Abstract
Background
The treatment of talus avascular necrosis (AVN) is challenging owing to its unique anatomical features. Despite decades of studies, till date, there is no appropriate treatment for talus AVN. Therefore, surgeons need to develop newer surgical methods. In the present study we introduce a new surgical method, 3D printed partial talus replacement (PTR), to treat partial talus necrosis and collapse (TNC).
Methods
A male patient with talus AVN underwent PTR in our hospital. The morphology of the talus was quantified using 3D computed tomography (CT) imaging. A novel 3D printed titanium prothesis was designed and manufactured according to the findings of the CT imaging. The prosthesis was applied during talus replantation surgery to reconstruct the anatomical structure of the ankle. The follow-up period for this patient was 24 months. The visual analog scale (VAS) scores before and after surgery, American Orthopedic Foot and Ankle Score (AOFAS), ankle range of motion, and postoperative complications were recorded to evaluate the prognosis.
Results
The anatomical structure of the talus was reconstructed. The patient was satisfied with the effects of treatment, recovery, and function. The VAS score decreased from 5 to 1. The AOFAS improved from 70 to 93. The range of motion remained the same as that during the pre-operation. The patient returned to a normal life.
Conclusion
3D printed PTR is a new surgical method for talus AVN that can provide satisfactory outcomes. In future, PTR might be an effective and preferential treatment for the treatment of partial talus AVN and collapse.
Introduction
Talar avascular necrosis (AVN) is a challenging problem owing to its unique anatomical features. The talus is prone to osteonecrosis as over 60% of its surface is articular cartilage with minimal soft tissue attachments and blood supply [Citation1]. AVN can be resulted by trauma or systemetic disease because of the damage of the blood supply. Whether a patient would have a posttraumatic talar AVN depends on the severity of the injury [Citation2]. Nontraumatic causes include chemotherapy, high levels of steroids, alcoholism, hyperlipidemia, and thrombophilia [Citation3]. Unfortunately, there are only a few options for the treatment of talar AVN.
Treatment for talar AVN begins with conservative measures, including bracing, protected weightbearing, or shock wave therapy [Citation4]. Despite conservative treatment, many patients continue to complain of pain and require surgical intervention. Patients with talar AVN without dome collapse are recommended joint-preserving surgery methods, such as core decompression or bone grafting [Citation5]. Bone marrow stimulation may best be reserved for osteochondral lesions of the talus sizes less than107.4 mm2 in area and/or 10.2 mm in diameter [Citation6,Citation7]. While osteochondral autograft is mainly required for the ipsilateral knee to repair talar defects with native hyaline cartilage, osteochondral allograft transplantation is suitable for large cystic lesions accompanied by a lack of subchondral bone integrity [Citation8]. Fresh frozen allogenic osteochondral transplantation has been applied for talar osteochondral injuries greater than 20 mm [Citation9]. Allografts are more expensive than autografts, and they carry a small risk of disease transmission [Citation10]. Further, fresh frozen allogenic osteochondral transplantation is not allowed in our country. Tibiotalocalcaneal (TTC) is usually performed as a salvage treatment toward setting the talar dome collapse [Citation11]. Although TTC arthrodesis has been reported to have an excellent prognosis in some studies, the incidence of complications including nonunion and infection continue to occur [Citation12]. Restriction of joint mobility can greatly interfere with the quality of life and performance of routine functions. Although total ankle replacement can reserve the function of ankle, but it is more appropriate for the end-stage arthritis [Citation13].
Total talus replacement (TTR), which was recently introduced to cure talar AVN, preserves the tibiotalar articulation [Citation14]. Previous studies had focused on the use of prostheses to perform talar body replacement. Harnroongroj documented good self-reported and functional outcomes in 33 patients who underwent talar body replacement [Citation15]. In contrast, Taniguchi found that among 22 patients who underwent talus body replacement, only 50% had good or excellent outcomes [Citation16]. Thus, it is still unclear whether talar body replacement is a good surgical treatment option for talar AVN. More recently, another study by Taniguchi et al. valuated 55 patients who underwent TTR with a ceramic prosthesis [Citation17]. According to them, this kind of prothesis could improve both the Ankle Osteoarthritis Scale (AOS) and Japanese Society for Surgery of the Foot (JSSF) ankle-hindfoot scale.
