 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To study the changes of motor and cognitive function of pituitary tumor rats after the operation. Methods: The experiment was divided into three groups: control group, model group and operation group (30 animals for each group). Female Fischer344 rats were selected. Model group rats were subcutaneously embedded with 10 mg estrogen sustained-release pump to induce a pituitary tumor model, and control group rats were subcutaneously embedded with a normal saline sustained-release pump as control. The operation group was successfully treated by microsurgery after the model was established. The quantitative expressions of PTTG, FGF-2 and VEGF were detected by Western blot. Morris test was used to detect the spatial learning and memory ability of rats. Western blot results showed that compared with the model group, the expression of the operation group was decreased, but still higher than that of the control group (p < 0.05). The water maze test results showed that the incubation period of searching the safe island in the model group was significantly longer than that in the control group on the 8th and 9th day after the injury, and the difference was statistically significant (p < 0.05). The incubation period of searching the safe island on the 8th and 9th day after injury in the operation group was significantly shorter than that in the control group. Through the detection of behavioral-related experimental and protein, the motor memory of rats after pituitary tumor surgery can be improved to some extent.
Introduction
Pituitary tumor is a common benign intracranial tumor, accounting for about 10% of intracranial tumors [Citation1]. Except for some prolactin adenomas, surgical treatment is the most fundamental and effective treatment at present, but postoperative recurrence remains the main difficulty at present [Citation2]. Now, transnasal sphenoidal microscopic pituitary tumor resection and transnasal sphenoidal neuroendoscopic pituitary tumor resection are commonly used for pituitary tumor resection. Microscopic surgery has a strong sense of three-dimensional space under direct vision, and neuroendoscopy has wide-angle lighting and a panoramic field of vision, so the surgical field can be more clearly and intuitional by adjusting the angle of the mirror [Citation3]. Pituitary tumor transforming gene (PTTG) is a recognized tumor gene closely related to the occurrence of pituitary tumors. Previous report has shown that PTTG and cell proliferation cycle nuclear antigen Ki-67 may be related to the invasive growth of pituitary tumors and pituitary apostrophia [Citation4]. In addition, the report has shown that PTTG can stimulate the generation of fibroblast growth factor 2(FGF-2) and VEGF by up-regulating the expression of growth factor receptors, and then promote the growth and invasion of pituitary adenomas. Therefore, the recurrence of pituitary tumors is probably related to the abnormal expression of PTTG/FGF-2/VEGF signaling pathway [Citation5]. This study aims to establish a rat model of the pituitary tumor to explore the changes in motor memory function after surgery, so as to provide a certain experimental basis for the recovery of clinical patients’ postoperative movement and memory.
Materials and methods
Animals
Female Fischer344 rats (4 weeks, 80–100 g), 90 SPF grade young male SD rats were selected and fed for one week at room temperature (25 ± 2)°Cand humidity (50 ± 5) % before the experiment. Provided by Beijing Vitong Lihua Animal Company. They were randomly divided into three groups: control group, model group and operation group. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication NO.80-23, revised in 1996). After the rats were sacrificed, the brain tissues were taken out and used for experiments [Citation6].
Animal models
Model group rats were subcutaneously embedded with 10 mg estrogen sustained release pump to induce a pituitary tumor model, and control group rats were subcutaneously embedded with normal saline sustained release pump(Beijing Jitai Yuancheng Technology Co., LTD) as control. The room temperature was 18 °C∼20 °C, and the relative humidity was 40%∼70%. After 8 weeks of modeling, the formation of pituitary tumors was observed by color ultrasonography. If there was no obvious pituitary tumor formation, the sustained release pump was placed or replaced with a new sustained release pump to continue modeling. Pituitary tumor tissue and normal pituitary tissue were collected from the observation group and the control group, respectively, for the next experiment [Citation6]. General anesthesia and endotracheal intubation were performed in the supine position, facial and bilateral nasal disinfection was performed with iodine, and a neuroendoscope was guided into the nasal cavity. The right nostril approach was generally chosen to find the middle turbinate and nasal septum, and epinephrine saline cotton tablets were soaked in the southern mucosa of the nasal septum to shrink the blood vessels, reduce bleeding and expand the nasal cavity. After the dural membrane at the bottom of the saddle was illuminated by bipolar electrolysis, the thin knife was cut across and the endoscope was illuminated, and the saline operation site was intermittently cleaned. At this time, the suction device, forceps, stripper and tumor curets of ethmoid sinus should be prepared to remove the tumor. After resection of most tumors, the endoscope will be inserted into the tumor cavity to observe residual tumor and bleeder, the residual tumors in and above the sella were further removed under direct vision, and the bleeding points could be controlled by electrocoagulation or gelatin sponge compression. When there is no bleeding, gelatin sponge was applied to the opening of the ear sphenoid sinus, which was an important anatomical mark of the surgical approach and should be confirmed strictly.
