buds per inflorescence. No significant relation was found between the grassland patch area and the number of flowering Spiranthes spiralis specimens per grassland patch (ρ = 0.181, p = 0.376).
Vegetation height and Spiranthes spiralis frequency and density
The vegetation height directly reflects the time since mowing, shown in Figure . The mean vegetation height per grassland patch varies from 5.1 cm to 15.8 cm (Table ). Patch area and vegetation height show a significant positive relation (ρ = 0.522, p = 0.006). No direct relation was found between the Spiranthes spiralis frequency and vegetation height (ρ = –0.209, p = 0.307). The mean density, expressed as the number of flowering plants per m2, varies from 0.0013 to 0.3560 (Table ). The vegetation height negatively affects the orchid density (ρ = –0.374, p = 0.041).
Robust morphological traits
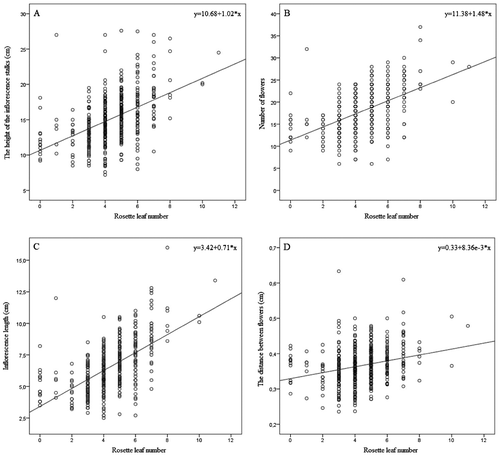
In total, 427 flowering orchid specimens were measured for their robust morphological traits (Table ). The maximum number of rosette leaves (Figure D) recorded in the field was 11. On average, 4.4 rosette leaves per flowering plant were recorded. Forty-eight per cent of the measured flowering Spiranthes spiralis specimens had five or more rosette leaves. Only eight flowering plants (out of 427) were without developed rosette leaves at the time of sampling. The number of the rosette leaves (Table ) proved to be an important morphological trait and could be used as a proxy for the general plant fitness of this orchid species. This trait shows a statistically significant, strong positive relation to (1) the height of the inflorescence stalks (ρ = 0.592, p = 0.001), (2) the number of flowers (ρ = 0.597, p = 0.001), (3) inflorescence length (ρ = 0.719, p = 0.000) and (4) the distance between the flowers (ρ = 0.534, p = 0.005) (Figure ).
Plant fitness and vegetation height
As mentioned, the vegetation height negatively affects the orchid density. We identified that the rosette-leaf number, which reflects the general plant fitness of the orchid specimens, is negatively related to the vegetation height (ρ = –0.382, p = 0.047) which reflects early mowing, but is also negatively related to grassland patch area (ρ = –0.501, p = 0.009).
Discussion
Rosette-leaf number: a suitable proxy for the general fitness of flowering Spiranthes spiralis specimens
In the case of Spiranthes spiralis, the number of the rosette leaves was found to be a suitable proxy for the general fitness of the flowering individuals. Other authors (Janečková et al. Citation2006; Wells et al. Citation1998) used the total leaf area of a plant as the indicator of plant fitness when studying European terrestrial orchids. Previous studies on orchids have already shown that the pollination success of flowers is bound to depend simultaneously on overall plant height, leaf number (a proxy for plant vigour) and population density (Kropf and Renner Citation2005).
The number of rosette leaves shows a significant, positive relation to the mean distance between the flowers. Non-random arrangement of flowers in a spiral was discussed already by Darwin (Citation1862) with respect to the orchid Spiranthes spiralis. Iwata et al. (Citation2012) found that the inflorescence architecture of Spiranthes sinensis (Pers.) Ames (Pers.) greatly affects the pollination success. The same is true for the studied species (Scopece, Gravendeel, and Cozzolino Citation2017). During the field research in Goričko Natural Park, the authors observed Apis mellifera (Linnaeus 1758) specimens regularly visiting the flowers (Fig. C). According to Iwata et al. (Citation2012), a larger distance between flowers increases the pollination success. We observed the vertical arrangement (mean distance between flowers) of flowers. Larger values indicate loosely twisted inflorescences. On the other hand, a small vertical distance between flowers indicates tightly twisted inflorescences. Since the number of rosette leaves was positively related to the vertical distance between the flowers as a trait of inflorescence architecture, we can conclude that the number of rosette leaves is also positively related to the pollinator behaviour and mating success, which leads to a higher fruit set production.
