Abstract
Objective: To evaluate the usefulness of Hisense Computer Assisted Surgery System (Hisense CAS) in pre-operative surgical planning and intra-operative navigation for resection of pediatric giant hepatic mesenchymal hamartoma (HMH).
Methods: Five children with HMH underwent hepatectomy in our hospital. Pre-operative abdominal enhanced CT was performed for diagnosis and treatment planning. Using CT DICOM files, three-dimensional reconstruction was performed in three cases for operation planning and intra-operative navigation, with SID carrying out precise liver resection during the operation with Hisense CAS.
Result: Two patients underwent right and left lobe hepatectomy, respectively, based only on enhanced CT. In 3 patients, by using the Hisense CAS system, three-dimensional reconstruction of the liver and tumors was successfully completed, and virtual hepatectomy performed successfully according to surgical plans. Hisense CAS could clearly and directly indicate the HMH location and shape, as well as its relationship with the intra-hepatic Glisson system, assisting safe hepatectomy. All five patients recovered well from surgery without any complications, and pathological examinations confirmed that all cases were HMH. No recurrence was observed during the follow-up period of 3 months to 5 years.
Conclusion: Hisense CAS system is useful for preoperative planning and intra-operative navigation, assisting safer hepatectomy.
Introduction
Hepatic mesenchymal hamartoma (HMH) is the second most common benign pediatric liver tumor; approximately 85% of HMH cases are children younger than 3 years. HMH is clinically characterized by abdominal distension and/or an upper abdominal mass; physical examination reveals a large, nontender and smooth liver tumor. Due to the possibility that HMH has a histogenetic relationship with undifferentiated embryonal sarcoma (UES) and could develop into UES within the preexisting HMH tumor, complete excision is the best treatment [Citation1,Citation2]. However, hepatectomy remains a complex operation even for experienced doctors.
Precise liver resection has been proposed to improve the concept of anatomic liver resection, suggesting anatomic liver resection should be a surgical maneuver removing the territories of one or several peripheral portal vein branches. Anatomic liver resection should not be confined to tumor-bearing segment removal, but extended to remove just enough of the tumor-bearing portal tributary area that may be confined within or across the segment regardless of Couinaud’s segment [Citation3]. Indocyanine green fluorescent imaging [Citation4] and contrast-enhanced ultrasound [Citation5] are newly developed navigation strategies for anatomic liver resection. In addition, simulation of surgical maneuvers using 3-dimesional (3D) simulation techniques is especially helpful in cases where dye injection is difficult. These novel techniques are expected to improve the quality of anatomic liver resection and further the development of laparoscopic liver resection.
3D simulation techniques have been widely used in assisting liver resection [Citation6] or living donor liver transplantation [Citation7] in recent years. In liver surgery, accurate assessments of remnant liver volume and anatomical variation are necessary for preoperative planning of safe curative hepatectomy. 3D simulation software programs enable rapid and easy rendering of high-definition 3D images of the liver, tumor and vessels, using previously captured CT or MRI DICOM files, providing highly practical analytic functions [Citation8,Citation9]. We recently developed a novel virtual hepatectomy simulation software named Hisense CAS. Using contrast-enhanced CT images, Hisense CAS could display 3 D liver images simply, quickly, and accurately. This software provides 4 basic functions: (1) 3 D viewing of the liver from arbitrary directions to confirm the organ anatomy and spatial relationships between the tumor and surrounding Glisson; (2) extraction of each vessel territory in the liver, mimicking precise liver resection or non-anatomical hepatectomy; (3) automatic calculation of remnant liver volume; (4) navigation of liver resection during operation. Besides extracting the portal vein territory to mimic precise liver resection, extracting each segmental hepatic venous perfusion area using Hisense CAS appeared to prevent congestion occurrence during the operation. Additionally, volumetric measurements using the Hisense CAS software were shown to be accurate based on pig experiments comparing the expected preoperative remnant liver volume and actual resected liver weight. Our 3 D simulation system, based on hepatic circulation, provided accurate volumetric and stereotactic information for preoperative planning of curative hepatectomy.
Here we present our experience of applying 3 D simulation in assisting giant cystic HMH resection in children.
