Abstract
To investigate 3D printing navigation system in pediatric epiphyseal complex lesion surgery. 10 children with epiphyseal complex lesions of the lower limb were recruited. After collecting imaging data such as CT and MRI in children with epiphyseal complex lesions of the lower limb, a three-dimensional model of bone was constructed using 3D printed computer modeling technologies for the localization of the lesion area. The extent of bone bridges was less than 30%, and all of them met the indications for bone bridge resection surgery. 3D printed navigation templates guided lesion resection. Epiphyseal block growth regulation with a figure-of-eight plate was also used in cases with preexisting abnormal alignment. During the operation, the average surgical incision was 4.0 cm, the bone bridge positioning was accurate, and the bone bridge tissue could be successfully and completely removed. As a result of follow-up, no cases had residual bone bridge tissue, no iatrogenic epiphyseal injury was found, and the epiphyseal plate was open in all children. 3D printing navigation system improved the accuracy of resection of lower limb epiphyseal complex lesions, significantly reduced the need for intraoperative fluoroscopy, avoided iatrogenic injury to the epiphyseal complex due to positioning errors, thereby reducing postoperative complications and considerably improving the prognosis of a series of lower limb epiphyseal complex lesion diseases in children.
Background
The most significant difference in the skeletal system between children and adults lies in the epiphysis and physeal plate and the epiphyseal complex [Citation1]. Epiphyseal injury is a general term involving damage to the longitudinal growth mechanism of bone, including epiphysis, epiphyseal plate, ring around the epiphyseal plate (Ranvier area), growth-related articular cartilage, and metaphyseal injury. The incidence of epiphyseal injuries in children under 16 years of age ranges from 6% to 30% [Citation2]. Innate metabolic diseases, infections, tumors and fractures may lead to epiphyseal damage [Citation1,Citation3]. According to statistics, about 5 to 10% of children experience growth failure after epiphyseal injury [Citation2]. It mainly involves two aspects: abnormalities in limb length and abnormalities in limb alignment that will seriously affect children’s joint quality and walking function and then affect life quality [Citation4–9]. They will be secondary to abnormalities in the spine, hip joints, and other joints over time [Citation10].
Damage to the epiphyseal complex from failed surgery is sometimes much more significant than the lesion itself. In the traditional surgery of epiphyseal complex lesions in children, the difficult points of surgery lie in the difficulty of localization, the difficulty in determining the lesion boundary, and the difficulty in selecting the surgical approach. Specifically, in most cases, the location of the lesion can only be determined with an intraoperative C-arm X-ray machine. However, the radiograph cannot precisely display the boundary of the lesion during the operation and does not display the lesion at all, making the operation unable to proceed. In addition, the actual extent of the lesion is often larger than that shown on X-ray radiographs. The extent shown on the intraoperative C-arm X-ray and on the preoperative magnetic resonance imaging (MRI)/Computed tomography (CT) are also often inconsistent. At this point, the surgeon can only rely on experience to decide on the extent of surgical resection. The subjective decision may result in a high risk of under-resection or over-injury. In the selection of surgical approach, the epiphyseal complex is only located near the joints and is surrounded by important vessels and nerves. Clinicians urgently need a GPS-like positioning and navigation device that allows precision-guided scalpels to directly hit the lesion through a safe surgical approach while protecting the surrounding normal epiphyseal complex. Then, the application of such a navigation device can increase the indications for the medial tibial incision, which will cause less injury. Therefore, to solve the difficulties of the above epiphyseal complex lesion surgery, we hope to design a positioning guide that can be used for epiphyseal complex lesion resection in children through this study. This study comprehensively applied 3D printing technology, including image registration, reverse engineering, and rapid prototyping, as a total solution for pediatric epiphyseal complex lesion surgery.
Methods
Ethical standards
This clinical trial protocol is designed by the Declaration of Helsinki and China’s Good Clinical Practice. It was implemented after approval from the Ethics Committee of our university.