However, in the case of talus necrosis and collapse (TNC) in the talar trochlea, there may be no benefit from the former methods. We, therefore, introduced a new prothesis using 3D printed technique, that could repair the defect as well as restore the appearance of the talus. Bone defects were measured using computed tomography (CT) scanning and based on this, we designed and produced a patient-specific partial titanium prosthesis using the 3D technique. In this study we report the preliminary results of a partial talus replacement for the treatment of TNC.
Materials and methods
Patient information
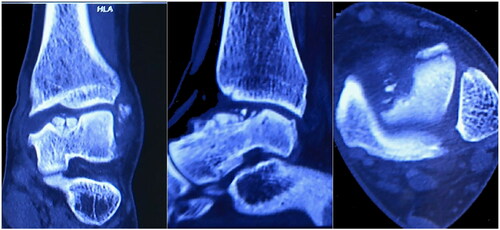
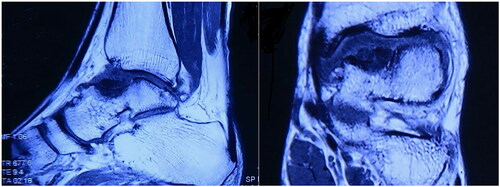
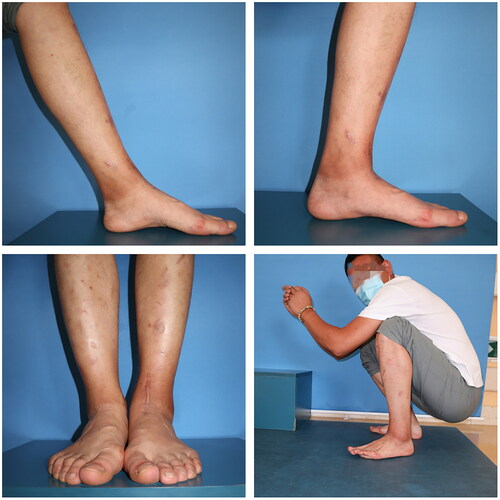
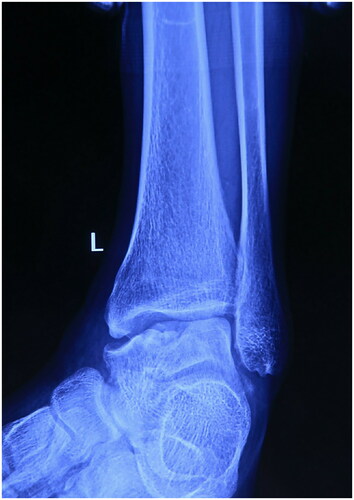
A 28-year-old male patient came to our clinic and complained of pain and swelling of the left ankle because of ankle sprain while walking 1 year ago. He stopped working, rested and controlled pain and swelling with external applications in local clinic. Though the pain gradually subsided, pain in his left ankle developed again after a mild sprain. So, he came to our clinic for help. Physical examination of the left ankle demonstrated a swollen ankle and restricted range of flexion and extension. He manifested 10° of passive ankle dorsiflexion and 30° of plantar flexion. Plain radiographs of the left ankle showed necrosis and collapse of the talar medial trochlea (). Bilateral ankle CT scans demonstrated the area and size of the talar medial trochlea collapse (). An MRI scan further confirmed necrosis of the talus (). The AOFAS was 70. Based on these findings, the patient was diagnosed with TNC by the surgeons in our hospital.
Figure 1. Lateral and anteroposterior x-rays of left ankle showing the necrosis and collapse of medial talus shoulder.
Preoperative planning
Radiographic results were evaluated by surgeons in our department. The images showed necrosis of the talus medial trochlea, which influenced the tibia-talus joint.