Materials and methods
Patients
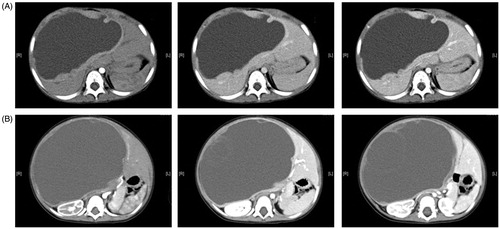
From September 2010 to October 2015, five children with HMH have been admitted to our hospital, including 2 boys and 3 girls aged 8–24 months (averaging 16 months). Main complaints were abdominal mass (2 cases) and abdominal distension (3 cases). Ultrasonography and upper abdominal enhanced CT scan revealed a liver mass in each of the five patients. Representative CT images from two cases are shown in . Three patients (Case 3, 4 and 5) underwent 3 D reconstruction with CT DICOM files using Hisense CAS (Hisense Medicine, Qingdao, China). Clinicopathological factors are shown in .
Table 1. Clinicopathological factors of all 5 patients.
This study was approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University, and written informed consent was obtained from all parents.
3 D simulation
3 D simulation steps were descried in a previous report by our team [Citation10]. Simply, DICOM data obtained from enhanced CT were uploaded to the Hisense CAS system; liver structures were reconstructed, including liver parenchyma, portal vein, hepatic veins, and tumors, in a 3 D context, by extracting neighboring voxels with a similar CT density, automatically calculating tumor and liver volumes; a virtual hepatectomy was performed using the software (automatic calculation of the remnant volume); the optimal surgical procedures were assessed based on virtual hepatectomy findings. During the operation, the Hisense surgical intelligent display system can be used to navigate the surgical procedure comparing to the intra-operative anatomy ().
Surgical procedure and follow-up
The surgical procedure for the five patients, operation time, intra-operative bleeding, time of intermittent Pringle maneuver and in-hospital time are shown in . During the operation, cysts were punctured with 20 G needles, aspirating the intra-tumor fluid; this is helpful for large tumors and impedes the operation process. A Cavitron ultrasonic surgical aspirator (CUSA) system was used to separate liver parenchyma, and ligate and cut the hepatic vascular system. No surgical complications (e.g. biliary fistula or bleeding) occurred after the operation in all five patients. Intermittent Pringle maneuver was useful to reduce bleeding, and Pringle time should be less than 20 min. The patients were followed up until October 2016; all five children were well without recurrence.
Results
Preoperative 3D simulation and surgical outcome
Enhanced CT from two cases were shown in , indicating giant cysts with solid areas, septa, and peripheral regions, may be enhanced after intravenous contrast. Tumors in two cases were located in the right lobe, two cases showed left lateral lobe tumors, and the middle lobe was affected in one patient. Tumor diameters ranged from 62 to 200 mm (averaging 121.8 mm). Abdominal enhanced CT examination was performed only in two cases, in whom it was difficult to accurately evaluate the relationship between tumors and surrounding vessels, with surgical removal of the tumors mainly relying on experience. In three children, successful 3 D model reconstruction was obtained with the Hisense CAS system ().
During the operation, all five HMH cases were found to have single cystic masses with clear margins, which were successfully removed. In the three cases, intraoperative findings were consistent with the 3 D reconstruction, with surgical procedures highly predictable. The intra-operative blood loss, operation time, and post-operative data are shown in . With pre-operative 3 D simulation, intra-operative blood loss tended to be reduced as well as operation time.
Representative case
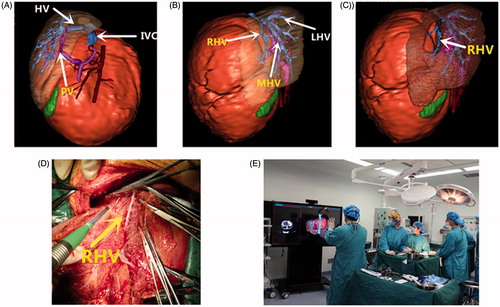
An 11-month-old male infant was admitted because of abdominal distension and loss of appetite for 2 months (Case 5). The relationship of HMH with the intrahepatic vasculature was revealed in a 3 D context. The right hepatic vein (RHV), middle hepatic vein (MHV), and left hepatic vein (LHV) converged into a common trunk (). There was no obvious infiltration or encasement to hepatic veins (HVs), portal veins (PVs), and the inferior vena cava (IVC) (). Volumes of both the functional liver and HMH were automatically calculated. The anatomical relationship between intrahepatic vascular and HMH could be confirmed from any direction. Different virtual surgical plans mimicking HMH resection were performed to assess the risk and difficulty during the actual operation. Finally, optimal surgical plan was developed using the 3 D simulation software, preserving RHV. During the operation, the Hisense surgical intelligent display system can be used to navigate the surgical procedure comparing to the intra-operative anatomy (). By gesture recognition in the Hisense surgical intelligent display system, the operator could control the 3 D images without touching the screen, comparing the pre-operative images with intra-operative anatomy.