Western blot analysis
Briefly, rats were anesthetized and underwent intracardiac perfusion with 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4). Take 100 mg of rats’ hippocampal tissue, and made them rapidly isolated, total proteins were extracted and protein concentration was determined by the BCA reagent (Solarbio, Beijing, China) method. Samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins on the gel were transferred onto PVDF membranes (Roche Diagnostics, Mannheim, Germany). Blots were blocked with 5% fat-free dry milk for 1 h at room temperature. Following blocking, the membrane was incubated with indicated primary antibodies overnight at 4 °C, including rabbit anti- PTTG polyclonal antibodies (Santa Cruz Biotechnology; Santa Cruz, CA, USA, diluted 1:500), rabbit anti- FGF-2 polyclonal antibody (Santa Cruz Biotechnology; Santa Cruz, CA, USA, diluted 1:500), rabbit anti- VEGF polyclonal antibody (Santa Cruz Biotechnology; Santa Cruz, CA, USA, diluted 1:500), mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology; Santa Cruz, CA, USA, diluted 1:500). And then incubated with horseradish peroxidase conjugated anti-rabbit IgG and anti-mouse IgG (Cell Signaling Technology, Inc., Danvers, MA, USA, diluted 1:5000) for 2 h at room temperature. Following incubation with a properly titrated second antibody, the immunoblot on the membrane was visible after development with an enhanced chemiluminescence (ECL) detection system and densitometric signals were quantified with an imaging program. Immunoreactive bands of all proteins expression were normalized to the intensity of corresponding bands for β-actin. The Western blot results were analyzed with National Institutes of Health Image 1.41 software (Bethesda, MD, USA) [Citation6].
Evaluation of brain edema
Brain edema was evaluated by analysis of brain water content as described previously. Rat brains were separated and weighed immediately with a chemical balance to get wet weight (WW). Following drying in a desiccating oven for 24h at 100 °C, dry tissues were weighed again to get constant dry weight (DW) data. The percentage of water in the tissues was calculated according to the formula:
% brain water = (WW - DW)/WW) ×100(6).
Morris water maze test
The spatial learning ability was assessed in a Morris water maze as described previously. The Morris water maze consists of a black circular pool (180 cm diameter, 45 cm high) filled with water (30 cm depth) at 26 °C and virtually divided into four equivalent quadrants: north (N), west (W), south (S) and east (E). A 2-cm submerged safe island (with a diameter 12 cm, height 28 cm, made opaque with paint) was placed in the middle of one of the quadrants equidistant from the sidewall and the center of the pool. Rats were trained to find the platform before pituitary adenoma or sham operation. For each trial, the rat was randomly placed into a quadrant start points (N, S, E or W) facing the wall of the pool and allowed a maximum of 180 sec to escape to the platform, rats that failed to escape within 90 sec were placed on the platform for a maximum of 20 sec and returned to the cage for a new trial (inter-trial interval 20 sec). Maze performance was recorded by a video camera suspended above the maze and interfaced with a video tracking system (HVS Imaging, Hampton, UK). The average escape latency of a total of five trials was calculated [Citation6].