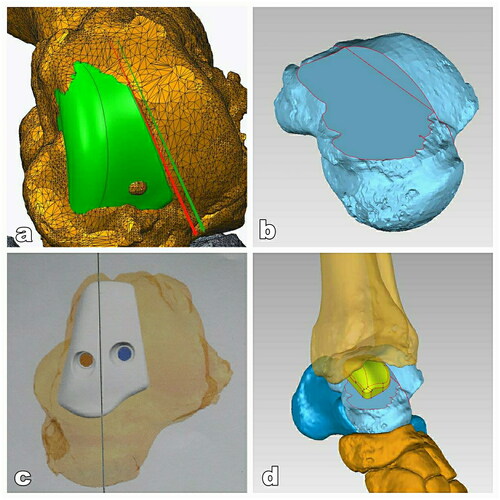
Reconstructive CT scans showed defective parts to be located in the lower limbs, including the tibia, fibula, and talus. DICOM files obtained from the CT scan were used to segment target bones via the software Mimic Medical 20.0. The bony regions around the defect were labeled manually based on the Hounsfield Unit to identify accurate regions. Thus, the affected bone was separated from the surrounding bones using a region-growing algorithm. The analysis showed that the defect region measured 2.8 cm × 1.7 cm × 1.5 cm, which was approximately the medial trochlea of talus. Next, professional software tools were used to design 3D implants ().
Figure 4. The design of 3D-printed prothesis. (A) The affected area of medial talar trochlea (green area). (B) The view of the talus after the osteotomy of affected medial talar trochlea. (C) Top view of the prosthesis placed on the talus and pre-drilled holes for screws in the prothesis. (D) The view of ankle after installation of prosthesis.
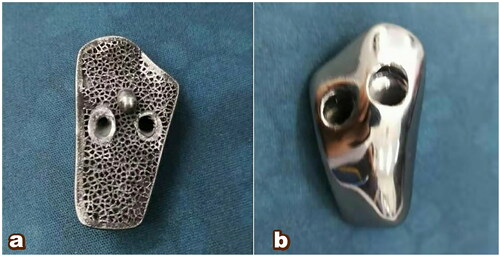
To adjust the prothesis continuously, our team co-ordinated with engineers of 3D printing corporations whose products met the Chinese Food and Drug Administration (CFDA) standards. Titanium alloy powder was used as the talar structure material. The specific casting process included mirror polishing of talar articular surface, ultrasonic cleaning, fine cleaning, and drying. The bottle of the prosthesis is a rough surface that facilitates bone ingrowth. The implant was equipped with two holes and one leg. The damaged part was replaced with a 3D-printed partial talar prosthesis ().
Figure 5. 3D printed finished product. (a) processing of porous talus and leg into the remained talus and establishment of the fixation screw channel; (b) bright polishing of articular facet of the talus prothesis.
Surgical techniques
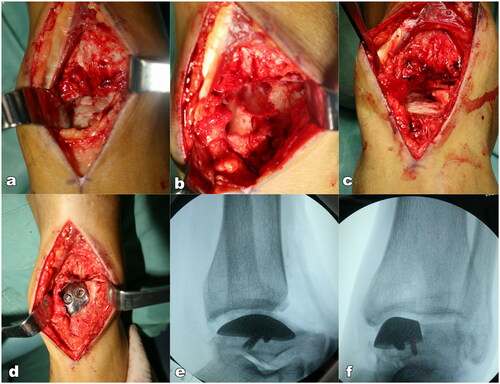
The patient was placed on the operating table in the supine position. General anesthesia was administered by the anesthetic team. An incision was made between the extensor hallucis longus and the tibialis anterior tendon. The joint of the tibial-talus articular joint was then clearly exposed. It was seen that the lesion area of the talus was covered by chondroid tissue. Following the removal of the chondroid tissue, a defect remained in the medial talar trochlea. The ankle was placed in the extreme plantarflexion position by the assistant. Osteotomy was then performed according to the 3D design. The damaged medial talus trochlea was removed slice by slice using the oscillating saw until the talar prosthesis is matched with the rest of joint articular. Soft tissue and ligaments were reserved. After resection of the affected medial talus trochlea, a trial talar prosthesis was implanted with manual traction. The articulation range of motion was assessed through plantarflexion, dorsiflexion, inversion, and eversion. An X-ray was used to determine the fitness. Finally, a 3D printed talus prosthesis was implanted and fixed with two 3.5 mm screws through the pre-drilled hole in the prosthesis (). The incision was closed layer by layer. The duration of operation was 1.5 h and the blood loss was about 50 ml.
