ABSTRACT
Cannabidiol (CBD)-infused pet treats are becoming a huge market for pet owners as they turn to this supplement for a non-traditional therapeutic option. However, CBD's short-term or long-term effects on companion animals remain largely unknown. We conducted a targeted literature search about the mechanism, efficacy, and safety of these treats in order to highlight the gaps in knowledge of CBD products. This communication elucidates some of the common misperceptions regarding CBD pet treats, and proposes suggestions for further research based on the status of knowledge in this field. With the emergence of these treats and identified gaps in knowledge, the veterinary research community needs to determine the pharmacokinetic parameters for short- and long-term duration and conduct rigorous clinical trials to assess CBD's and other cannabinoids' impact on various diseases.
KEYWORDS:
Introduction
Despite the fact that little is known about the safety and efficacy of cannabinoids in pets, the cannabinoid pet treat industry is booming. Companies like Canna-Pet, Treatibles, and Hemp Genix offer CBD-infused pet treats that are easily accessible to pet owners online [Citation1–3]. With recent state-wide legalizations in Colorado, California, Washington and Oregon [Citation4], it is not surprising that many pet owners are beginning to give cannabinoid-containing pet products to their companion animals in hopes that they provide therapeutic benefits. Cannabidiol (CBD) is the primary cannabinoid listed on pet treats. CBD is the major non-psychoactive compound in the marijuana plant, Cannabis sativa [Citation5]. Because CBD has been shown not to exhibit psychotropic effects [Citation6], it has been marketed as a herbal supplement [Citation1,Citation7]. Companies producing and offering CBD pet treats allude that these treats may help pets (canines and felines) with anxiety, arthritis, and even cancer [Citation7]. However, at this time, CBD products lack FDA approval and lack any conclusive clinical studies in animals [Citation8,Citation9].
Although significant controversy surrounds CBD products, the treats continue to find success among pet owners. A recent study investigated public perception in a large cohort of pet owners from Colorado about the administration and effects of CBD treats on their pets [Citation10]. While this study provided information on many aspects of the CBD pet product perceptions (including perceived efficacy and product safety), one particular statistic stood out: of the 632 consumers sampled, only 7% of pet owners reported that they felt the CBD products did not perform as well compared to other standard care medications or therapies [Citation10]. A staggering 93% felt that the CBD treats performed equally or better than these standards of care [Citation10]. These claims rest upon on pet owner observations as there are very limited data on safety or efficacy of CBD pet treats following short- or long-term administration. We illustrate some of the common misperceptions regarding CBD pet treats, highlight the gaps in knowledge, and propose suggestions for further research based on the status of knowledge in this field.
What is CBD?
CBD, or cannabidiol, is a compound of the cannabinoid family. It is the product of decarboxylation of cannabidiolic acid, triggered by UV exposure, heat or prolonged storage. Cannabinoids occur naturally in the C. sativa plant. Presently, over 100 cannabinoid compounds have been identified in C. sativa, with Δ9-THC (tetrahydrocannabinol or THC) and CBD considered the major phytoendocannabinoids found in highest concentrations [Citation5]. The endocannabinoid profile varies with subspecies and strains of plants. Cannabinoids may be either psychoactive, like Δ9-THC [Citation11], or non-psychoactive, like CBD [Citation6]. It is for this reason that many modern therapeutics have attempted to harness the proposed properties of CBD and other non-psychoactive cannabinoids, as these compounds do not alter mental state as does Δ9-THC [Citation11].