Spiranthes spiralis plant fitness in relation to late mowing
Since the number of rosette leaves is negatively related to vegetation height, directly connected to the time since last mowing and positively related to the distance between the flowers (and also with the other observed morphological traits), we can conclude that late seasonal mowing also enhances the reproductive success of Spiranthes spiralis specimens. We identified that the density of the orchids on studied grassland patches is correlated to the vegetation height, which affects the growth of rosette leaves. The rosette-leaf number is negatively affected by the size of the grassland patch. We can conclude that Spiranthes spiralis, when occupying smaller, traditionally managed and typical semi-dry grassland patches, tends to increase the production of rosette leaves. According to Ziegenspeck (Citation1936) and Willems (Citation1989), Spiranthes spiralis flowering will only take place when a plant has exceeded the production of a threshold rosette-leaf area, but exact figures have not been provided (Jacquemyn and Hutchings Citation2010).
The performance of orchid populations seems to be determined by light availability and competition with the surrounding vegetation (Dorland and Willems Citation2002; Willems, Balounová, and Kindlmann Citation2001). Typically, mowing favours low-growing rosette forming plant species as well as species with annual and biennial life forms (Hellstrӧm et al. Citation2006). The time of mowing critically affects the vegetation development (Smith and Jones Citation1991). Huhta et al. (Citation2001) observed that late mowing mainly maintains the prevailing vegetation. It has also been demonstrated that early-summer mowing has a detrimental effect on the species richness of flowering plants, as it hampers completion of the reproductive cycle, while later mowing is generally found to be more favourable for vascular plant biodiversity (Smith et al. Citation2000, Citation2002). Previous studies have shown (Bissels et al. Citation2006; Kotorová and Lepš Citation1999; Zobel et al. Citation2000) that the effects of disturbance (mowing) differ among individual species. Generally, mowing regimes affect seedling recruitment, while species respond differently to the mowing regimes (Bissels et al. Citation2006).
Our results imply that one of the most important factors that influence the overall population fitness of Spiranthes spiralis in central European semi-dry acid grasslands is the seasonal timing of mowing. Larger grassland patches are in general mowed earlier in the late season than the smaller ones, and this is what the positive correlation between the patch area and the vegetation height also showed. This basically means that the vegetation on earlier mowed grasslands grows higher for the second time in the late season. The influence of shadow created by adjacent plants has a negative effect on Spiranthes spiralis population fitness. On earlier mown grassland patches, the vegetation remains higher during the winter and early spring while decreasing the amount of light available for photosynthesis.
Spiranthes spiralis, a good flagship species for further conservation guidelines
A major issue in ecology is understanding the drivers of temporal changes in the spatial distribution of species (Vogt-Schilb et al. Citation2015). Population monitoring is an essential element of studies of threatened and endangered terrestrial orchids. Without an adequate monitoring programme, it would not be possible to follow the responses of populations to natural and anthropogenic changes to their habitats (Whigham and Willems Citation2003). Willems (Citation1989) found that populations of the endangered Spiranthes spiralis decreased dramatically in the Limburg area of the Netherlands from about 50 sites before 1950 to only one site at present. The decline of this species was the direct result of increased shading following grassland abandonment. Reintroduction of grazing and removal of invading woody species has led to an increase in Spiranthes spiralis population size (Whigham and Willems Citation2003).
Fruit set tends to be greater where the Spiranthes spiralis inflorescence density is high. On the other hand, fruit production decreases when other species are flowering in the immediate neighbourhood of flowering Spiranthes spiralis plants, thus indicating that different species compete with Spiranthes spiralis for pollinators (Willems and Lahtinen Citation1997). Although seeds are dispersed by wind, experiments have shown that seed dispersal in Spiranthes spiralis is limited to distances from a few centimetres to a few decimetres from maternal plants (Machon et al. Citation2003). Thus, the species is very limited when colonising new, distant habitats.
This feature could have important implications in the strategy of grassland conservation. It takes more than a decade for an individual plant to produce flowers (13–14 years) and a fruit set for the first time (Wells Citation1967), which is why populations with many individuals that were observed during our field work, mainly on smaller grassland patches, tend to be very old. Such permanent grassland patches expressing viable populations must be properly and continuously managed for longer periods of time.