Neuromotor function score
[Citation1] Pick up the rat and hold the rat’s torso with the thumb and forefinger around the rat’s forearm. Lightly brush the rat’s hind limbs between the first and second digits. After contact, the normal rats would show the grasping reaction of the hind limbs, which was counted as 1 point. No response indicates the presence of injury, and the score is 0. Each rat was tested 3 times and the total score was recorded. Bilateral hind limb tests score out of 6 [Citation2]. Vibrissae-evoked forelimb placing. The rat trunk and hind limbs were fixed. Then the antennae of the rats were placed in contact with the edge of the plate-like platform to induce the positioning response of forelimb extension. Reaction within 5 s indicating good reflection, which counted as 1 point. No response or failure to locate, which counted as 0 points. Each rat was tested by 3 times, with a maximum score of 6 [Citation3]. Lateral stepping. The function of all limbs of rats was examined. The rats were placed in the center of a circular table, and the rats were allowed to perceive the change of position by the touch of their limbs. The rats were slowly pushed to the side in the opposite direction of each limb movement to observe the adjustment of their gait. The normal rats were able to adjust their gait repeatedly, which counted as 1 point. If not, which counted as 0 point. All limbs were tested by three times, with a maximum score of 12.
The total score above was 24 points, and the scores of the control group, model group and operation group were recorded for statistical analysis.
Field test
The experiment needs to be carried out in a relatively quiet and soundproof room, and each test should be conducted at the same time. The rats were gently placed in the central square, and then the experimental analysis software was used to detect the total distance of walking, central activity time, standing times and modifying behaviors of the rats. After each rat was tested, the experimental area was cleaned with 75% alcohol to ensure that the next experiment would go on as planned.
Statistical analysis
All data were presented as mean ± SD. SPSS 16.0 (SPSS, Chicago, IL) was used for the statistical analysis of the data. Statistical analysis was performed with ANOVA and followed by the Student-Newman-Keuls posthoc tests. Statistical significance was inferred at p<0.05.
Results
Western blot
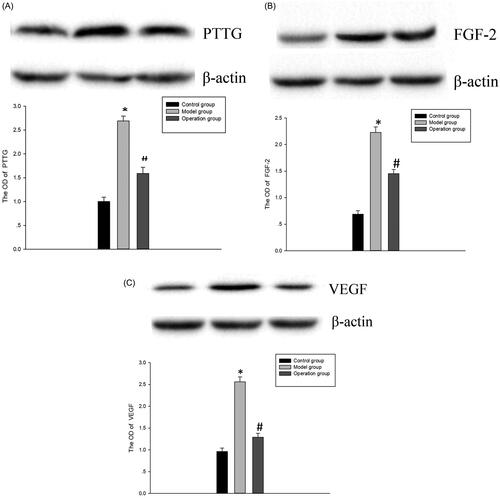
Compared with the control group, the protein expressions of PTTG, FGF-2 and VEGF in the model group were significantly increased (p < 0.05). Compared with the model group, the expression of histone was significantly decreased (p < 0.05), but it was still higher than the control group. This suggests that PTTG, FGF-2 and VEGF proteins are involved in the development of pituitary tumors ().
Figure 1. (A) The protein expression of PTTG in each group. Model group vs Control group, *p < 0.05; Operation group vs Model group, #p < 0.05. (B) The protein expression of FGF-2 in each group.Model group vs Control group, *p < 0.05; Operation group vs Model group, #p < 0.05. (C) The protein expression of VEGF in each group. Model group vs Control group, *p < 0.05; Operation group vs Model group, #p < 0.05.
Evaluation of brain edema
In the control group, the water content of brain tissue after the pituitary tumor of rats had no difference at all time points. The water content of brain tissue in the model group increased with time, which was significantly higher than that in the control group (p < 0.05), indicating that there was obvious brain edema in the brain tissue after the pituitary tumor. Compared with the model group, the water content in the operation group decreased at each time point but was significantly higher than that in the control group (p < 0.05). (, ).
Figure 2. Brain water content was determined as percentage of dry/wet ratio. *p < 0.05 vs Control group; #p < 0.05 vs Model group.
