ABSTRACT
Arsenical keratosis, a keratotic skin disorder caused by chronic arsenic exposure, generally presents as hyperkeratosis and hyperpigmentation. Here, we report a rare case of arsenical keratosis coexisted with chloracne in a 56-year-old man who presented with generalized multiple polymorphic eruptions: poikiloderma, punctate keratosis, keratosis plaques, verruca-like rashes and nail opacity. Chloracne were simultaneously observed. To our knowledge, this is the first case showed arsenical keratosis combined with chloracne. As skin eruptions induced by pesticide exposure might present variously and are related to several types of cutaneous malignancy, clarifying exposure history and toxicants detection are necessary to avoid misdiagnosis.
Introduction
Arsenical keratosis is a keratotic skin disorder caused by chronic arsenic exposure. Despite numerous efforts to decreased environmental contaminant in drinking water and occupational hazard, sporadic cases among farmers and prolonged arsenic medication ingestion still occur [Citation1]. We here describe a rare case of arsenical keratosis presented with multiple polymorphic eruptions simultaneously combined with chloracne.
Case
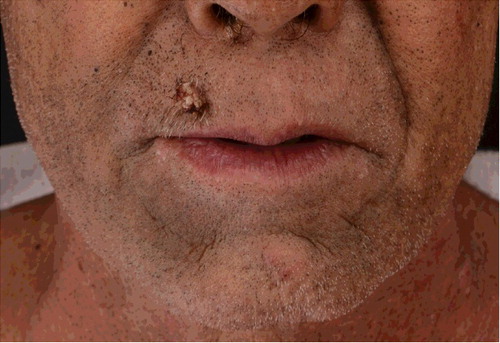
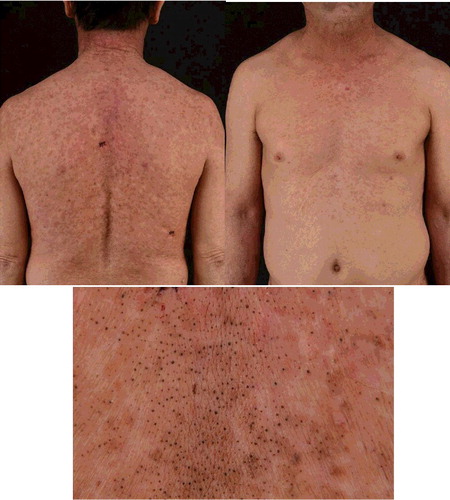
In February 2016, a 56-year-old man presented to us with an eight-month history of non-itchy skin eruption on face, truck, extremities and palms. He was without any signs of acute toxicity or neuropathy. He has the exposure history of every month contact with pesticide containing dichlorodiphenyltrichloroethane, lead arsenate, calcium arsenate, sodium fluorosilicate, pyrethrum, rotenone tobacco carbaryl and carbofuran in his occupation as farmer for more than five years. Eight months ago, keratosis papules and plaques appeared on the dorsum of both hands and feet (). Seven months ago, punctate keratosis on both palms, nails opacity and verruca-like keratoses on the upper lip were observed (). Four months ago, he developed comedonal acne-like rashes on face which gradually involved the trunk and extremities combined with skin color anomaly ( and ).
Figure 1. Arsenical keratosis lesions. Keratosis plaques, verruca-like keratoses and nails opacity on the dorsum of both hands and feet.
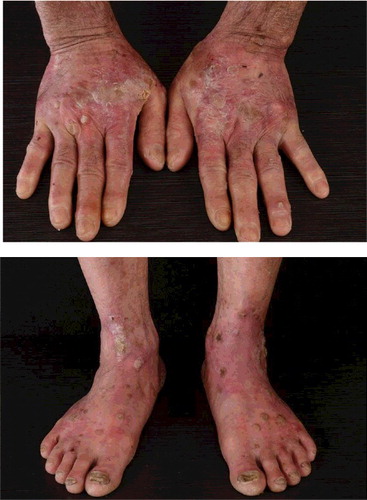
Figure 3. Chloracne lesions on trunk. Comedone-like lesions combined with skin color anomaly on trunk.