In the vast areas of Central, Eastern and Southern Europe, a millennia-long co-evolution of Spiranthes spiralis to the animal-driven maintenance of grasslands stopped at the beginning of the nineteenth century. Our study species experienced drastic changes in the ecological condition of its growing sites. Grazing, predominantly by domesticated animals, is absent in NE Slovenia (Goričko Natural Park, our study site) where large populations of Spiranthes spiralis were found by the authors. Also, in central Slovenia, the grasslands are “driven” by mowing exclusively (grazing by wild animals provides little impact). The same, changed habitat conditions appear in southern Austria, Hungary, north Croatia, Slovakia and Czech Republic – actually in quite a large proportion of Central and Eastern Europe (Keenleyside and Tucker Citation2010; Köhler, Hiller, and Tischew Citation2016; Newton et al. Citation2009, 5; Török et al. Citation2016; Varga et al. Citation2016; Vassilev et al. Citation2011). Mowing is the only driver that ensures the existence of the species-rich grasslands with Spiranthes spiralis. Only small grassland areas in the Karst plateau (W Slovenia) and Haloze region (NE Slovenia) are both periodically grazed and mown. From that point of view, the “new” habitat of our study species (in most of Central and Eastern Europe) is regularly mown (at the proper time) nutrient-poor grasslands. It is of great importance to study species response to mowing, which is the only driver that ensures the proper vegetation height and photosynthesis for this particular species, and ensures viability of Spiranthes spiralis populations. We should study the relatively new habitat conditions to ensure the species’ presence in Slovenia in the future at the local and regional scale.
Unlike several other terrestrial orchids with a broader ecological range like Anacamptis morio (L.) R. M. Bateman, Pridgeon & M. W. Chase (Hornemann, Michalski, and Durka Citation2012; Paušič and Kaligarič Citation2015), Spiranthes spiralis is a good indicator for species-rich, nutrient-poor grasslands or better for proper long-term management of the grasslands. In Slovenia, this species is protected by law and included in the Red List (Anonymus Citation2002) as a vulnerable taxon (VU). That is why preservation of semi-dry grasslands of Goričko is of great importance for ensuring the existence of the populations inside the Goričko Natural Park.
Our main conclusion is that the overall population viability of Spiranthes spiralis (here measured as species density and estimated plant fitness) in Goričko Natural Park depends on the time of last mowing (here measured as vegetation height). Nowadays, grazing is virtually absent in the area of Goričko Natural Park, so the timing of the late mowing, 10–15 days before the flowering begins, depending on climatic conditions, is the crucial factor that could explain or determine the distribution of the species in the future.
Notes on contributors
Igor Paušič is a teaching assistant at the University of Maribor, Slovenia. He completed his PhD at the University of Maribor. He is working on flora and vegetation of dry grasslands with a special interest in native orchids. He is also involved in different aspects of archaeobotanical research and landscape ecology research of grasslands in Slovenia. Contribution: He has undertaken the research work and is responsible for the field research and statistical analysis, he wrote the article and contributed to the figures and tables.
Mitja Kaligarič is professor of botany at the University of Maribor, Slovenia. He completed his MSc and PhD in the University of Ljubljana. He is working on flora and vegetation of dry grasslands between the Alps, Mediterranean and central Europe. He is also a specialist for halophyte vegetation and ecology. Contribution: The author was responsible for correction and improvement of the manuscript.
Branko Bakan is a teaching assistant at the University of Maribor, Slovenia. He completed his bachelor’s degree at the University of Maribor. Branko Bakan mainly works on flora and vegetation of the Prekmurje region, NE Slovenia. He is also involved in landscape ecology research of grassland areas in Slovenia. Contribution: The author contributed in the field research, he also contributed to the figures and tables.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
We thank two anonymous reviewers for their constructive, insightful comments, which helped us to improve the manuscript. The research for this paper was partly funded by the P1-0164 grant, provided by the Slovenian Research Agency.
References
- AHO Bayern. 2015 “Arbeitskreis Heimische Orchideen [Bayern Native Orchids Working group].” Accessed February 24 2016. www.aho-bayern.de.