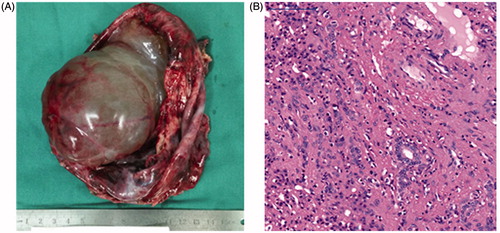
During the operation, the fluid was aspirated using a 20 G needle to decompression [Citation11]. The resection line at the HMH borderline was revealed by the preoperative virtual hepatectomy. The intermittent Pringle maneuver was used to reduce the risk of bleeding. The HMH was safely removed, with the right hepatic vein successfully protected (). There was almost no anatomical discrepancy between the operation and 3 D simulation. The infant recovered uneventfully. The resected tumor () and histopathology staining confirmed the diagnosis of mesenchymal hamartoma ().
Discussion
HMH is the second most frequent benign pediatric liver tumor in children [Citation11]. It tends to present as a giant, progressively increasing, non-tender abdominal mass. We summarized the surgical treatments of five cases and explored the usefulness of 3 D simulation in assisting liver resection with giant HMH.
Ultrasound, computed tomography (CT), and magnetic resonance imaging demonstrate that HMH appears as a large, well-defined, multilocular or solid-cystic mass [Citation5]. In US, the presence of thin mobile septa and/or round hyperechoic parietal nodules within the cyst is highly suggestive of MHL [Citation12]. Solid areas, septa, and peripheral regions may be enhanced by intravenous contrast; delayed images may show persistent peripheral and septal enhancement with centripetal fill-in of solid areas [Citation13]. Typically, tumors have a low signal intensity on T1-weighted magnetic resonance sequences, but a variable signal intensity on T2-weighted sequences [Citation14]. Beside diagnosis, CT scan and MRI allow better anatomical definition and planning of possible surgical approaches; magnetic resonance angiography is useful for preoperative surgical planning. Moreover, intraoperative US may be used to guide resection and define vascular anatomy [Citation11]. However, CT, MRI and US are 2 dimensional images, which sometimes are difficult for surgeons to ascertain the anatomical relationship of the tumor and intrahepatic vascular structure, especially for giant tumors like HMH. Interestingly, the 3 D simulation software was developed to transform 2 dimensional CT or MRI data into 3 D models, including liver parenchyma, tumor and Glisson systems. This 3 D simulation software makes hepatectomy easier and safer [Citation8,Citation15,Citation16,].
At present, several software programs from different countries are available for computer-assisted liver surgery, including OVA (Hitachi Medical Corporation, Japan), Ziostation (Ziosoft, Japan), VirtualPlace (AZE, Japan), SYNAPSE VINCENT (FUJIFILM, Japan), Hepa Vision (Mevis, Germany), and VR-Render (IRCAD, France). SYNAPSE VINCENT was reported to be feasible and useful for preoperative simulation and navigation of surgical procedures [Citation7]. The angle of 3 D images composed by most software programs can be changed freely, revealing the relationship between the tumor and vessels. Moreover, preoperative 3 D virtual endoscopic simulation with SYNAPSE VINCENT can contribute to successful guidance of liver lesion surgery based on a virtual laparoscope or thoracoscope. We have developed a similar software named Hisense CAS based on children’s CT DICOM data, which tends to be more accurate than those developed from adult CT data. The constructed images are displayed in the operating room on a monitor (), and can be used for deciding surgical strategy and navigation during intraoperative surgical manipulations. Preoperative 3 D simulation could also reduce the surgeon’s stress, particularly when highly skilled techniques are needed to operate on lesions difficult to reach and widely spaced areas for giant tumors, as well as in pediatric patients. This 3 D simulation technique has also been tested for robot-assisted thoracic operations by thoracic surgeons, and could lead to greater safety and precision in operative settings, and easier manipulation, by creating appropriate port positioning and direction of instrument arms [Citation9]. Most recently, a newly developed novel 3 D virtual hepatectomy simulation software named Liversim that can visualize real-time liver deformation, and is thought to be helpful for performing safe hepatectomy as well as educating surgery residents and medical students.
