ABSTRACT
This case study follows a professional internationally capped female soccer player’s two-year journey from eumenorrhea, through injury, to amenorrhea, and the challenges faced by the player and nutritionist. The two years are split into three sections: (Areta et al. 2013) longitudinal profiling of the player, (Baker et al. 2020) nutrition to support her return from injury, and (Beato et al. 2018) investigation into the observed secondary amenorrhea. The cause of amenorrhea was investigated through the assessment of energy availability via doubly labelled water, remote food photography, blood biomarkers and resting metabolic rate. Despite having secondary amenorrhea and anovulatory cycles, the player did not have low energy availability. This study shows the importance for practitioner’s, particularly nutritionists, to not assume that all menstrual irregularities are caused by low energy availability and could be caused by a combination of factors (e.g., clinical, physiological, and psychological), which requires a multi-disciplinary investigation and intervention team. This study also showed that education needs to be provided about menstrual health to elite female soccer players as the player (i) believed that not having a period was beneficial for performance and unsure of possible health implications; (ii) was convinced that a one-day bleed indicated a regular menstrual cycle; and (iii) was reluctant to waste the practitioners time discussing menstrual issues and was nervous of finding out if she had an actual health issue. It is therefore crucial that players feel comfortable in discussing their menstrual status with practitioners to support their performance and long-term health.
Introduction
Low energy availability (LEA) is the cornerstone of both the Female Athlete Triad (Joy et al. Citation2014) and Relative Energy Deficiency in Sport (RED-S) (Mountjoy et al. Citation2014) and results in a myriad of negative health and performance outcomes (Dipla et al. Citation2021, Logue et al. Citation2020). LEA is often identified and treated by nutritionists as they are largely responsible for an athlete’s dietary energy intake (DEI). Menstruation, or rather the absence of menstruation (i.e., secondary amenorrhea), is commonly used as an early warning sign for LEA, meaning that nutritionists are now routinely encountering and dealing with menstrual irregularities, often without specialist training. As such, where should athletes go when their periods go?
Methods
Athlete
The player was a Caucasian, professional, internationally capped female soccer player. She previously played soccer in international leagues, before moving to England to play in the Women Super League (WSL).
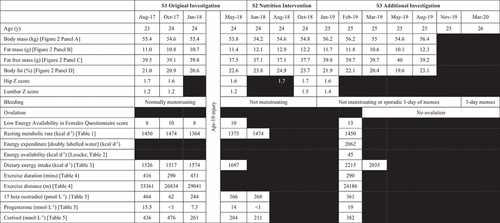
Research design
shows the timeline and outcomes of the study. In brief, the player was involved in a longitudinal profiling study (S1) before becoming injured [mid-substance full-thickness tear of the anterior cruciate ligament, plus a partial tear of the popliteal attachment of the posterior inferior meniscal popliteal fascicle and of the popliteofibular ligament of left knee]. Following the injury and resultant surgery [semitendinosus autograft], a nutrition intervention was undertaken to assist in recovery from surgery (S2), which lead to the identification of secondary amenorrhea. An investigation into the underlying cause of the secondary amenorrhea was subsequently instigated (S3). The study ended in August 2019 when the player left the area/club, thus preventing further detailed investigation, although the player stayed in remote contact with the research team, thus facilitating additional non-invasive measures. The player read the case study and provided a written record of approval for publication. Ethical approval was granted by the Wales Research Ethics Committee [approval number: 17/WA/0228].
Nutrition intervention
The focus of the post-injury nutrition intervention was to maintain the players lean mass and improve healing time, with a long-term goal of returning the payer to peak performance for the FIFA Women’s World Cup in May/June 2019. Nutrition education was provided with a food plan focused on overall energy balance (2200 kcal), lower carbohydrate (<2.5 g∙kg−1) and higher protein (2–2.5 g∙kg−1) (Close et al. Citation2019), ingesting 25 g protein every 3 hours throughout the day (Areta et al. Citation2013). Supplementation included omega-3 fatty acids (n-3FA) (1500 mg eicosapentaenoic acid and 750 mg docosahexaenoic acid) (Marques et al. Citation2015), creatine (5 g∙d−1) (Johnston et al. Citation2009) and collagen (20 g∙d−1) (Shaw et al. Citation2016). The player was provided the nutrition strategy and supplements to take at home and whilst it was not possible to measure adherence the player verbally reported that she followed the protocols provided.