- Anonymus. 2002. Pravilnik o uvrstitvi ogroženih rastlinskih in živalskih vrst v rdeči seznam [Rules on the Inclusion of Endangered Plant and Animal Species in the Red List]. Priloga 1: Rdeči seznam praprotnic in semenk (Pteridophyta & Spematophyta). Uradni list RS 82/2002, 8893–8910.
- ARSO. 2015. Ministrstvo za kmetijstvo in okolje, Agencija Republike Slovenije za okolje, klimatski podatki za 30 letno obdobje, Veliki Dolenci [Ministry of Agriculture, Forestry and Food, Slovenian Environment Agency, 30-year climate data for Veliki Dolenci]. Accessed February 9 2015. http://www.arso.gov.si/vreme/napovedi%20in%20podatki/veliki_dolenci.htm.
- Bakan, B. 2006. Slikovni pregled višjih rastlin Prekmurja [Overview of vascular plants of Prekmurje region], 245. Lendava, Slovenia: Razvojni center, S-Tisk.
- Bissels, S., T. W. Donath, N. Hölzel, and A. Otte. 2006. “Effects of Different Mowing Regimes on Seedling Recruitment in Alluvial Grasslands.” Basic Applied Ecololgy 7 (5): 433–442.10.1016/j.baae.2005.10.002
- Bohner A., H. Kerschbaumsteiner, and F. Starlinger. 2010. “Ein bemerkenswerter Fund von Spiranthes spiralis (Orchidaceae) im Steirischen Salzkammergut (Steiermark, Österreich) [Spiranthes spiralis- A Remarkable Discovery in Salzkammergut (Steiermark, Austria)].” Joannea Botanik 8: 5–18.
- Böhnert W., G. Hecht, and H. J. Stapperfenne. 1986. “Orchideen des Bezirkes Halle. Einst und jetzt [Orchids of Halle District, Germany].” Naturschutzarbeit in den Bezirken Halle und Magdeburg (23) (Supplementary issue).
- Činč Juhant, B., and M. Planjšek. 2002. O geologiji Pomurja in Goričkega [A contribution to geology of Pomurje region and Goričko]. Ljubljana: Narava Slovenije. Mura in Prekmurje, Prirodoslovni muzej.
- Claessens J., and J. Kleynen. 2016. “The Pollination of European Orchids Part 4: Goodyera and Spiranthes.” Journal of the Hardy Orchid Society 13: 54–61.
- Darwin, C. R. 1862. On the Various Contrivances by which British and Foreign Orchids are Fertilised by Insects. London: John Murray.
- Darwin, C. R. 1877. The Various Contrivances by Which British and Foreign Orchids are Fertilized by Insects. London, UK: John Murray.
- Dolinar, B. 2015. Kukavičevke Slovenije [Orchids of Slovenia], 184. Ljubljana: Pipinova knjiga.
- Dorland, E., and J. H. Willems. 2002. “Light climate and plant performance of Ophrys insectifera: a 4-year field experiment in The Netherlands 1998–2001.” In Trends and Fluctuations and Underlying Mechanisms in Terrestrial Orchid Populations, edited by P. Kindlmann, J. H. Willems and D. F. Whigham, 225–238. Leiden: Backhuys Publishers.
- Dorland E., and J. H. Willems. 2006. “High Light Availability Alleviates the Costs of Reproduction in Ophrys insectifera (Orchidaceae).” Journal Europäischer Orchideen 38: 369–386.
- ESRI. 2010. ArcGIS Desktop, Release 9.3. Redlands, CA: Environmental Systems Research Institute.
- Gill, D. E. 1996. “The Natural Population Ecology of Temperate Terrestrials: Pink Lady’s-Slippers, Cypripedium acaule.” In North American Native Terrestrial Orchid Conference, conference, edited by C. Allen and R. Colema, 91–106. Germantown, MD: North American Native Terrestrial Orchid Conference. Proceedings, March 16 & 17, 1996, [held at the National Arboretum, Washington, D.C]. https://doi.org/10.5962/bhl.title.131246.
- Grubb, P. J. 1986. “Problems Posed by Sparse and Patchily Distributed Species in Species Rich Communities.” In Community Ecology, edited by J. Diamond and T. J. Case, 207–255. New York: Harper and Row.
- GURS 2010. Digitalni model višin 5 x 5 m [Digital elevation model 5 x 5 m]. Ljubljana: Geodetska Uprava Republike Slovenije [ The surveying and mapping authority of the Republic of Slovenia, Ministry of Infrastructure and Spatial Planning].