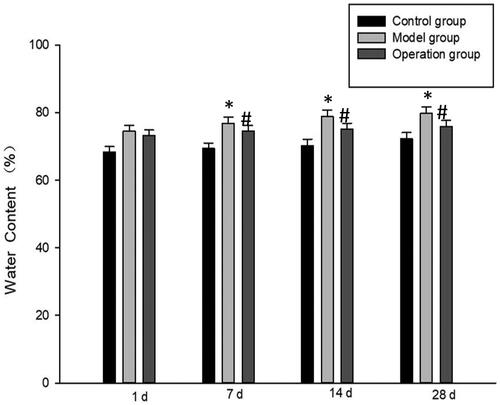
Table 1. Results of determination of water content in brain tissue (%).
Morris water maze test
Because the exercise ability of rats would be affected to some extent after pituitary tumor, the experimental time was set to conduct the Morris water maze test on the 6th, 7th, 8th and 9th day after each group of models. The experimental results showed that the incubation period of searching the safe island in the model group was significantly longer than that in the control group on the 8th and 9th day after the injury, and the difference was statistically significant (p < 0.05). The incubation period of searching the safe island in the operation group on the 8th and 9th day after injury was significantly shorter than that in the control group, indicating that the memory function of rats had been restored to a certain extent, and the difference was statistically significant (p < 0.05) ().
Figure 3. (A) The moving track of Control group in Morris water maze. A: 6 d, B: 7 d, C:8 d, D: 9 d. (B) The moving track of Model group in Morris water maze. A: 6 d, B: 7 d, C: 8 d, D: 9 d. (C) The moving track of Operation group in Morris water maze. A: 6 d, B: 7 d, C: 8 d, D: 9 d. (D) The comparison of delitescence in water maze among groups after injury.*p < 0.05 vs Control group; #p < 0.05 vs Model group.
Neuromotor function score
The score of the control group was 24 points, the score of the model group was 9.56 ± 0.61 points, and the score of the surgery group was 17.89 ± 0.52 points. Compared with the control group, rats in the model group showed abnormal performance in the tests of hindlimb grasping reflex, tentacle-induced forelimb positioning and lateral padding, leading to decreased functional scores, with statistical significance (p < 0.05). Compared with the model group, the functional scores of each item in the operation group were significantly increased, indicating that the motor function of the rats was improved to a certain extent (p < 0.05) (, ).
Figure 4. Comparation of the neurologic function scores. *p < 0.05 vs Control group; #p < 0.05 vs Model group.
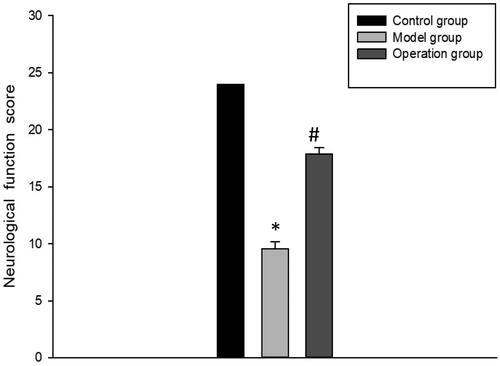
Table 2. Results of neurological function score( ± s).
Field test
The total walking distance, time of central frame activity, number of standing up and modifying behaviors in control group were the least, while that in the model group were less than the control group (p < 0.05). Compared with the model group, the total distance, the time of central cell activity, the number of upright and the number of grooming behaviors in the operation group were decreased, but higher than those in the control group (p < 0.05) ().
Discussion
The cognitive function of pituitary tumor patients is generally impaired, which is manifested in many aspects. The reason for cognitive decline in patients with pituitary tumor are not only the compression of peripheral brain tissue structure by tumor and high hormone levels, which can damage the brain tissue structure and cause cognitive impairment of patients [Citation7]. Surgery is also considered to be one of the factors leading to cognitive impairment in patients with pituitary tumor [Citation8,Citation9]. The treatment of pituitary tumor includes surgery, radiotherapy, drug therapy and other comprehensive treatments. However, surgery is still the main treatment for pituitary adenoma [Citation10,Citation11]. The surgical methods of pituitary adenoma are changing all the time, which is also a reflection of the development of surgical techniques. At present, according to the researches, even if we achieved very good treatment of pituitary adenoma patients (including total excision of the tumor, hormone level disorder completely or mostly improved, eased symptoms caused by tumor), in the long term run, patients’ cognitive function still reflects obvious damage symptom compared with normal people [Citation12]. There are many factors that cause cognitive impairment in patients after treatment, and some studies suggest that the treatment process is often one of the factors that cause cognitive ability decline in patients [Citation13].