Postoperative treatment and follow-up
The incision was maintained clean by changing the wound dressings every other day and there was no infection or necrosis. The drainage tube was removed 2 days after surgery. Short-leg cast immobilization was required in the first 4 weeks. The patient was allowed to exercise under non-weight-bearing conditions for this period, and further allowed to bear weight in the ankle boot for 8 weeks. The patient returned to a regular shoe and started physical therapy for range of motion and strength exercises after 12 weeks.
Results
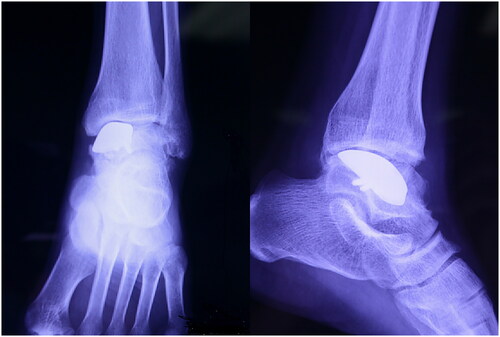
No postoperative complications were observed. The patient returned to our hospital for review at 1, 2, 3, 6, 12 and 24 months for follow-up. Radiography showed no signs of prothesis loosening or sinking after 24-month follow-up ().
With regard to the range of motion, the patient showed an improvement in his active dorsiflexion; the plantarflexion angle was 45°. The VAS score significantly improved postoperatively from 5 to 1. The AOFAS also improved from 70 to 93. The affected ankle joint showed a normal appearance and he could walk without difficulty and returned to normal life (). These indicated that partial talus replacement in the affected ankle was successful.
Discussion
The treatment of talus TNC continues to remain a challenging problem [Citation18]. Options are relatively limited and highly dependent on the disease severity. Conservative treatment usually requires patients to sustain a non-weight-bearing position for a long period which may not result in a satisfactory outcome. Although there are some joint-preserving surgery methods for the treatment of talar AVN, progressive disease is still frequently treated with arthrodesis and allografts. However, neither of them could achieve a good prognosis because of long recovery periods and a considerable risk of pseudarthrosis [Citation19–21].
To address this problem, a total talar prosthesis was introduced in 1997 [Citation15]. Harnroongroj reported the outcomes in 16 patients who underwent talar body prosthesis replacement, which was made of stainless steel [Citation22]. Although replacement of the talar body relieved patients’ symptoms, abrasion between the tibia and the prosthesis can cause damage to the articular surface of the tibia. Taniguchi et al. designed two different types of talar implants [Citation17]. The first generation was implanted into the talar head using a screw. Loosening around the peg was noted in all patients in the first generation. The second generation was designed to completely replace the talus. Among the 14 patients who received total talus replacement, eight had excellent results. The authors suggested that total talus replacement was better than talar body replacement. Total talus replacement is currently considered the gold standard for extensive aseptic necrosis of the talar body. However, it is still unclear whether total talus replacement would really fit all patients with talus AVN.
The talus has a unique structure, including being covered by approximately 60% articular cartilage and no muscle or tendon attachments. Therefore, blood vessels only travel in a limited area, which enables the talus to be a ‘lonely island’—lacking collateral circulation. The talus is prone to osteonecrosis when its vascular supply is disturbed. The structural features remind us that the less destroying the blood supply, the better the prognosis of the talus. In addition, all ligaments are destroyed in the TTR. Although the range of motion of the ankle is better than that of arthrodesis, ankle instability remains a potential problem. Considering these anatomical characteristics, our team suggested that partial talus replacement may be better than total talar replacement, especially for young and active patients with high demands for sport related activities. Partial talar replacement avoids aggressive destruction of the vascular supply and ligament stability is preserved, which alleviates the instability of ankle joint as much as possible. We believe that residual ligaments and capsules could provide sufficient stability. It is our opinion that, to some degree, the partial talar prosthesis is similar to unicompartmental knee arthroplasty, and good long-term results without prominent osteoarthritic changes can be expected. However, replacement of partial talus trochlea has not been reported in the literature.