- Hellstrӧm, K., and A. P. Huhta., P. Rautio, and J. Tuomi. 2006. “Search for Optimal Mowing Regime – Slow Community Change in a Restoration Trial in Northern Finland.” Annales Botanici Fennici 43: 338–348.
- Hornemann, G., S. G. Michalski, and W. Durka. 2012. “Short-Term Fitness and Long-Term Population Trends in the Orchid Anacamptis morio.” Plant Ecology 213 (10): 1583–1595.10.1007/s11258-012-0113-6
- Huhta, A. P., P. Rautio, J. Tuomi, and K. Laine. 2001. “Restorative Mowing on an Abandoned Semi-Natural Meadow: Short-Term and Predicted Long-Term Effects.” Journal of Vegetation Science 12 (5): 677–686.10.2307/3236908
- Hutchings, M. J. 1987. “The Population Biology of the Early Spider Orchid, Ophrys shegodes Mill. I. A Demographic Study From 1975 to 1984.” Journal of Ecology 75 (3): 711–727.10.2307/2260201
- Iwata, T., O. Nagasaki, H. S. Ishii, and A. Ushimaru. 2012. “Inflorescence Architecture Affects Pollinator Behaviour and Mating Success in Spiranthes sinensis (Orchidaceae).” New Phytologist 193 (1): 196–203.10.1111/j.1469-8137.2011.03892.x
- Jacquemyn, H., and M. J. Hutchings. 2010. “Biological Flora of the British Isles: Spiranthes spiralis (L.) Chevall.” Journal of Ecology 98 (5): 1253–1267.10.1111/jec.2010.98.issue-5
- Jacquemyn, H., R. Brys, M. Hermy, and J. H. Willems. 2007. “Long-Term Dynamics and Population Viability In one of the Last Populations of the Endangered Spiranthes spiralis (Orchidaceae) in the Netherlands.” Biology Conservation 134 (1): 14–21. 10.1016/j.biocon.2006.07.016
- Jacquemyn, H., R. Brys, D. Adriaens, O. Honnay, and I. Roldán-Ruiz. 2009. “Effects of Population Size and Forest Management on Genetic Diversity and Structure of The Tuberous Orchid Orchis mascula.” Conservation Genetics 10 (1): 161–168. 10.1007/s10592-008-9543-z
- Janečková, P., K. Wotavová, I. Schödelbauerová, J. Jersáková, and P. Kindlmann. 2006. “Relative Effects of Management and Environmental Conditions on Performance And Survival of Populations of a Terrestrial Orchid, Dactylorhiza majalis.” Biology Conservation 129 (1): 40–49.10.1016/j.biocon.2005.09.045
- Jatinova, M., and J. Schmitak. 1996. Verbreitung und Schutz der Orchideen in Mähren and Schlesien [Distribution and protection of Orchids in the Moravian-Silesian region]. Trebic, Czech Republic: Verlag Arca JiMfa.
- Jogan, N., T. Bačič, B. Frajman, I. Leskovar, D. Naglič, A. Podobnik, B. Rozman, S. Strgulc Krajšek, and B. Trčak. 2001. Gradivo za Atlas flore Slovenije [Materials for the Atlas of Flora of Slovenia]. Miklavž na dravskem polju: CKFF.
- Kaligarič, M., J. Sedonja, and N. Šajna. 2008. “Traditional Agricultural Landscape in Goričko Landscape Park (Slovenia): Distribution and Variety of Riparian Stream Corridors and Patches.” Landscape and Urban Planning 85 (1): 71–78.10.1016/j.landurbplan.2007.09.012
- Keenleyside, C., and G. M. Tucker. 2010. Farmland Abandonment in the EU: an Assessment of Trends and Prospects. Report prepared for WWF. London: Institute for European Environmental Policy.
- Kocjan, J. M. 2014. “– Prispevek k poznavanju razširjenosti nekaterih redkih, ogroženih ali drugače zanimivih taksonov v flori Slovenije – II. [Contribution to the Knowledge of the Distribution of Some Rare, Threatened or Otherwise Interesting Taxa in the Flora of Slovenia].” Hladnikia 33: 31–63.