Research protocol
Anthropometrical assessments
Body mass was assessed in minimal clothing and without shoes (SECA, model-875, Hamburg, Germany). Body composition was assessed using whole-beam dual-energy X-ray absorptiometry (DXA) (Hologic QDR Series, Discovery A, Bedford, MA, USA). All scans were performed and analysed by the same trained operator in accordance with the best practice guidelines (Nana et al. Citation2016). All scans were performed at the same time of day and in a fasted and euhydrated state.
Menstrual function
Menstrual function and risk of LEA was assessed using the Low Energy Availability in Females questionnaire (LEAF-Q) (Melin et al. Citation2014). Menses were tracked (electronic calendar) and verbally confirmed prior to DXA scanning. Ovulation was assessed using a urinary detection kit (Clearblue, Digital Ovulation Test, SPD, Development Company, Bedford, UK).
Resting metabolic rate (RMR)
RMR was measured at the same time of day in a fasted state having avoided strenuous exercise for at least 24 hours. The measurement was carried out via open-circuit indirect calorimetry (GEM Nutrition Ltd, UK) using a standard protocol (Bone and Burke Citation2018). Before starting data collection, the player relaxed for 10 minutes under a transparent ventilated hood, in a supine position, in a dark, quiet, thermoneutral room. Data were collected over a 20-minute period (2 × 10-minute duplicates). Data for the second 10-min period were used to determine RMR and analysed as previously described (Hannon et al. Citation2020). Based on the work done by O’Neil et al (O’Neill et al. Citation2022), the following equations were used to predict RMR: Cunningham (CRMR) (Cunningham Citation1980), Ten Haaf (ten Haaf et al. Citation2014) and Watson (Watson et al. Citation2019). Ten Haaf (ten Haaf et al. Citation2014) was used as the primary comparative equation.
Measurement of total energy expenditure using the doubly labelled water method
Measurement of total energy expenditure was quantified using doubly labelled water (DLW) (Lifson and McClintock Citation1966, Speakman and Hambly Citation2016) over 14 days. DLW was administered using methods described previously (Hannon et al. Citation2020).
Energy availability (EA)
EA was calculated using the equations of Loucks, Kiens (Loucks et al. Citation2011).
Assessment of dietary energy intake (DEI)
The player electronically tracked dietary intake for 3 days (S1 and S2) or 14 days (S3) using the validated remote food photographic method (RFPM (Costello et al. Citation2017). The player completed at least one 24-hour dietary recall every other day (using the triple pass method) to ensure the player did not omit food/drinks and to cross-check the dietary intake information (Capling et al. Citation2017) Dietary intake was analysed by a British Dietetic Association and Sports and Exercise Nutrition Register (SENr) accredited dietitian using dietary analysis software (Nutritics, v5, Ireland). To ensure reliability, a second SENr accredited nutritionist independently analysed the players dietary intake (Nutritics, v5, Ireland).
Exercise training
The player’s training load was monitored using global positioning system (GPS) technology (Apex, STATSports, Newry, Northern Ireland). The portable GPS unit sampled at 10 Hz (Beato et al. Citation2018). The training load was captured using methods previously described (Hannon et al. Citation2020)
Blood biomarkers
All venous blood samples were obtained by an accredited phlebotomist between 8.00 and 9.00 hours in a rested and fasted state. Blood samples were collected into vacutainers containing ethylenediaminetetraacetic acid (EDTA), lithium heparin, thixotropic gel, fluoride/oxalate, and silica and stored on ice. All samples (except full blood count) were centrifuged immediately and separated. Samples were either stored at −20°C until analysed or tested on the same day. A full blood count, electrolytes, liver function, iron profile, and endocrine panel (including luteinising hormone, follicle-stimulating hormone, prolactin, progesterone, oestradiol, testosterone, sex hormone-binding globulin, cortisol, insulin growth factor-1 thyroxine, triiodothyronine, and thyroid stimulating hormone) were measured.