For patients enrolled in this study, the study director is responsible for systematically and comprehensively introducing the purpose, procedures, and possible risks of this study to the patient or his/her designated legal representative or impartial witness in written form. Patients have the right to withdraw from the study at any time. All informed consent forms signed and dated by the subject or legally acceptable representative, and the investigator in the study files retained the person who conducted the informed consent process.
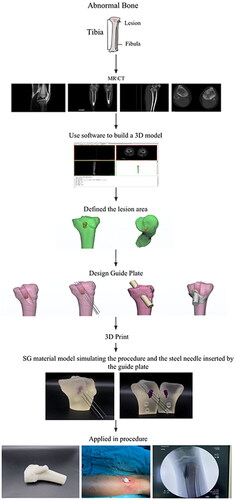
Clinical flowchart and technology roadmap
In the clinical process, MR/CT radiography is first performed on the abnormal bone. 3D modeling is established based on radiograph data. The navigation template is designed according to the lesion location. 3D printing is performed for the template and bone model. In vitro simulation was used to verify that the Kirschner needle can accurately reach the lesion location under a guide plate. After verification, the guide plate is applied in the procedure to remove the bone bridge. presented the clinical flowchart.
Inclusion criteria
(A) Imaging confirmed the presence of lesions in the epiphyseal complex of the lower limb. (B) Age less than 14 years, regardless of male or female. (C) Patients who received lesion excision surgery or lesion biopsy surgery. (D) The subject or legal representative voluntarily accepts the treatment and signs the Informed Consent Form;
Exclusion criteria
(A) Radiographically confirmed that the epiphysis of the patient’s lesion had closed. (B) Patients with highly suspected malignant lesions and distant metastasis on imaging were excluded from this study. however, patients with only suspected malignant tumors and no distant metastasis could still be included in this study, and a lesion biopsy was performed in strict accordance with the principle of no tumor. (C) Preoperative X-ray, CT, and MRI imaging data were incomplete. (D) Patients with active infection, poor general condition, and other conditions, unable to undergo surgery. (E) Patients do not agree to participate in this clinical trial study, and other investigators believe that it is not appropriate to participate in this study.
Withdrawal criteria
The investigator medically considered that the subject needed to discontinue the trial and the patient requested to terminate the trial.
Accuracy evaluation
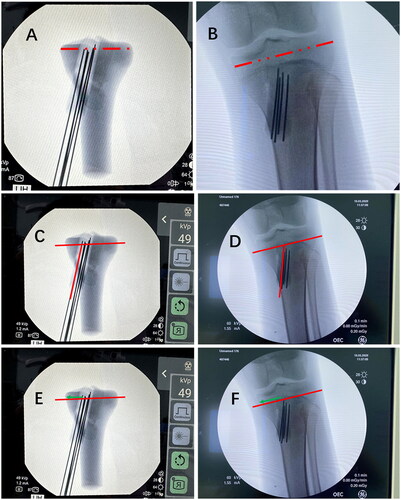
First, in the anteroposterior and lateral position films, the size of the 3D model and actual bone was corrected by scale (). Then, the angle and distance difference between the preoperative 3D model (planned insertion trajectories) and the actual operation (real insertion trajectories) were recorded. We measured the angle between the steel needle and epiphyseal line in the preoperative 3D model simulation (). Intraoperatively, the corresponding angle between the steel needle and the epiphyseal line was also recorded (). The difference between the above two angles was calculated as the angle error. Additionally, the distance from the intersection of the steel needle and the epiphyseal line to the edge of the epiphyseal line was recorded in the 3D model (). The corresponding intraoperative distance was recorded (). The difference between the above two distances was calculated as the distance error.
Figure 2. The angle and distance difference between the preoperative 3D model (planned insertion trajectories) and the actual operation (real insertion trajectories). The size of the 3D model (A) and actual bone (B) was corrected by scale. The angle was measured between the steel needle and epiphyseal line in the preoperative 3D model (C) and in the actual bone (D). The distance from the intersection of the steel needle and the epiphyseal line to the edge of the epiphyseal line (Green line) was recorded in the 3D model (E) and in actual bone (F).