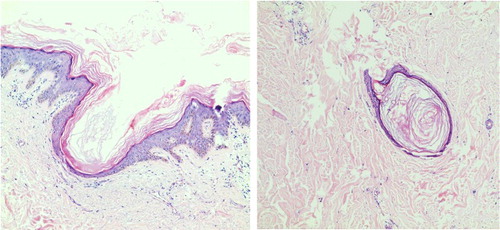
Systemic examination was normal and lymphadenectasis not found. Urine arsenic test was 198 μg/L (reference value 2.6–22.7 μg/L). Laboratory blood tests were within normal range (). Fungal microscopic and culture examination were negative on both thumbs nails, palms and feet (KOH−). Multiple punch skin biopsy was done on the right-hand dorsum (keratosis popular), upper lip (verruca-like keratoses) and back (comedone-like lesion). Skin pathology of keratosis popular showed epidermis hyperplasia, lymphocytic infiltrating and collagen fiber eosinophilic changes in dermis (). Histopathology of verruca-like eruption above the upper lip showed epidermal hyperplasia, lymphocytic infiltrating in superficial dermis and without vacuoles. Epidermal hyperplasia, mild papillary hyperplasia and pseudo-angle cyst were found in comedone-like lesions by histopathology (). Cutaneous malignancy and skin virus infection were excluded.
Table 1. Patient blood biochemical examination results.
Figure 4. Arsenical keratosis. Histopathology of keratosis plaques on acral limbs showed epidermis hyperplasia, diffuse lymphocyte infiltration and collagen fiber eosinophilic changes in the dermis.
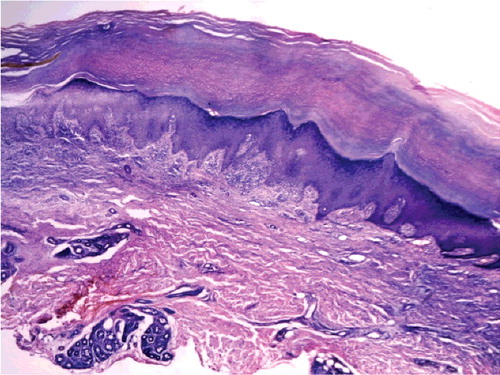
Figure 5. Chloracne. Histopathology of comedone-like lesions on back showed epidermal hyperplasia, mild papillary hyperplasia and pseudo-angle cyst (HE 100×).
The clinical and histopathological findings led to a diagnosis of arsenical keratosis combined with chloracne. The patient was given 10% sodium thiosulfate intravenous injection (0.64 g/day for 7 days) as antitoxic treatment. Verruca-like keratoses above the right upper lip was taken out by operation. Adapalene gel was topically used on both chloracne and arsenical keratosis lesions twice daily for 6 months. One month after treatment, the keratosis plaques and punctate keratosis on hands and foots became flattened and smaller, and the omedone-like lesions on trunk and face were decreased. Urine arsenic re-test after 3 months treatment was within normal rage (19.1 μg/L, reference value 2.6–22.7 μg/L). This patient, on a regular follow-up monthly for 15 months until now, has not presented new eruptions.
Discussion
Dibenzofurans, dioxins and sodium 3,5,6-trichloropyridin-2-ol (STCP) are the main toxicants that cause chloracne in China. As early as 1993, Cheng et al. surveyed 109 Chinese workers and reported that the prevalence of chloracne was 73.4% (80/109) in total and 95.2% (20/21) in a trichlorobenzene (TCB) tank area where dioxin and dibenzofurans levels were in thousands of ppm [Citation1]. Recently, Wu et al. and Niu et al. reported that the workers who worked in an STCP factory in China developed chloracne and peripheral nerve damage [Citation2,Citation3]. For pathogenesis investigation, Liu et al. found that the activation of mitogen-activated protein kinase pathway and upregulation of CK17 and TGK might play roles in the pathogenesis of chloracne related to dioxin exposures [Citation4]. Tang et al. investigated the gene expression of Chinese chloracne patients and found that AhR, CYP1A1, GSTA1 and c-fos transactivations were significantly induced in chloracne lesions [Citation5]. In this case, we report a Chinese chloracne patient, who exposed to pesticides which might contain a variety of toxic chemicals, simultaneously presented arsenical keratosis.