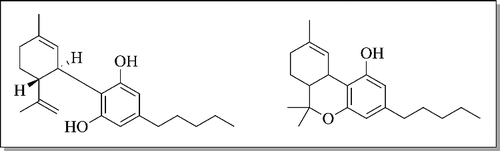
There are limited data available on the pharmacokinetics and pharmacodynamics of CBD. However, CBD has similar pharmacokinetic properties to Δ9-THC, and greatly differs in its pharmacodynamic properties as compared to Δ9-THC. Similarity in structure () between Δ9-THC and CBD may explain the compounds' similar pharmacokinetic properties. Human and laboratory animal pharmacokinetic studies have been used historically to extrapolate canine pharmacokinetics. In humans, oil vehicles, such as hemp seed oil, improve oral bioavailability of individual cannabinoids, yet even with the administration of the same vehicle, peak concentrations and rates of absorption vary considerably [Citation12]. In canines, however, CBD has been shown to have a low oral bioavailability ranging from 13% to 19% [Citation13]. Intravenous CBD administration in canines has superior bioavailability as compared to oral administration, as CBD is thought to undergo first pass metabolism in the liver [Citation13]. Canines differ from humans in their metabolism of cannabidiol, specifically in the difference in urine metabolites [Citation14,Citation15]. Canines primarily oxidize cannabidiol via the 6 β-hydroxylation pathway, versus humans that produce 7-oic acid group metabolites of cannabidiol [Citation14]. In humans, peak plasma concentration of CBD after oral exposure occurs at 1–4 hours [Citation16], while after sublingual administration of 10 mg Δ9-THC and CBD, analytes in serum were detectable at 1 hour with CBD concentrations <2 ng/mL [Citation12]. Tissue distribution information is not available for CBD, but based on its reported volume of distribution of 6.9–10.4 L/kg [Citation13], it is expected to distribute into adipose, liver, lung, spleen, brain and muscles [Citation17]. Interestingly, brain tissue of rats even after administration of CBD alone contained small amounts of Δ9-THC [Citation16]. These various findings may have implications on the use of repeated administration of CBD treats in companion animals and warrants further investigation.
Function and conservation of the cannaboid receptors
The two known receptors of the endocannabanoid receptor system are the CB1 and CB2 receptors. The CB1 receptor has wide distribution in the central nervous system, specifically in central and peripheral neuron terminals [Citation18]. Less is known about the CB2 receptor, but it exists in parts of the central nervous system and immune system cells, and other tissue such as testes [Citation18]. However, a definitive function of the CB2 receptor remains largely unknown. One hypothesis suggests that diseased brain tissue express the CB2 receptor, possibly linked to a degenerative immune response. However, this controversial idea remains unconfirmed within the field [Citation18]. Both the CB1 and CB2 receptor exist in canines [Citation19,Citation20]. The CB1 receptor exists in felines [Citation21]; however, no data confirm the presence of the CB2 receptor in felines. The roles of the CB1 and CB2 receptors in diseases in companion animals remain unexplored.
Other phytocannabinoid receptors
Though CBD interacts with the CB1 and CB2 receptors, recent research has shown that other receptors may contribute to CBD's physiological effects. These other receptors include, but are not limited to, the peroxisome proliferator-activated receptor-γ (PPAR-γ), the α-3 glycine receptor (GLRA3), the 5-HT1A and 5-HT3A receptors, and vanilloid (VR1) receptors. The PPAR-γ receptor may be involved in CBD's anti-inflammatory effects, as PPAR-γ receptor activation suppresses aggravated immune response [Citation22]. The GLRA3 receptor participates in nociceptive regulation and may be a contributing factor in CBD's analgesic effects [Citation23]. The 5-HT receptors in the CNS are potential therapeutic targets for regulating depressive and anxiolytic symptoms [Citation24]. The VR1 receptor also contributes to nociceptive regulation and may also contribute to CBD's analgesic effects [Citation25]. CBD also regulates mitochondrial Ca2+ levels, which may prevent mitochondrial-linked neurotoxicity diseases like Huntington's disease [Citation26]. Based on this specific effect, CBD has been shown to be successful as an anticonvulsant in the treatment of epilepsy [Citation27].
Although CBD is the main component in pet treats, preliminary studies have confirmed the presence of other cannabinoids including Δ9-THC in treats (Dr Ingrid Gennity, personal communication). These other cannabinoids may have direct pharmacological effects or alter the metabolism of CBD. Therefore, administration of a mixture of cannabinoids instead of CBD alone may influence the therapeutic outcome.
Potential toxicity concerns
Initial toxicity studies have shown that CBD has a favorable safety margin when administered orally. The only LD50 study of cannabidiol by Thompson et al. included rhesus monkeys, dogs, and rats [Citation28]. Animals received oral Δ9-THC and crude marijuana extract (CME) with observation for toxic effects. The LD50 in beagle dogs was >3000 mg/kg as no deaths occurred as a result of Δ9-THC toxicity even at the highest administered dose [Citation28]. In rhesus monkeys, a CME dose of 5000 mg/kg failed to illicit any sign of toxicity [Citation28]. In primates, Δ9-THC doses of up to 9000 mg/kg had no significant toxicity effects [Citation28]. From this paper, the estimated LD50 was >10,000 mg/kg in primates [Citation28].