Follow up
Patients were followed up at 1, 3, 6 and 12 months after surgery and then annually. With mobile phones, network terminals, and network database platforms, standardized and convenient cross-platform follow-up can be realized, and clinical data can be recorded in a standardized manner. Various postoperative complications were routinely recorded.
Results
Follow-up results
From 2016 to 2018, a total of 38 cases of lower limb epiphyseal complex lesions were treated in our center. Among them, ten patients were included in this study, and the predisposing factors of the bone bridge were trauma in 5 cases, infection in 2 cases, and unknown causes in 3 cases. The extent of bone bridges was less than 30%, and all of the patients met the indications for bone bridge resection surgery. 3D-printed navigation templates were used to guide lesion resection, and figure-of-eight plate epiphyseal block growth regulation was also used in cases with preexisting abnormal alignment. The mean age was 7.7 (6, 10) years, and the mean follow-up was three years. During the operation, the average surgical incision was 4.0 cm, the bone bridge was accurately located, and the bone bridge tissues could be successfully and completely removed. The average number of radiographs was 23 in the first three cases and 14 in the last seven cases during the operation (). Follow-up results showed that there was no case of residual bone bridge tissue and iatrogenic epiphyseal injury. On follow-up radiographs, the physis was open in all children. In patients using figure-of-eight plates, the lower limb’s gravity line also gradually returned to normal ().
Figure 3. (A) The medial focus of the tibia. X-ray, CT, and full-length radiography of the lower limb showed varus of the right knee; (B) Bone bridge resection combined with lateral figure-of-eight plate epiphyseal block. Two years after surgery, the bone bridge was removed entirely without iatrogenic epiphyseal injury. Full-length radiography of the lower limbs showed that the right lower limb’s gravity line returned to normal.
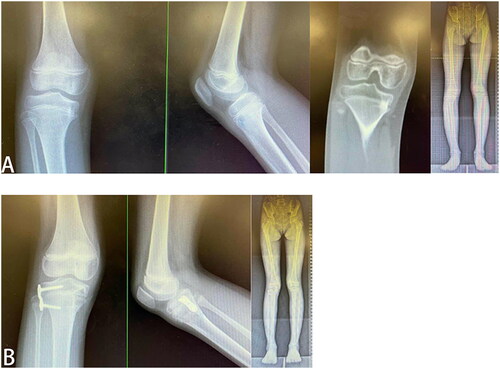
Table 1. Characteristics of 10 cases with lower limb epiphyseal complex lesions.
Case
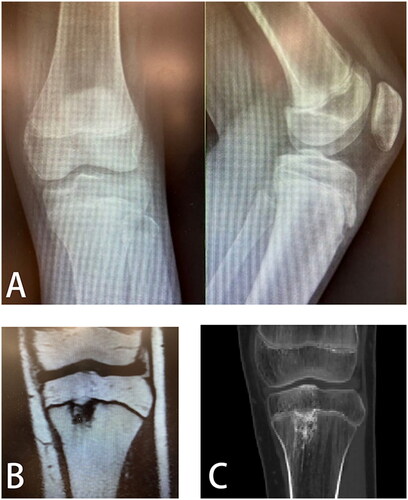
A 13-year-old male child presented with left knee pain and limping on walking. Imaging examination revealed a lesion in the left tibial epiphyseal complex, which may damage the epiphysis. Therefore, the attending physician decided to remove the lesion in the epiphyseal complex surgically. However, the lesion was not visualized on X-ray radiographs (). Traditional surgical methods rely on intraoperative C-arm X-ray machine positioning, so it is difficult to use conventional procedures in this case. However, this lesion and its extent can be visualized on CT and MRI ().
Figure 4. (A) Lesions are not visualized on anteroposterior and lateral X-ray radiographs of the knee; (B) CT images of the lesion; (C) MRI images of lesions.