Common skin manifestations of arsenical keratosis are generally classified as hyperkeratosis and hyperpigmentation. Hyperkeratotic lesions include punctate keratoses, papule keratoses, corns-shaped keratosis mostly found predominantly on palms and soles [Citation6]. There were other less common skin lesions reported, such as verrucous hyperplasia, lichen planus-like keratosis, folliculitis-like lesion and capillaries expansion [Citation7]. Changes of finger nails often present as Mees’ lines or koilonychia. High incidence of multiple carcinomas such as squamous-cell carcinoma and basal cell carcinoma are documented [Citation8]. In this case, the patient presented with both typical and unusual lesions that included punctate keratoses, papule keratoses, keratosis plaques, poikiloderma and nail opacity.
Pathogenesis of arsenic-induced cutaneous changes includes disturbing epidermal keratinocyte differentiation and pigmentary processes, vitiating nucleotide excision repairing mechanisms, regulating DNA methylation, interacting with unfolded protein response signaling and MAP kinase pathways. Recent research shows that oxidative stress, chromosomal abnormality and altered growth factors are also involved in arsenic-induced carcinogenesis [Citation9]. Thompson et al. found that arsenic was a co-carcinogen with UV in skin carcinogenesis, likely by adversely influencing DNA repairing system and or ROS-mediated damages [Citation10].
Antitoxic sodium thiosulfate which could delay toxicants-induced hemolysis and promote toxins excretion from kidney has been used in treating acute and chronic chemical poisoning conditions [Citation11], including arsenic [Citation12]. Rael et al. found that sodium thiosulfate could delay arsine-induced hemolysis in human erythrocytes in vitro [Citation13]. Our case proposed that sodium thiosulfate could be used as one of the treatment options for arsenic associated with chlorine poisoning patients, but more clinical validation is needed and the pharmacological mechanisms are still unclear and need further investigation.
Here we report a rare case of arsenical keratosis presented with multiple polymorphic eruptions combined with chloracne. As skin eruptions induced by pesticide exposure might present variously and are related to several types of cutaneous malignancy, clarifying exposure history and toxicants detection are necessary to avoid misdiagnosis.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Cheng WN, Coenraads PJ, Hao ZH, et al. A health survey of workers in the pentachlorophenol section of a chemical manufacturing plant. Am J Ind Med. 1993;24(1):81–92.
- Wu N, Hao F, Yu X. Peripheral nerve and skin damage associated with working in a STCP factory: report of four cases. Clin Toxicol (Phila). 2012;50(6):514–517.
- Niu YM, Hao FT, Xia YJ. Sodium 3,5,6-trichloropyridin-2-ol poisoning: report of four cases. Toxicol Ind Health. 2014;30(5):475–479.
- Liu J, Zhang CM, Coenraads PJ, et al. Abnormal expression of MAPK, EGFR, CK17 and TGk in the skin lesions of chloracne patients exposed to dioxins. Toxicol Lett. 2011;201(3):230–234.
- Tang NJ, Liu J, Coenraads PJ, et al. Expression of AhR, CYP1A1, GSTA1, c-fos and TGF-alpha in skin lesions from dioxin-exposed humans with chloracne. Toxicol Lett. 2008;177(3):182–187.
- Das, S. An introspection into the cutaneous manifestations of chronic arsenicosis as reported in a tertiary care centre in Kolkata. 2014;24(4):286–291.
- Ahmed TS, Misbahuddin M. Role of linoleic acid in arsenical palmar keratosis. Int J Dermatol. 2016;55(3):289–295.
- Torchia D, Massi D, Caproni M, et al. Multiple cutaneous precanceroses and carcinomas from combined iatrogenic/professional exposure to arsenic. Int J Dermatol. 2008;47(6):592–593.
- Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J Biomed Sci. 2006;13(5):657–666.
- Thompson BC, Halliday GM, Damian DL. Nicotinamide enhances repair of arsenic and ultraviolet radiation-induced DNA damage in HaCaT keratinocytes and ex vivo human skin. PLoS One. 2015;10(2):e0117491.
- Bebarta VS, Brittain M, Chan A, et al. Sodium nitrite and sodium thiosulfate are effective against acute cyanide poisoning when administered by intramuscular injection. Ann Emerg Med. 2017;69(6):718–725.
- Koyama K, Tanaka K, Sakamoto N, et al. Serum and urine total arsenic concentration in a case of acute arsenic intoxication. Chudoku Kenkyu. 2002;15(2):167–170.
- Rael LT, Ayala-Fierro F, Carter DE. The effects of sulfur, thiol, and thiol inhibitor compounds on arsine-induced toxicity in the human erythrocyte membrane. Toxicol Sci. 2000;55(2):468–477.