Despite satisfactory ankle and foot function restoration, prosthesis replacement failure can occur. Implant mismatch is the most common reason for replacement failure. 3D printed talus prosthesis is a novel method to treat talus AVN, which has been increasingly used in TTR [Citation23–26]. 3D printing technology is more suitable for manufacturing small products with a complex shape and structure [Citation27]. This approach could provide a perfect match between the bony defect and the individual prosthesis, which could restore the anatomy of the talus more appropriately [Citation28]. Different materials were used for the prosthesis production, every which has its advantage and disadvantage. In total talus replacement, assembled modular design with two different material was designed [Citation29,Citation30]. In our study, the prosthesis of medial talar trochlea is small and difficult for the assembled design. Titanium and its alloys are widely used in biomedical materials due to their good biocompatibility, low elastic modulus and high specific strength, especially in 3D printed prosthesis. So, we choose titanium for the material of prothesis, which could maximize the restoration of joint movement function and the service life of the prostheses. Inaccurate osteotomy can indeed lead to failure of prosthesis installation. This is a problem that must be considered in this operation, especially for 3D printing customized prosthesis. Each patient has only one prosthesis to choose from, so preoperative design is particularly important. Before the operation, we made a detailed plan according to the patient’s CT three-dimensional reconstruction. However, there are still some deficiencies because it is the first time to perform this operation. In order to ensure the accurate placement of the prosthesis during the operation, we still choose a more conservative method, which is multiple osteotomy with small amount and repeated comparison of the prosthesis to ensure that the prosthesis is consistent with the talus.
As a result, a significant improvement in patient-reported outcomes was noted after 24 months of follow-up with a concurrent improvement in the AOFAS scores. A customized 3D printed implant enabled treatment of the partial talus necrosis and collapse, similar to the hemicondyle replacement of the knee joint. This procedure restores native talar kinematics and reserves the other healthy half of the talar articular cartilage. Furthermore, it significantly reduces postoperative pain, decreases opioid requirements, permits earlier weightbearing activity, and reduces duration of hospital stay. Our experience indicates that 3D printed implant is a feasible option for patients with part necrosis and collapse of the talus.
Conclusion
Customized 3D printed PTR is a promising new surgical method for talus AVN that can achieve a satisfactory outcome. PTR might be an effective and preferential treatment for partial talus necrosis and collapse. Definitive and convincing conclusions are difficult to draw because of the limited number of cases in this study. Another limitation is the follow-up period which was 2 years. For this patient, we planned an annual follow-up that included physical evaluation and radiography. In future, a prospective randomized controlled study should be conducted to manage the clinical surgical treatment of talus AVN and collapse.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Pearce DH, Mongiardi CN, Fornasier VL, et al. Avascular necrosis of the talus: a pictorial essay. Radiographics. 2005;25(2):399–410.
- Biz C, Golin N, De Cicco M, et al. Long-term radiographic and clinical-functional outcomes of isolated, displaced, closed talar neck and body fractures treated by ORIF: the timing of surgical management. BMC Musculoskelet Disord. 2019;20(1):363.
- Gross CE, Haughom B, Chahal J, et al. Treatments for avascular necrosis of the talus: a systematic review. Foot Ankle Spec. 2014;7(5):387–397.
- Gross CE, Sershon RA, Frank JM, et al. Treatment of osteonecrosis of the talus. JBJS Rev. 2016;4(7):e2.
- Hoffmann M, Petersen JP, Schröder M, et al. Retrograde drilling of talar osteochondritis dissecans lesions: a feasibility and accuracy analysis of a novel electromagnetic navigation method versus a standard fluoroscopic method. Arthroscopy. 2012;28(10):1547–1554.
- Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698–1705.
- van Bergen CJA, Baur OL, Murawski CD, et al. Diagnosis: history, physical examination, imaging, and arthroscopy: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1_suppl):3S–8S.