Step one
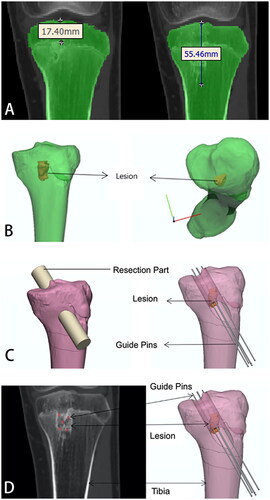
The standard anteroposterior CT data of the left tibia of the 13-year-old child were selected. Two-dimensional images determined the lesion’s regional extent with a distance of 17.41 mm from the tibial plateau to the highest end of the lesion area and 55.46 mm from the tibial plateau to the lowest end of the lesion area ().
Step two
Three-dimensional lesion models were built by two-dimensional CT data using Mimics, or Medraw software, and the built three-dimensional tibial model and three-dimensional lesion model were imported into 3-Matic. Preoperative planning was performed based on the patient’s three-dimensional model of the tibia and the three-dimensional model of the lesion ().
Step three
The surgeon simulated the resection of the tibial block in the lesion area according to the surgical approach planned preoperatively ().
Step four
The placement of guiding steel needles were simulated according to the patient’s CT image and the three-dimensional image of the tibial and tibial lesion area. Five guiding steel needles with a diameter of 1.5 mm were simulated (the steel needles with a diameter of less than 2.0 mm will not damage the epiphysis). The simulated five guide steel needle positions are determined by comparing the two-dimensional CT data ().
Step five
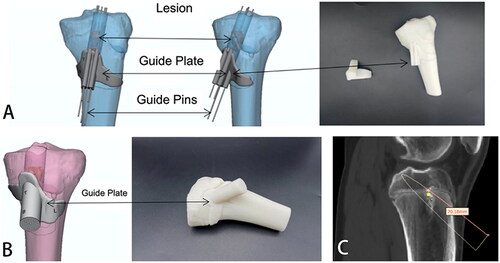
The 3-Matic software was used to design the external tibial surface onlay position and the guide plate wrapping the guidewire according to the region of the tibia cut and the guidewire’s position. In this case, the cut range and selected onlay surface for the first design were shown in . The cutting range of was more excellent for cutting soft tissue during surgery. The guide printed behind the onlay surface of cannot be reasonably applied in surgery. Hence, we improved the preliminary scheme by redesign, changed the cutting range in the tibial region and changed the range and size of the onlay surface in the position (). This can reduce the extent of soft tissue injury in children so that the designed guide plate can be reasonably used in surgery. In the guide plate design, the length from the tibia’s physis to the tail end of the steel needle was 7 cm, which allowed the depth of the steel needle to be accurately calculated during surgery to avoid damage to the physis ().
Figure 6. (A) Accurate positioning and placement of steel needle and lesion range simulated by guide plate (Regimen 1); (B) Accurate positioning and placement of steel needle and lesion range simulated by guide plate (Regimen 2); (C) The length from the physis of the tibia to the tail end of the steel needle was 7 cm.
Step six
We first used photosensitive resin 128 material to print three-dimensional bone models for solid printing. It was found in the application that we could not determine the accuracy of the guide plate. It could not accurately verify the guide steel needle’s positional relationship, inserted according to the guide plate and the lesion (). Thus, in the subsequent preliminary experiment, we used SG biocompatible materials and precisely hollowed the lesion part according to its location, size, and shape. The three-dimensional bone model was also divided for printing, which solved the problem of guide accuracy verification.
Figure 7. (A) Photosensitive resin No. 128 material printed three-dimensional bone model and positioning guide plate; (B,C) Three-dimensional bone model of SG material (transparent) with lesion area hollowed out and split in its position; (D,E) Sectional view of the guidewire and lesion impacted with the guide plate; (F) Guidewire impacted through the guide plate; (G,H) Comparison of preoperative CT planning and intraoperative guidance steel needle; (I) Complete excision of the lesion through the minimally invasive incision; (J) The steel needle was used to explore the excavated lesion’s edge without damaging the normal physis.