Results
Body composition
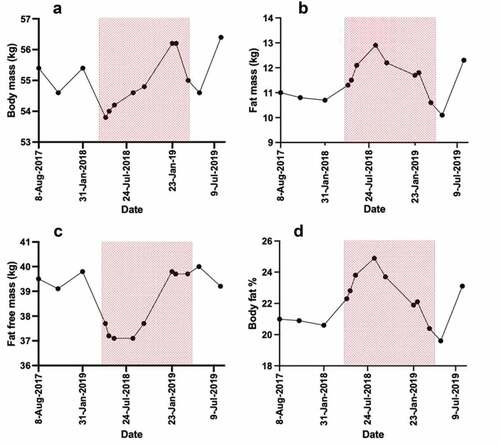
Fat-free mass decreased by 2.6 kg within three weeks of injury, returning to pre-injury levels within nine months ().
Resting metabolic rate
The players RMR varied by 7% over the two-year period. All three prediction equations were within 10% of the measured RMR and >0.90 ratio.
Energy expenditure and availability
The mean daily energy expenditure for week 1, week 2, and over the 14-day period was 2054, 2070, and 2062 kcal∙d−1. shows the average and range of relative EA per day using the Loucks et al. (Citation2011) equation.
Table 1. Relative mean and per day energy availability.
Daily estimated intake
Estimated energy intake increased by 30% post intervention (February and May 2019), with the increase being made up from protein and fat intake.
Exercise training
provides an overview of combined absolute training and match load during data collection periods.
Table 2. An overview of combined absolute training and match load during a 7-day data collection period. The first three time-points in were undertaken with the team pre-injury, with the last two time-points undertaken post-injury as individual sessions. These data [February 2019] were aligned with the DLW assessment.
Biomarkers
Iron markers were out of range (low) at the second testing time-point, otherwise all markers were within the normal clinical ranges ().
Discussion
Findings
This case study provides a detailed two-year examination of a professional internationally capped female soccer player. The major finding from this study was that although the player had secondary amenorrhea and anovulatory menstrual cycles, she did not have LEA (defined as ≤30 kcal/kg LBM/day (Loucks et al. Citation2011). This study shows that practitioners, particularly nutritionists, should not assume that menstrual irregularities are always caused by LEA, and are likely caused by a combination of factors (e.g., clinical, physiological, and psychological), which requires a multi-disciplinary investigation and intervention team.
During the initial study (S1), the player was classified as ‘at risk’ of LEA [LEAF-Q score ≥8; calorie intake 1517–1697 kcal∙d−1; EA 34 kcal∙kg∙d−1], placing her in the subclinical EA range (Loucks et al. Citation2011), despite reporting regularly menstruating () and her blood profile supporting this (). One possible reason for this is that this level of EA can be tolerated for short periods of times without any negative outcomes (Loucks et al. Citation2011). The other option is that DEI was underreported, despite employing measures to reduce underreporting including dietary recalls and multiple daily reminders (Capling et al. Citation2017). Measuring DEI in free-living athletes is difficult and places a great burden on athletes and practitioners (Burke et al. Citation2018).
During the nutrition intervention (S2) the player did not menstruate (i.e., since being injured and undergoing surgery). Eight months of amenorrhea had little impact on the player’s bone health (; Z scores), which might be due to the loading nature of soccer (Baker et al. Citation2020) and the fact that the player was not suffering from long-term LEA. In addition, at this time (S2 into S3), DEI increased (1526 kcal∙d−1 pre-injury to 2215 kcal∙d−1 post injury) and provided greater than 45 kcal∙kg∙d−1 (i.e., above the threshold for LEA). In contrast, her LEAF-Q score was 13, categorising her ‘at risk’ of LEA. This score has its limitations as the LEAF-Q was designed as a screening tool in female endurance runners. Recent evidence suggests that it should not be used to classify ‘at risk’ athletes or as a surrogate diagnostic tool in soccer due to its low specificity and validity in team sports (Rogers et al. Citation2021).