- Looze CA, Capo J, Ryan MK, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8(1):19–30.
- Yañez Arauz JM, Del Vecchio JJ, Bilbao F, et al. Osteochondral lesions of the talus treatment with fresh frozen allograft. Foot Ankle Surg. 2017;23(4):296–301.
- Hannon CP, Smyth NA, Murawski CD, et al. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B(2):164–171.
- Biz C, Hoxhaj B, Aldegheri R, et al. Minimally invasive surgery for tibiotalocalcaneal arthrodesis using a retrograde intramedullary nail: preliminary results of an innovative modified technique. J Foot Ankle Surg. 2016;55(6):1130–1138.
- Dhillon MS, Rana B, Panda I, et al. Management options in avascular necrosis of talus. Indian J Orthop. 2018;52(3):284–296.
- Usuelli FG, Maccario C. Pearls and pitfalls for a surgeon new to ankle replacements. Foot Ankle Clin. 2017;22(2):477–489.
- Mittwede PN, Murawski CD, Ackermann J, et al. Revision and salvage management: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1_suppl):54S–60S.
- Harnroongroj T, Vanadurongwan V. The talar body prosthesis. J Bone Joint Surg Am. 1997;79(9):1313–1322.
- Taniguchi A, Takakura Y, Sugimoto K, et al. The use of a ceramic talar body prosthesis in patients with aseptic necrosis of the talus. J Bone Joint Surg Br. 2012;94(11):1529–1533.
- Taniguchi A, Tanaka Y. An alumina ceramic total talar prosthesis for avascular necrosis of the talus. Foot Ankle Clin. 2019;24(1):163–171.
- D’Ambrosi R, Di Silvestri C, Manzi L, et al. Post-traumatic ankle osteoarthritis: quality of life, frequency and associated factors. Muscle Ligaments and Tendons J. 2019;09(03):363–371.
- Chu AK, Wilson MD, Houng B, et al. Outcomes of ankle arthrodesis conversion to total ankle arthroplasty: a systematic review. J Foot Ankle Surg. 2021;60(2):362–367.
- Juels CA, So E, Seidenstricker C, et al. Complications of En Bloc osteochondral talar allografts and treatment of failures: literature review and case report. J Foot Ankle Surg. 2020;59(1):149–155.
- Conti MS, Ellington JK, Behrens SB. Osteochondral defects of the talus. Clin Sports Med. 2020;39(4):893–909.
- Harnroongroj T, Harnroongroj T. The talar body prosthesis: results at ten to thirty-six years of follow-up. J Bone Joint Surg Am. 2014;96(14):1211–1218.
- Huang J, Xie F, Tan X, et al. Treatment of osteosarcoma of the talus with a 3D-printed talar prosthesis. J Foot Ankle Surg. 2021;60(1):194–198.
- Kadakia RJ, Akoh CC, Chen J, et al. 3D printed total talus replacement for avascular necrosis of the talus. Foot Ankle Int. 2020;41(12):1529–1536.
- Scott DJ, Steele J, Fletcher A, et al. Early outcomes of 3D printed total talus arthroplasty. Foot Ankle Spec. 2020;13(5):372–377.
- Akoh CC, Chen J, Adams SB. Total ankle total talus replacement using a 3D printed talus component: a case report. J Foot Ankle Surg. 2020;59(6):1306–1312.
- Wang G, Lin J, Zhang H, et al. Three-dimension correction of charcot ankle deformity with a titanium implant. Comput Assist Surg. 2021;26(1):15–21.
- Tracey J, Arora D, Gross CE, et al. Custom 3D-printed total talar prostheses restore normal joint anatomy throughout the hindfoot. Foot Ankle Spec. 2019;12(1):39–48.
- Fang X, Liu H, Xiong Y, et al. Total talar replacement with a novel 3D printed modular prosthesis for tumors. Ther Clin Risk Manag. 2018;14:1897–1905.
- Mu MD, Yang QD, Chen W, et al. Three-dimension printing talar prostheses for total replacement in talar necrosis and collapse. International Orthopaedics (SICOT). 2021;45(9):2313–2321.