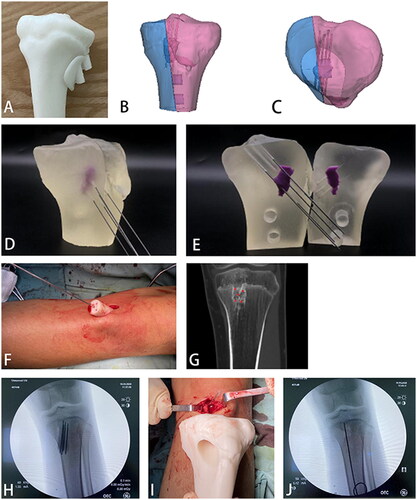
Step seven
During the operation, a small incision was made, a guide plate was placed, and a guide steel needle was inserted through the guide plate (). presented the comparison of preoperative CT planning and intraoperative guidance steel needle. The lesion location was accurately located by the guide plate, the lesion was completely excavated, and postoperative radiographs confirmed successful surgery without harming the normal physis ().
Accuracy evaluation
The results of angle and distance errors show that in the anteroposterior film, the angle error between the 3D model and actual operation was 2.68° ± 0.53°, and the distance error was 1.45 ± 0.28 mm. In the lateral film, the angle error was 3.03° ± 0.80°, and the distance error was 1.45 ± 0.40 mm ().
Discussion
The most significant difference between the pediatric and adult skeletal systems is the presence of the epiphyseal complex. The normal epiphyseal complex ensures the normal length and line of force of the extremities in children. Epiphyseal complex lesions in the lower limbs can cause epiphyseal injury and produce bone dysplasia, resulting in abnormal lower limb alignment and length in children, and ultimately arthritis and walking dysfunction. Excision of epiphyseal complex lesions requires special attention to avoid iatrogenic damage to the normal epiphysis. However, it is difficult for the operator to accurately define the lesion boundary by the naked eye and intraoperative fluoroscopy in conventional surgery. CT and MRI examinations cannot be performed in the operating room, and intraoperative immediate three-dimensional navigation has a high price and challenging transformation. Therefore, it is urgent to develop tools that can economically, simply, and accurately locate epiphyseal complex lesions during surgery.
This study intended to collect CT and MRI imaging data of children with lower limb epiphyseal complex lesions and construct a three-dimensional bone model using 3D printed computer modeling technology to localize the lesion area. We designed a personalized positioning and aiming guide for the lesion’s resection, which achieved accurate resection of the lesion and protected the normal epiphyseal complex from avoiding iatrogenic injury.
In recent years, applying the above orthopedics techniques has gradually increased, mainly focusing on three aspects: physical models, individualized guides, and implants [Citation11–13]. Since Goffin [Citation14] reported the application of the guide plate technique in pedicle screw placement in 2001, it has been successively reported in the literature that a guide plate is used to assist the accurate positioning of the acetabular cup in hip replacement [Citation15]. The navigation of bone tumor boundary cutting, 3D printed individualized guide technique, and clinical application of personalized implants have been reported in China, which clinically confirmed the accuracy of navigation templates and reduced the complexity of surgery [Citation11,Citation15]. Preoperative planning and 3D printing technology based on 2D/3D registration can effectively overcome navigation shortcomings without increasing operation time. The design and manufacture of surgical guidance devices and bone substitutes can be achieved at one time.
In addition, the preoperative planning techniques in this study comprehensively applied 3D printing technology, including image registration, reverse engineering, and rapid prototyping. Therefore, we propose a total solution for the precise resection of lesions in pediatric epiphyseal complex lesion surgery. The 3D printing of organs and lesions, the design of guide plates to fit on the surface of the organs, and the preoperative simulation of the surgical procedure included in this technology can provide a reference value for orthopedic and other surgical procedures in which lesions cannot be precisely located and resected intraoperatively.