Therefore, this experiment was designed to explore the changes in cognitive function of rats after pituitary tumor operation. It can be seen from the experimental results that, compared with the model group, the protein expression of the operation group decreased significantly, but was still higher than that of the control group, indicating that PTTG, FGF-2 and VEGF are involved in the occurrence and development of pituitary tumors. The mine field and water maze test results indicated that the incubation period of searching the safety island in the model group was significantly longer than that in the control group on the 8th and 9th days after injury, and the incubation period of searching the safety island in the operation group was significantly shorter than that in the model group on the 8th and 9th days after injury, suggesting that the spatial memory function of rats had been improved to some extent. Neurological function score results showed that compared with the control group, rats in the model group had abnormal performance in the tests of hindlimb grasping reflex, antenna-induced forelimb positioning and lateral pad. Compared with the operation group and the model group, the functional scores of each group were significantly higher, indicating that the neural motor function of rats was also restored to a certain extent after surgical treatment.
To sum up, through the study of this experiment, we can draw the conclusion that PTTG, FGF-2 and VEGF proteins are involved in the occurrence and development of pituitary tumors, and it also proves that pituitary tumors will have a certain impact on cognitive function, which can be improved and recovered to a certain extent through surgical treatment, providing theoretical support for timely clinical treatment.
Ethics approval and consent to participate
All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
ARRIVE guidelines
The study was carried out in compliance with the ARRIVE guidelines.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Donangelo I, Melmed S. Implication of pituitary tropic status on tumor development. Front Horm Res. 2006;35:1–8.
- Loyo-Varela M, Herrada-Pineda T, Revilla-Pacheco F, et al. Pituitary tumor surgery: review of 3004 cases. . [World Neurosurg. 2013;79(2):331–336.
- Hardy J. Reflections on the evolution of pituitary tumor surgery with emphasis on the transsphenoidal approach. Transsphenoidal Surgery. 2010;(12)1–3.
- Salehi F, Kovacs K, Scheithauer BW, et al. Immunohistochemical expression of pituitary tumor transforming gene (PTTG) in pituitary adenomas: a correlative study of tumor subtypes.. Int J Surg Pathol. 2010;18(1):5–13.
- Cho SY. Pituitary tumor-transforming gene (PTTG) induces both vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Bull- Korean Chem Soc. 2005;26(11-13)
- Chang-Meng C, Jun-Ling, et al. Chloroquine exerts neuroprotection following traumatic brain injury via suppression of inflammation and neuronal autophagic death. Mol Med Rep. 2015;(6):2323–2328.
- Wang J, Song DL, Deng L, et al. Extraventricular neurocytoma of the sellar region: case report and literature review. Springerplus. 2016;5(1):987.
- Hendrix P, Griessenauer CJ, Hans E, et al. Cognitive function surrounding resection of nonfunctioning pituitary adenomas with suprasellar extension: a prospective matched-control study. J Clin Neurosci. 2017;40:109–114.
- Petry C, Rios MC, Leaes C, et al. Assessment of cognitive function, mood and quality of life in hypopituitary patients after pituitary adenomectomy with or without radiotherapy. Int J Endocrinol Metab. 2012;9(2):306–310.
- Liu Y, Zheng T, Lv W, et al. Ambulatory surgery protocol for endoscopic endonasal resection of pituitary adenomas: a prospective single-arm trial with initial implementation experience. Sci Rep. 2020;10(1):478–482.
- Marino AC, Taylor DG, Desai B, et al. Surgery for pediatric pituitary adenomas. Neurosurg Clin N Am. 2019;30(4):465–471.
- Tooze A, Gittoes N, Jones C, et al. The effects of radiotherapy on neurocognitive function in patients treated for non-functioning pituitary adenoma Endocrine. 2007;13(27):299.
- Yedinak CG, Fleseriu M. Self-perception of cognitive function among patients with active acromegaly, controlled acromegaly, and non-functional pituitary adenoma: a pilot study. Endocrine. 2014;46(3):585–593.