During S3, the player’s RMR ratio (measured vs predicted RMR >0.90) and biomarkers (LH, FSH, oestradiol, prolactin, FT3, FT4, and IGF-1) were within normal ranges ( and ) indicating that the player was not in LEA. During this time, she transitioned from amenorrhea to anovulatory cycles with sporadic bleeding. During S3, the player participated in three matches for her country at the 2019 World Cup. After the World Cup, the player left her WSL club and the area, however, due to the continued menstrual irregularities the research team kept supporting the player. The research team had arranged for further medical investigations (e.g., polycystic ovary syndrome, hyperprolactinemia, primary ovarian insufficiency) (Elliott-Sale et al. Citation2021); however, the COVID-19 global pandemic prevented these tests from occurring. In November 2020, the player left England for Australia, preventing any further exploration and resolution for this case study.
Table 3. Resting metabolic rate indirect measurements, predictions, and ratios.
Table 4. Mean energy and macronutrient intake expressed in absolute and relative terms during a 3-day* and 14-day** data collection timescale.
Table 5. Blood biomarker data over an 18-month study timescale.
Nutritionists’ reflections
The player did not initially present with menstrual disturbances, meaning that the focus of this case-study changed over time; from a profiling study (S1), to a post-injury/surgery anthropometric-based nutrition intervention (S2), to a menstrual irregularities investigation (S3). As such, nutritionists need to be adaptive to support players in a timely and dynamic fashion.
Sport nutritionists presented with a player with more than eight months without menses might default to investigating DEI-induced LEA without considering other options. The culture of soccer (and potentially other sports) was to turn to LEA as the initial cause rather than ruling out other medical issues. This can lead to costly (i.e., DLW) and burdensome (e.g., training and food diaries) investigations, without resolution (as in this case study). On reflection, a thorough medical assessment by other relevant domains (e.g., physician, endocrinologist and gynaecologist) should have been carried out before exploring LEA in such detail. As such, nutritionists must work as part of a multi-disciplinary team, maintaining an open dialogue with players and other support staff (e.g., clinicians, psychologists, and coaches) in order to gain a 360° perspective of the athlete’s behaviours and health.
Sports nutritionists and support staff need to cautious when using or interpreting the LEAF-Q due to it being designed for female endurance runners and not being validated in female soccer players.
Apart from iron markers, the players biomarkers were within normal clinical ranges. These clinical ranges are inflated by between-subject variability. Hecksteden and Meyer (Hecksteden and Meyer Citation2020) work around intraindividual analysis of fatigue markers provides an insight into the future of longitudinal biomarker interpretation. However, due to only having six time points over an eighteen-month period and with the athlete presenting with clinical symptoms, we did not feel confident interpretating the data to this level.
Due to the nutritional intervention (S2) and DLW results (S3) the player changed her eating habits (e.g., increasing total calories, protein, and fat intake and adherence to protein shakes around training sessions) whilst reducing her fat mass and increasing her fat-free mass. This highlights the importance and impact of nutrition interventions/awareness/education in players.
The following verbatim quotes by the player highlight the need for menstrual cycle education: (i) talking about not having a period - “its handy not having a period coming up to a World Cup”, “what harm can it really do”, “I can get my period back later”; (ii) talking about her periods - “I bled for 1 day this month again, isn’t that a period”; talking about further investigation into the cause of her menstrual disturbances - “I’m waiting on my period at the moment. I just feel like surely my period is just a one-day bleed so don’t want to waste your time”, “Don’t know if I’m just nervous to find out”.
During this case study, the player sustained a career threatening injury, moved club (which involved moving away from her home and boyfriend), had a run of poor results (e.g., conceded 23 goals in three games) and travelled internationally (encountered different foods). As such, nutritionists also need to consider ‘stress’ and signpost athletes to appropriate resources rather than assume menstrual disturbance is always linked to LEA.
Conclusion
Players should be encouraged to discuss their menstrual status with appropriate support staff. Nutritionists should be mindful that secondary amenorrhea is not exclusively caused by LEA and should work as part of a multi-disciplinary team to support female players with menstrual disturbances.