There were several limitations to this study. Due to the early phase of this study, adequate experience is lacking. Therefore, all cases were selected as children with the tibial bone bridge. The tibia structure is clear, which makes the positioning guide plate easy to be designed and applied. It is hoped that through the experience in this study, the positioning guide plate will be further designed for the focus of the distal tibial, distal femoral epiphyseal complex
In summary, this study is based on the disease of pediatric epiphyseal complex lesions and innovatively designs high-precision personalized guides suitable for individual anatomy. 3D printing navigation system improved the accuracy of resection of lower limb epiphyseal complex lesions, significantly reduced the need for intraoperative fluoroscopy, avoided iatrogenic injury to the epiphyseal complex due to positioning errors, thereby reducing postoperative complications and considerably improving the prognosis of a series of lower limb epiphyseal complex lesion diseases in children. This study has a broad prospect of clinical application and great socio-economic significance in pediatric orthopedics.
Author contributions
Haiqing Cai was dedicated to the guarantor of integrity of the entire study and study concepts; Haoqi Cai carried out the study design, definition of intellectual content, literature research, clinical studies, experimental studies, data acquisition, data analysis, statistical analysis and manuscript editing; Zhigang Wang handled the manuscript review. All authors have read and approved this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shaw N, Erickson C, Bryant SJ, et al. Regenerative medicine approaches for the treatment of pediatric physeal injuries. Tissue Engineering Part B. 2017;24(2):85–97.
- Eastwood DM, Gheldere AD. Physeal injuries in children. Surgery. 2011;29:146–152.
- Mallick A, Prem H. Physeal injuries in children. Surgery. 2017;35:10–17.
- Dabash S, Prabhakar G, Potter E, et al. Management of growth arrest: current practice and future directions. J Clin Orthop Trauma. 2018;9(Suppl 1):S58–S66.
- Tsang KY, Chan D, Cheah KS. Fate of growth plate hypertrophic chondrocytes: death or lineage extension? Dev Growth Differ. 2015;57(2):179–192.
- Dodwell ER, Kelley SP. Physeal fractures: basic science, assessment and acute management. Orthop Trauma. 2011;25:377–391.
- Barrett JP Jr. Rashkoff E, Sirna EC, et al. Correlation of roentgenographic patterns and clinical manifestations of symptomatic idiopathic osteoarthritis of the knee. Clin Orthop Relat Res. 1990;253(4):179–183.
- Kristensen KD. Degenerative arthritis of the knee secondary to fracture malunion. Clin Orthop Relat Res. 1989;245(8):311–312.
- Hernborg JS, Nilsson BE. The natural course of untreated osteoarthritis of the knee. Clin Orthop Relat Res. 1977;123(2):130–137.
- Deriu L, Eastwood DM. Physeal injury, epiphysiodesis and guided growth. In: Alshryda S, Huntley JS, Banaszkiewicz PA, editors. Paediatric Orthopaedics: An Evidence-Based Approach to Clinical Questions. Cham, Switzerland: Springer International Publishing; 2017. p. 451–473
- Chen X, Xu L, Wang Y, et al. Image-guided installation of 3D-printed patient-specific implant and its application in pelvic tumor resection and reconstruction surgery. Comput Methods Programs Biomed. 2016;125:66–78.
- Zhang YZ, Lu S, Yang Y, et al. Design and primary application of computer-assisted, patient-specific navigational templates in metal-on-metal hip resurfacing arthroplasty. J Arthroplasty. 2011;26(7):1083–1087.
- Ai ST, Qu Y, Fang XD, et al. Application of 3D printing models in undergraduate medical imagine teaching. Chin J Med Edu Res. 2017;16(9):904–908.
- Goffin J, Van Brussel K, Martens K, et al. Three-dimensional computed tomography-based, personalized drill guide for posterior cervical stabilization at C1–C2. Spine. 2001;26(12):1343–1347.
- Qu Y, Ai ST, Yang F, et al. Application of CT/MRI image registration and fusion combined with 3D printing technique in pre-surgical planning of refractory pelvic tumors. J Shanghai Jiaotong Univ (Med Sci). 2017;37(9):1239–1244.