Toxicokinetic parameters are important to consider when evaluating the potential for toxicity with varying doses and length of administration. CBD is a small molecule with a molecular weight of 314.2 g/mol. With a logP value estimated to be 6.5, CBD is highly lipophilic [Citation29]. This raises concerns over potential long-term tissue build-up and toxicity. After a 45 mg IV dose of CBD, the volume of distribution in canines ranged from 6.9 to 10.4 L/kg [Citation13]. Unfortunately, there are no toxicokinetic, safety, and efficacy data on the chronic use of CBD in companion animals.
CBD may cause significant and potentially dangerous drug–drug interactions when taken simultaneously with other drugs because of its inhibition of cytochrome P450 enzymes, specifically CYP450 2C19 [Citation30,Citation31]. The preliminary data on CBD CYP450 2C19 interactions involved recombinant human proteins. Canines and felines also express CYP 450 2C19 [Citation32], but interaction data with CBD are lacking.
While CBD may have attractive therapeutic properties such as anti-emetic, analgesic, anti-inflammatory and immunosuppressant effects [Citation24], long-term cannabis use has deleterious immune and cognitive effects in humans, including visual processing and executive functioning [Citation33]. CBD's interaction with CB2 receptors located on immune cells suggests important therapeutic implications for autoimmune diseases [Citation34]. While this may prove useful in treating, CBD's immunosuppressant effects may pose a risk for immunocompromised animals. While CBD might have effects, safety and efficacy in companion animals remain unclear.
The challenges
The cannabis pet treat industry is continually expanding, creating a larger disconnect between scientific evidence and public opinion and perception. The essential challenge is that reports of cannabis use in pets are observational and uncontrolled, and steeped in optimism by enthusiastic pet owners. Previous clinical studies in humans suggest that CBD possesses possible benefits for patients with certain medical conditions. For example, Epidiolex™, a CBD-based drug, is in phase 3 human clinical trials for treatment of Dravet syndrome [Citation35]. A stark contrast to the clinical efficacy studies currently conducted with Epidiolex™ is the fact that marijuana, including CBD, is still a Schedule I drug. These suggest a great divide in knowledge and opinion that exist in the field of cannabis research and medicine. This gap in knowledge is even greater in veterinary medicine and will motivate research. Hence, further pharmacokinetic and pharmacodynamic studies, including long-term dosing studies, are necessary in companion animals to ensure the safety and efficacy of CBD treat products. Ideally, veterinary clinical trials should evaluate the effects with short- and long-term term use of CBD or other cannabinoids. Potential target conditions may include cancer, pain, arthritis, glaucoma, and immune diseases. Until these studies are completed, pet owners should exercise caution when administering these treats to their pets, especially for long-term usage. Research must be carefully conducted considering that the federal and state laws regarding the medical use of cannabis and cannabinoids are in conflict [Citation36].
The current popularity of CBD treats for companion animals may reflect marketing strategies, anecdotal evidence from fellow pet owners, and public perception. After extensive review of the literature on the safety and efficacy of CBD and CBD pet treats for dogs and cats, we found more gaps than knowledge. Furthermore, there is a critical need for increased owner education and awareness regarding the composition, consistency, and safety of various CBD pet treats.
Acknowledgments
The authors would like to thank Dr Ingrid Gennity and Ms Mehdia Zaidi for their preliminary assessment of cannabinoids in pet treats.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- The Science Oakland, CA: Treatibles; [cited 2017 Nov 18]. Available from: https://www.treatibles.com/pages/new-the-science
- Hemp Genix. 2016. Available from: https://hempgenix.us/checkout/
- Canna-Pet. 2018. Available from: https://canna-pet.com/
- State Medical Marijuana Laws: National Conference of State Legislatures; [updated 2017 Sept 9; cited 2017 2017 Oct 9]. Available from: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx-4
- Small E, Cronquist A. A practical and natural taxonomy for cannabis. Taxon. 1976;25(4):405.
- Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras PsiquiatrRev Bras Psiquiatr. 2008;30(3):271–280.
