?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Decorative coral species capable of producing palytoxin (PTX) frequently adorn marine aquariums. PTX is an ultra-potent toxin with significant morbidity and mortality. Toxicity from PTX results in a spectrum of symptoms depending on route of exposure. We report the case of a 30-year-old man who accidentally aerosolized PTX while cleaning his saltwater fish tank, resulting in precipitous respiratory distress.
Introduction
An estimated 700,000 households in the United States feature a saltwater aquarium [Citation1]. Enthusiasts often decorate these with colorful exotic animals, including coral. Zoanthid corals are colonizing animals of the phylum Cnidaria, thus phylogenetically related to venomous species of jellyfish such as the box jellyfish. Some zoanthids are capable of synthesizing or bioaccumulating a heat stable, ultra-potent toxin which the animal releases under duress. We present a case of inhalational exposure to aerosolized palytoxin (PTX) from zoanthid coral.
Case
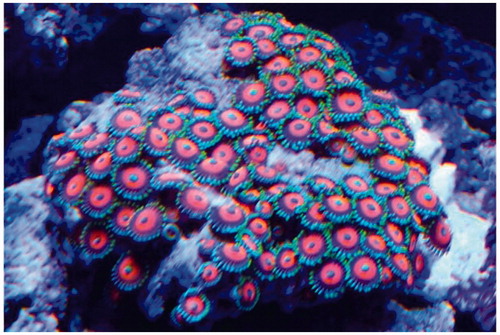
A 30-year-old man without pertinent past medical history presented to the Emergency Department (ED) with complaints of rigors, fever (home temperature of 102 °F), dyspnea, pharyngitis, emesis, myalgias, and headache. His symptoms started within an hour of inhaling steam released while eradicating overgrown Zoantharia from a rock in his saltwater fish tank () by dousing it with boiling water. His partner who was in a separate room developed similar, milder symptoms.
Figure 1. Zoanthid corals from the patient’s home aquarium which he attempted to eradicate prior to the onset of his symptoms. Photo: Paige Wood, reproduced with permission.
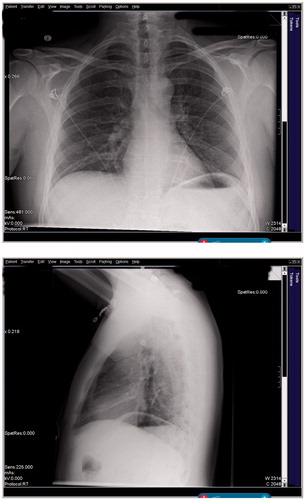
Upon arrival to the ED he was tachycardic (140 bpm), tachypneic (30 rpm), and hypoxemic (pulse oximetry 90% on room air). His blood pressure was 135/89 mmHg and temperature 99.2 °F (oral) after self-administration of 500 mg acetaminophen orally prior to arrival. He exhibited sub-costal retractions, wheezing, accessory muscle use, and appeared ill. His ECG showed sinus tachycardia with appropriate electrical intervals and no changes consistent with pharmacologic cardioactive steroid effects. His chest X-ray showed a small right basilar infiltrate ().
He received supplemental oxygen, intravenous crystalloids, corticosteroids, and nebulized albuterol with ipratropium. After poor initial response to these interventions he also received magnesium sulfate. Laboratory results showed initial mild leukocytosis of 11.21 k/L, normal creatine phosphokinase of 149 IU/L, elevated lactate of 3.0 mmol/L, and initial elevated procalcitonin of 0.35 ng/mL which increased to 2.59 ng/mL over an 8-h interval. Influenza and respiratory syncytial virus PCR were negative. The patient received empiric, broad spectrum antibiotics, yet blood cultures remained negative. During the patient’s course in the ED, his blood pressure decreased to 104/54 mmHg and he required additional IV fluids, inhaled β2-agonists, and oxygen by non-rebreather mask.
Based on the history and presentation, the treating clinician suspected PTX toxicity but elected to empirically treat bacterial pneumonia. The consulting toxicologist recommended supportive therapy. The patient improved as an inpatient over the subsequent day. Outpatient therapy included systemic corticosteroids and inhaled β2-agonist therapy.
Discussion
PTX is a highly complex non-protein compound first isolated in 1971 from Palythoa toxica, an anemone of the phylum Cnidarian, order Zoantharia. Several species of the dinoflagellate genus Ostreopsis are also capable of producing PTX. Consumption of coral or dinoflagelattes facilitates bioaccumulation in predatory marine animals, including fish and crabs. Multiple analogues to PTX exist, including homopalytoxin, bishomopalytoxin, neopalytoxin, deoxypalytoxin, and 42-OH palytoxin [Citation2].