Epilogue
Upon final drafting of the case study, we sent the final version to the player to gain permission to submit for publication. At this time, the player reported that her menstrual cycle had returned (January 2021) to that of pre-injury (April 2018) and she was ovulating (confirmed using an ovulation kit). She highlighted ‘eating foods I grew up with’ and ‘am much happier’ as reasons for this. The player had returned close to home, family and friends and was enjoying her football again. Therefore, the menstrual dysfunction was unlikely due to the pathology we were unable to measure and likely down to other factors such as nutrition and psychology. It also emphasises that the return of menstruation may take a prolonged period of time. As of May 2022, the player was not diagnosed with any clinical condition, is still playing topflight football with menses returning to that of pre-injury.
Acknowledgements
The study was designed by LP, KES, JM and GC and data were collected and analysed by LP, KES, MH, JM and GC. The authors would like to thank Jack Clover and Matthew Taberner for their help with data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, GC upon reasonable request.
Additional information
Funding
References
- Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, et al. 2013. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 591(9):2319–2331. doi:10.1113/jphysiol.2012.244897.
- Baker BS, Chen Z, Larson RD, Bemben MG, Bemben DA. 2020. Sex differences in bone density, geometry, and bone strength of competitive soccer players. J Musculoskelet Neuronal Interact. 20(1):62–76.
- Beato M, Coratella G, Stiff A, Iacono AD. 2018. The validity and between-unit variability of GNSS units (STATSports Apex 10 and 18 Hz) for measuring distance and peak speed in team sports. Front Physiol. 9:1288. doi:10.3389/fphys.2018.01288.
- Bone JL, Burke LM. 2018. No difference in young adult athletes’ resting energy expenditure when measured under inpatient or outpatient conditions. Int J Sport Nutr Exerc Metab. 28(5):464–467. doi:10.1123/ijsnem.2016-0315.
- Burke LM, Lundy B, Fahrenholtz IL, Melin AK. 2018. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab. 28(4):350–363. doi:10.1123/ijsnem.2018-0142.
- Capling L, Beck K, Gifford J, Slater G, Flood V, O’Connor H. 2017. Validity of dietary assessment in athletes: a systematic review. Nutrients. 9(12):1313. doi:10.3390/nu9121313.
- Close GL, Sale C, Baar K, Bermon S. 2019. Nutrition for the prevention and treatment of injuries in track and field athletes. Int J Sport Nutr Exerc Metab. 29(2):189–197. doi:10.1123/ijsnem.2018-0290.
- Costello N, Deighton K, Dyson J, McKenna J, Jones B. 2017. Snap-N-Send: a valid and reliable method for assessing the energy intake of elite adolescent athletes. Eur J Sport Sci. 17(8):1044–1055. doi:10.1080/17461391.2017.1337815.
- Cunningham JJ. 1980. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 33(11):2372–2374. doi:10.1093/ajcn/33.11.2372.
- Dipla K, Kraemer RR, Constantini NW, Hackney AC. 2021. Relative energy deficiency in sports (RED-S): elucidation of endocrine changes affecting the health of males and females. Hormones (Athens). 20(1):35–47. doi:10.1007/s42000-020-00214-w.
- Elliott-Sale KJ, Minahan CL, de Jonge XAKJ, Ackerman KE, Sipilä S, Constantini NW, Lebrun CM, Hackney AC. 2021. Methodological considerations for studies in sport and exercise science with women as participants: a working guide for standards of practice for research on women. Sports Med. 51(5):843–861. doi:10.1007/s40279-021-01435-8.
- Hannon MP, Carney DJ, Floyd S, Parker LJF, McKeown J, Drust B, et al. 2020. Cross-Sectional comparison of body composition and resting metabolic rate in Premier League academy soccer players: implications for growth and maturation. J Sports Sci:1–9.
- Hannon MP, Parker LJF, Carney DJ, McKeown J, Speakman JR, Hambly C, et al. 2020. Energy requirements of male academy soccer players from the English Premier League. Med Sci Sports Exerc.
- Hecksteden A, Meyer T. 2020. Blood-Borne fatigue markers during major international football tournaments – a retrospective analysis of data from the FIFA World Championships and UEFA European Championships 2006 – 2016. Sci Med Football. 4(2):135–141. doi:10.1080/24733938.2019.1692144.
- Johnston APW, Burke DG, MacNeil LG, Candow DG. 2009. Effect of creatine supplementation during cast-induced immobilization on the preservation of muscle mass, strength, and endurance. J Strength Condi Res. 23(1):116–120. doi:10.1519/JSC.0b013e31818efbcc.