- Phytochemistry & Compounds: Canna-Pet; [cited 2017 Nov 18]. Available from: https://canna-pet.com/our-products/phytochemistry-active-compounds/
- Nelson E. 2015. Canna Pet LLC 2/24/15 Silver Spring, MD: FDA; [cited 2017 18 Nov]. Available from: https://www.fda.gov/ICECI/EnforcementActions/WarningLetters/ucm435662.htm
- Administration USFaD. Warning letters test results for cannabidiol-related products; [cited 2018]. Available from: https://www.fda.gov/newsevents/publichealthfocus/ucm484109.htm
- Kogan LR, Hellyer PW, Robinson NG. Consumers' perceptions of hemp products for animals. J Am Holist Vet Med Assoc. 2016;42:40–48.
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64(1):21–47.
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–1804.
- Samara E, Bialer M, Mechoulam R. Pharmacokinetics of cannabidiol in dogs. Drug Metab Dispos. 1988;16(3):469–472.
- Samara E, Bialer M, Harvey DJ. Identification of urinary metabolites of cannabidiol in the dog. Drug Metab Dispos. 1990;18(5):571–579.
- Samara E, Bialer M, Harvey DJ. Pharmacokinetics of urinary metabolites of cannabidiol in the dog. Biopharm Drug Dispos. 1990;11(9):785–795.
- Nadulski T, Sporkert F, Schnelle M, et al., Simultaneous and sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extract.J Anal Toxicology. 2005;29(8):782–789.
- Brunet B, Doucet C, Venisse N, et al. Validation of large white pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forensic Sci Int. 2006;161(2–3):169–174.
- Howlett AC, Abood ME. Chapter five - CB1 and CB2 receptor pharmacology. In: Kendall D, Alexander SPH, editors. Advances in pharmacology. Academic Press; Elsevier, San Diego, CA, USA. 2017;80: 169–206.
- Freundt-Revilla J, Kegler K, Baumgartner W, et al. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS One. 2017;12(7):e0181064.
- Fernandez-Trapero M, Espejo-Porras F, Rodriguez-Cueto C, et al. Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Dis Model Mech. 2017;10(5):551–558.
- Gebremedhin D, Lange AR, Campbell WB, et al. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol Heart C. 1999;276(6):H2085–H2093.
- Esposito G, Scuderi C, Valenza M, et al. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS One. 2011;6(12):e28668.
- Xiong W, Cui T, Cheng K, et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J Exp Med. 2012;209(6):1121–1134.
- Schier A, Ribeiro N, Coutinho D, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. 2014;13(6):953–960.
- Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–852.
- Ryan D, Drysdale AJ, Lafourcade C, et al. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29(7):2053–2063.
- Shirazi-zand Z, Ahmad-Molaei L, Motamedi F, et al. The role of potassium BK channels in anticonvulsant effect of cannabidiol in pentylenetetrazole and maximal electroshock models of seizure in mice. Epilepsy Behav. 2013;28(1):1–7.
- Thompson GR, Rosenkrantz H, Schaeppi UH, et al. Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys. Toxicol Appl Pharmacol. 1973;25(3):363–372.
- PubChem Compound Database; CID = 644019; [cited 2018 Jan 8]. Available from: https://pubchem.ncbi.nlm.nih.gov/
- Jiang R, Yamaori S, Okamoto Y, et al. Cannabidiol is a potent inhibitor of the catalytic activity of Cytochrome P450 2C19. Drug Metab Pharmacokinet 2013;28(4):332–338.
- Yamaori S, Ebisawa J, Okushima Y, et al. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88(15):730–736.
- Sasaki K, Shimoda M. Possible drug–drug interaction in dogs and cats resulted from alteration in drug metabolism: a mini review. J Adv Res. 2015;6(3):383–392.
- Machado Bergamaschi M, Helena Costa Queiroz R, Waldo Zuardi A, et al. Safety and side effects of Cannabidiol, a Cannabis sativa constituent. Curr Drug Safety. 2011;6(4):237–249.
- Rieder SA, Chauhan A, Singh U, et al. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215(8):598–605.
- GW announces new Epidiolex® (CBD) positive phase 3 data in Dravet Syndrome and Lennox-Gastaut Syndrome [Internet]. London: GW Pharmaceuticals; 2016; [cited Nov 18]. Available from: https://www.gwpharm.com/about-us/news/gw-announces-new-epidiolex%C2%AE-cbd-positive-phase-3-data-dravet-syndrome-and-lennox
- Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017;70:288–291.