- Köhler, M., G. Hiller, and S. Tischew. 2016. “Year-Round Horse Grazing Supports Typical Vascular Plant Species, Orchids and Rare Bird Communities in a Dry Calcareous Grassland.” Agriculture, Ecosystems and Environment 234: 48–57.10.1016/j.agee.2016.03.020
- Kotorová, I., and J. Lepš. 1999. “Comparative Ecology of Seedling Recruitment in an Oligotrophic Wet Meadow.” Journal of Vegetation Science 10 (2): 175–186.10.2307/3237139
- Králik, T. 1998. “Monitoring of the Population of Spiranthes spiralis.” In The Nature Reserve Ostrovné Lúčky. Európske vstaváčovité, edited by J. Vlčko, and R. Hrivnák, pp. 51–56. Zvolene, Czech Republic: Technická Univerzita vo Zvolene.
- Kropf, M., and S. S. Renner. 2005. “Pollination Success in Monochromic Yellow Populations of the Rewardless Orchid Dactylorhiza sambucina.” Plant Systematics and Evolution 254 (3-4): 185–197.10.1007/s00606-005-0338-0
- Kull, T. 2002. “Population Dynamics of North Temperate Orchids.” In Orchid biology VIII: Reviews and Perspectives, edited by T. Kull and J. Arditti, 139–165. Dordrecht: Kluwer Academic Publishers.10.1007/978-94-017-2500-2
- Kull, T., and M. J. Hutchings. 2006. “A Comparative Analysis of Decline in The Distribution Ranges of Orchid Species in Estonia and the United Kingdom.” Biological Conservation 129 (1): 31–39.10.1016/j.biocon.2005.09.046
- Landi, M., F. Frignani, C. Lazzeri, and C. Angiolini. 2009. “Abundance of Orchids on Calcareous Grasslands in Relation to Community Species, Environmental, and Vegetational Conditions.” Russian Journal of Ecology 40 (7): 486–494.10.1134/S1067413609070066
- Lepš, J. 1999. “Nutrient Status, Disturbance and Competition: An Experimental Test of Relationships in a Wet Meadow.” Journal of Vegetation Science 10: 219–230.
- Maccherini, S. 2006. “Factors Associated With Species Richness in a Remnant Calcareous Grassland.” Grassland Science 52 (4): 181–184.10.1111/grs.2006.52.issue-4
- Machon, N., P. Bardin, S. J. Mazer, J. Moret, B. Godelle, and F. Austerlitz. 2003. “Relationship Between Genetic Structure and Seed and Pollen Dispersal in the Endangered Orchid Spiranthes spiralis.” New Phytologist 157 (3): 677–687.10.1046/j.1469-8137.2003.00694.x
- Meekers, T., and O. Honnay. 2011. “Effects of Habitat Fragmentation on the Reproductive Success of the Nectar-Producing Orchid Gymnadenia conopsea and the Nectarless Orchis mascula.” Plant Ecology 212 (11): 1791–1801.10.1007/s11258-011-9949-4
- Newton, A. C., G. B. Stewart, G. Myers, A. Diaz, S. Lake, J. M. Bullock, and A. S. Pullin. 2009. “Impacts of Grazing on Lowland Heathland in North-West Europe.” Biological Conservation 142: 935–947.10.1016/j.biocon.2008.10.018
- Paušič, I., and M. Kaligarič. 2015. “Dry grassland land use treatment regime explains the occurrence of the green winged orchid, Anacamptis morio (L.) R. M. Bateman, Pridgeon & M. W. Chase in the Goričko Nature Park, NE Slovenia.” Folia Biologica et Geologica 56 (3): 137–148.
- Pfeifer, M., K. Wiegand, W. Heinrich, and G. Jetschke. 2006. “Long-Term Demographic Fluctuations in an Orchid Species Driven By Weather: Implications for Conservation Planning.” Journal of Applied Ecology 43 (2): 313–324.10.1111/j.1365-2664.2006.01148.x
- Poschlod, P., and M. F. Wallis DeVries. 2002. “The Historical and Socioeconomic Perspective of Calcareous Grasslands: Lessons from the Distant and Recent Past.” Biological Conservation 108: 247–258.