- Joy E, De Souza MJ, Nattiv A, Misra M, Williams NI, Mallinson RJ, Gibbs JC, Olmsted M, Goolsby M, Matheson G, et al. 2014. 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr Sports Med Rep. 13(4):219–232. doi:10.1249/JSR.0000000000000077.
- Lifson N, McClintock R. 1966. Theory of use of the turnover rates of body water for measuring energy and material balance. J Theor Biol. 12(1):46–74. doi:10.1016/0022-5193(66)90185-8.
- Logue DM, Madigan SM, Melin A, Delahunt E, Heinen M, Donnell SM, Corish CA. 2020. Low energy availability in athletes 2020: an updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients. 12(3):835. doi:10.3390/nu12030835.
- Loucks AB, Kiens B, Wright HH. 2011. Energy availability in athletes. J Sports Sci. 29(Suppl 1):S7–15. doi:10.1080/02640414.2011.588958.
- Marques CG, Santos VC, Levada-Pires AC, Jacintho TM, Gorjão R, Pithon-Curi TC, Cury-Boaventura MF. 2015. Effects of DHA-rich fish oil supplementation on the lipid profile, markers of muscle damage, and neutrophil function in wheelchair basketball athletes before and after acute exercise. Appl Physiol Nutr Metab. 40(6):596–604. doi:10.1139/apnm-2014-0140.
- Melin A, Tornberg ÅB, Skouby S, Faber J, Ritz C, Sjödin A, Sundgot-Borgen J. 2014. The LEAF questionnaire: a screening tool for the identification of female athletes at risk for the female athlete triad. Br J Sports Med. 48(7):540–545. doi:10.1136/bjsports-2013-093240.
- Mountjoy M, Sundgot-Borgen J, Burke L, Carter S, Constantini N, Lebrun C, Meyer N, Sherman R, Steffen K, Budgett R, et al. 2014. The IOC consensus statement: beyond the female athlete triad—Relative Energy Deficiency in Sport (RED-S). Br J Sports Med. 48(7):491–497. doi:10.1136/bjsports-2014-093502.
- Nana A, Slater GJ, Hopkins WG, Halson SL, Martin DT, West NP, Burke LM. 2016. Importance of standardized DXA protocol for assessing physique changes in athletes. Int J Sport Nutr Exerc Metab. 26(3):259–267. doi:10.1123/ijsnem.2013-0111.
- O’Neill J, Walsh CS, McNulty SJ, Gantly HC, Corish ME, Crognale D, Horner K. 2022. Resting metabolic rate in female rugby players: differences in measured versus predicted values. J Strength Cond Res. 36(3):845–850. doi:10.1519/JSC.0000000000003634.
- Rogers MA, Drew MK, Appaneal R, Lovell G, Lundy B, Hughes D, Vlahovich N, Waddington G, Burke LM. 2021. The utility of the low energy availability in females questionnaire to detect markers consistent with low energy availability-related conditions in a mixed-sport cohort. Int J Sport Nutr Exerc Metab. 31(5):427–437. doi:10.1123/ijsnem.2020-0233.
- Shaw G, Lee-Barthel A, Ross ML, Wang B, Baar K. 2016. Vitamin C–enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am J Clin Nutr. 105(1):136–143. doi:10.3945/ajcn.116.138594.
- Speakman JR, Hambly C. 2016. Using doubly-labelled water to measure free-living energy expenditure: some old things to remember and some new things to consider. Comp Biochem Physiol a Mol Integr Physiol. 202:3–9. doi:10.1016/j.cbpa.2016.03.017.
- ten Haaf T, Weijs PJ, Alemany M. 2014. Resting energy expenditure prediction in recreational athletes of 18–35 years: confirmation of Cunningham equation and an improved weight-based alternative. PLoS One. 9(9):e108460. doi:10.1371/journal.pone.0108460.
- Watson AD, Zabriskie HA, Witherbee KE, Sulavik A, Gieske BT, Kerksick CM. 2019. Determining a resting metabolic rate prediction equation for collegiate female athletes. J Strength Cond Res. 33(9):2426–2432. doi:10.1519/JSC.0000000000002856.