Several limitations of this novel software should be mentioned. First, currently, this approach requires somewhat long time (approximately 1 h) to create detailed images. Second, certain small vessel branches may be missed by Hisense CAS because not all vessels can be visualized by CT for each patient. Third, this study involved only 3 patients.
In the present cases, CT images were uploaded to the Hisense CAS 3 D simulation software for liver reconstruction. The relationship of hamartoma with intrahepatic vasculature was revealed in a 3 D-context. Anatomically, three hepatic vein branches converged into a common trunk. Moreover, hepatic vein, portal vein, and inferior vena cava were displaced. The surgical plans were designed for RHV reconstruction using the 3 D simulation software. During hamartoma resection, the intrahepatic vessels assessed by the 3 D simulation software were dissected for protection, or ligated and divided. It took only 20 minutes to remove the giant cystic HMH; RHV was successfully preserved. There was no anatomical discrepancy between surgical findings and preoperative 3 D simulation ().
3 D simulation can facilitate the investigation of unknown fields that cannot be encompassed by conventional imaging studies, especially in giant HMH cases. With the help of the 3 D simulation software, optimal surgical management could become more available for infantile giant cystic HMH.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. 2005;40:1681–1690.
- Wildhaber BE, Montaruli E, Guerin F, et al. Mesenchymal hamartoma or embryonal sarcoma of the liver in childhood: a difficult diagnosis before complete surgical excision. J Pediatr Surg. 2014;49:1372–1377.
- Shindoh J, Hasegawa K, Kokudo N. Anatomic resection of hepatocellular carcinoma: a step forward for the precise resection of the tumor-bearing portal territory of the liver. Ann Surg. 2015;261:e145.
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr. 2016;5:322–328.
- Sen D, Gulati YS, Majumder A, et al. Hepatic cystic mesenchymal hamartoma. Med J Armed Forces India. 2015;71:S574–S5S7.
- Aoki T, Murakami M, Fujimori A, et al. Routes for virtually guided endoscopic liver resection of subdiaphragmatic liver tumors. Langenbecks Arch Surg. 2016;401:263–273.
- Mochizuki K, Takatsuki M, Soyama A, et al. The usefulness of a high-speed 3D-image analysis system in pediatric living donor liver transplantation. Ann Transplant. 2012;17:31–34.
- Oshiro Y, Yano H, Mitani J, et al. Novel 3-dimensional virtual hepatectomy simulation combined with real-time deformation. World J Gastroenterol. 2015;21:9982–9992.
- Kajiwara N, Akata S, Hagiwara M, et al. High-speed 3-dimensional imaging in robot-assisted thoracic surgical procedures. Ann Thorac Surg. 2014;97:2182–2184.
- Zhang G, Zhou XJ, Zhu CZ, et al. Usefulness of three-dimensional(3D) simulation software in hepatectomy for pediatric hepatoblastoma. Surg Oncol. 2016;25:236–243.
- Anil G, Fortier M, Low Y. Cystic hepatic mesenchymal hamartoma: the role of radiology in diagnosis and perioperative management. Br J Radiol. 2011;84:e91–e94.
- Koumanidou C, Vakaki M, Papadaki M, et al. New sonographic appearance of hepatic mesenchymal hamartoma in childhood. J Clin Ultrasound. 1999;27:164–167.
- Cetin M, Demirpolat G, Elmas N, et al. Stromal predominant type mesenchymal hamartoma of liver: CT and MR features. Comput Med Imaging Graph. 2002;26:167–169.
- Wholey MH, Wojno KJ. Pediatric hepatic mesenchymal hamartoma demonstrated on plain film, ultrasound and MRI, and correlated with pathology. Pediatr Radiol. 1994;24:143–144.
- Mise Y, Tani K, Aoki T, et al. Virtual liver resection: computer-assisted operation planning using a three-dimensional liver representation. J Hepatobiliary Pancreat Sci.2013;20:157–164.
- Simpson AL, Geller DA, Hemming AW, et al. Liver planning software accurately predicts postoperative liver volume and measures early regeneration. J Am Coll Surg. 2014;219:199–207.