- Preston, C. D., and M. O. Hill. 1997. “The Geographical Relationships of British and Irish Vascular Plants.” Botanical Journal of the Linnean Society 124 (1): 1–120.10.1111/boj.1997.124.issue-1
- Rasmussen, H. N. 1995. Terrestrial Orchids: From Seed to mycotrophic Plant, 444. Cambridge: Cambridge University Press.10.1017/CBO9780511525452
- Ravnik, V. 2002. Orhideje Slovenije [Orchids of Slovenia], 192. Ljubljana: Tehniška založba.
- Reinhard, H. R., P. Gӧlz, R. Peter, and H. Wildermuth. 1991. Die Orchideen der Schweiz und Angrenzender Gebiete [Orchids of Switzerland and neighbouring regions]. Egg, Switzerland: FotorotarAG.
- Schrautzer, J., A. Fichtner, A. Huckauf, L. Rasran, and K. Jensen. 2011. “Long-term Population Dynamics of Dactylorhiza incarnata (L.) Soó After Abandonment and Re-Introduction of Mowing.” Flora 206 (7): 622–630.10.1016/j.flora.2010.11.008
- Scopece, G., B. Gravendeel, and S. Cozzolino. 2017. “The Effect of Different Chiral Morphs on Visitation Rates and Fruit Set in the Orchid Spiranthes spiralis.” Plant Ecology and Diversity 10 (2-3): 97–104.10.1080/17550874.2017.1354093
- Sieg, C. H., and R. M. King. 1995. “Influence of Environmental Factors and Preliminary Demographic Analyses of a Threatened Orchid, Platanthera praeclara.” American Midland Naturalist 134 (2): 307–323.10.2307/2426300
- Škornik, S. 2003. “Suha travišča reda Brometalia erecti Koch (1926) na Goričkem (SV Slovenija) [Dry Grasslands from the Order Brometalia erecti Koch (1926) in Goričko (NE Slovenia)].” Hacquetia 2 (1): 71–90.
- Smith, R. S., and L. Jones. 1991. “The Phenology of Mesotrophic Grassland in The Pennine Dales, Northern England: Historic Hay Cutting Dates, Vegetation Variation and Plant Species Phenologies.” Journal of Applied Ecology 28 (1): 42–59.
- Smith, R. S., R. S. Shiel, D. Millward, and P. Corkhill. 2000. “The Interactive Effects of Management on The Productivity and Plant Community Structure of an Upland Meadow: An 8-Year Field Trial.” Journal of Applied Ecology 37 (6): 1029–1043.10.1046/j.1365-2664.2000.00566.x
- Smith, R. S., R. S. Shiel, D. Millward, P. Corkhill, and R. A. Sanderson. 2002. “Soil Seed Banks and the Effects of Meadow Management on Vegetation Change in A 10-Year Meadow Field Trial.” Journal of Applied Ecology 39 (2): 279–293.10.1046/j.1365-2664.2002.00715.x
- SPSS Inc.. 2006. SPSS Base 15.00 User’s Guide. Chicago, IL: SPSS Inc.
- Tamm, C. O. 1991. “Behaviour of Some Orchid Populations in a Changing Environment. Observations on Permanent Plots, 1943-1990.” In Population Ecology of Terrestrial Orchids. International Orchid Symposium (1990: South Kimburg, Netherlands), edited by T. C. E. Wells, and J. H. Williams, 1–13. The Hague: SPB Academic Publishing.
- Török, P., N. Hölzel, R. van Diggelen, and S. Tischew. 2016. “Grazing in European Open Landscapes: How to Reconcile Sustainable Land Management and Biodiversity Conservation?” Agriculture, Ecosystems and Environment 234: 1–4.10.1016/j.agee.2016.06.012
- Tutin, T. G., V. H. Heywood, N. A. Burgess, D. M. Moore, D. H. Valentine, S. M. Walters, and D. A. Webb. 1980. Flora Europaea V. Cambridge: Cambridge University Press.
- Tyteca, D. 2000. “The Orchid Flora of Portugal: Addendum n.3. Remarks on Spiranthes spiralis (L.) Chevall. and Three New Taxa to the Portuguese Flora.” Journal Europäischer Orchideen 32: 291–347.
- Van der Cingel, N. A. 1995. An Atlas of Orchid Pollination. European Orchids. Rotterdam, The Netherlands: A. A. Balkema.
- Varga, A., Z. Molnár, M. Biró, L. Demeter, K. Gellény, E. Miókovics, Á. Molnár, et al. 2016. “Changing Year-Round Habitat Use of Extensively Grazing Cattle, Sheep and Pigs in East-Central Europe Between 1940 and 2014: Consequences for Conservation and Policy.” Agriculture, Ecosystems and Environment 234: 142–153.10.1016/j.agee.2016.05.018
- Vassilev, K., H. Pedashenko, S. C. Nikolov, I. Apostolova, and J. Dengler. 2011. “Effect of land abandonment on the vegetation of upland semi-natural grasslands in the Western Balkan Mts., Bulgaria.” Plant Biosystems 145: 654–665.10.1080/11263504.2011.601337
- Vogt-Schilb, H., and F. Munoz., F. Richard, and B. Schatz. 2015. “Recent Declines and Range Changes of Orchids in Western Europe (France, Belgium And Luxembourg).” Biological Conservation 190: 133–141.10.1016/j.biocon.2015.05.002
- Vogt-Schilb, H., R. Pradel, P. Geniez, L. Hugot, A. Delage, F. Richard., and B. Schatz. 2016. “Responses of Orchids to Habitat Change in Corsica Over 27 Years.” Annals of Botany 118 (1): 115–123.10.1093/aob/mcw070
- Waite, S., and M. J. Hutchings. 1991. “The Effects of Different Management Regimes on the Population Dynamics of Ophrys sphegodes: Analysis and Description Using Matrix Models.” In Population Ecology of Terrestrial Orchids. International Orchid Symposium (1990: South Kimburg, Netherlands), edited by T. C. E. Wells, and J. H. Williams, 161–275. The Hague: SPB Academic Publishing.
- Wells, T. C. E. 1967. “Changes in a Population of Spiranthes spiralis (L.) Chevall. at Knocking Hoe National Nature Reserve, Bedfordshire, 1962–1965.” Journal of Ecology 55 (1): 83–99.10.2307/2257718
- Wells, T. C. E., P. Rothery, R. Cox, and S. Bamford. 1998. “Flowering Dynamics of Orchis morio L. and Herminium monorchis (L.) R.Br. at Two Sites in Eastern England.” Biological Journal of the Linnean Society 126: 39–48.
- Whigham, D. F., and J. H. Willems. 2003. “Demographic Studies and Life-History Strategies of Temperate Terrestrial Orchids as a Basis for Conservation.” In Orchid Conservation, edited by K. W. Dixon, S. P. Kell, R. L. Barrett and P. J. Cribb, 137–158. Kota Kinabalu, Sabah: Natural History Publications (Borneo).
- Willems, J. H. 1989. “Population Dynamics of Spiranthes spiralis in South-Limburg, The Netherlands.” Mémoires de la Société Royale de Botanique de Belgique 11: 115–121.
- Willems, J. H. 2006. Herfstschroeforchis. Portret van een Laatbloeier [Spiranthes spiralis - a Portrait of a Late Bloomer]. Maastricht, The Netherlands: Stichting Natuurpublicaties Limburg.
- Willems, J. H., and M. L. Lahtinen. 1997. “Impact of Pollination and Resource Limitation on Seed Production in a Border Population of Spiranthes spiralis.” Acta Botanica Neerlandica 46 (4): 365–375.10.1111/plb.1997.46.issue-4
- Willems, J. H., Z. Balounová., and P. Kindlmann. 2001. “The Effect of Experimental Shading on Seed Production and Plant Survival in the Threatened Species Spiranthes spiralis (Orchidaceae).” Lindleyana 16: 31–37.
- Wotavová, K., Z. Balounová, and P. Kindlmann. 2004. “Factors Affecting Persistence of Terrestrial Orchids in Wet Meadows and Implications for Their Conservation in a Changing Agricultural Landscape.” Biological Conservation 118 (3): 271–279.10.1016/j.biocon.2003.09.005
- Ziegenspeck, H. 1936. Orchidaceae. Lebensgeschichte der Blütenpflanzen Mitteleuropas [Orchidaceae. Life Story oft the Central European Flowering Plants]. Band 1, Abteilung 4. Stuttgart, Germany: Eugen Ulmer.
- Zobel, M., M. Otsus, J. Liira, M. Moora, and T. Möls. 2000. “Is Small-Scale Species Richness Limited By Seed Availability Or Microsite Availability?” Ecology 81 (12): 3274–3282.10.1890/0012-9658(2000)081[3274:ISSSRL]2.0.CO;2