The molecular target of PTX and its analogues is the Na+,K+-ATPase [Citation3]. This ubiquitous ion pump maintains normal electrochemical equilibria required for the function of multiple excitable tissues including the nervous system, cardiac conduction system, and contractile apparatus of myocytes. Binding of PTX to Na+,K+-ATPase occurs near the ouabain binding site and exerts a similar inhibitory effect [Citation3]. Unlike ouabain, PTX leads to arrest of the Na+,K+-ATPase in a conformational position that allows free flux of single-charge cations across the cellular membrane, thus effectively converting it into a non-selective cation channel [Citation4].
Toxicity occurs by various routes: ingestion of seafood containing PTX, dermal absorption after contact with P. toxica, or inhalation of aerosolized PTX during cleaning of aquaria or inhalation of sea spray during Osteropsis bloom. Ocular exposure to PTX may occur with inhalation or direct inoculation.
Ingestion of seafood containing PTX is often associated with metallic taste and leads to rapid development of symptoms over minutes to hours. Gastrointestinal distress and paresthesias are commonly described in the early course. Myalgias herald the beginning of rhabdomyolysis with associated laboratory abnormalities. Seizures and cardiotoxicity caused death in three patients with laboratory-proven PTX ingestions [Citation5,Citation6].
Topical absorption with subsequent systemic toxicity may occur in swimmers or divers who touch PTX-producing coral, or individuals handling these animals during maintenance work of aquaria. Clinically significant absorption of PTX occurs with minimal dermal injury, as was the case with a 32-year-old man who developed cardiotoxicity after sustaining a 5 mm cut [Citation7]. Visible dermal injury is not necessary for systemic toxicity to occur. A 25-year-old woman developed a metallic taste, paresthesia, diffuse urticaria, and perioral edema after touching Zoantharia coral with her bare hands without sustaining visible injury [Citation8]. A special case of topical exposure is ocular contact. During rapid closing (a protective reflex), a zoanthid sprayed water into the eye of a 63-year-old man, leading to corneal ulceration requiring in situ amniotic membrane transplantation. In addition, he developed myoglobinuria and mild rhabdomyolysis [Citation9]. A similar case resulted in keratitis and persistent corneal scarring in a 45-year-old woman [Citation10]. In a series of seven patients, four developed corneal melt, at times with perforation [Citation11]. Other patients experienced full recovery [Citation12].
Inhalational exposure to PTX occurs from two distinct sources: Osteropsis algal blooms and Zoantharia corals from costal environments or aquaria, respectively. During a dinoflagellate bloom in Genoa, Italy, beachgoers were exposed to ocean spray containing Ostreopsis. Over a period of ten days, 209 patients presented to local hospitals with respiratory complaints, over half were febrile on presentation [Citation13]. Alternatively, inhalational exposure occurs after aerosolizing PTX during aquarium maintenance. Zoantharia coral can overgrow a saltwater tank, and thus are often killed by pouring boiling water over the affected aquarium rock. The stress-response of the dying animal liberates PTX, which is heat stable and aerosolized with the steam.
PTX depolarizes bronchial smooth muscle and results in calcium influx [Citation14]. Thus, patients develop bronchospasm within minutes to hours of exposure. Pneumonitis occurs in the majority of patients with associated fever, leukocytosis, and patchy infiltrates on high resolution computed tomography pulmonary imaging [Citation15]. Patients can progress to acute respiratory distress syndrome (ARDS) with subsequent respiratory failure [Citation16]. The proximity of the exposure correlates to the severity of the symptoms [Citation17], but toxicity can affect individuals in different rooms or floors of a dwelling [Citation18]. The partner of our patient was in the adjacent room and developed only mild respiratory symptoms. A review of the Pubmed-indexed literature revealed 17 cases of respiratory symptoms due to PTX inhalation [Citation15–19]. There were no fatalities. Unlike other routes of exposure, clinically significant rhabdomyolysis has not been reported when inhalation was the primary route of exposure.
Inhalational exposure to PTX results in bronchospasm as well as airway inflammation. Inhaled β2-agonists and systemic corticosteroids appear to have some treatment benefit [Citation15], but data from rigorous trials establishing efficacy are not available. The benefit of antihistamines is unknown. The membrane-stabilizing and sedating properties of members of this drug class may theoretically complicate the clinical course of patients with severe PTX toxicity, and PTX toxicity is not primarily histamine-mediated.
Laboratory confirmation of PTX utilizes a variety of assays. Enzyme-linked aptamers (horseradish peroxidase) [Citation20], spectrophotometric hemolytic assays [Citation21], liquid chromatography with mass spectrometry [Citation22], and cytometry-based immunoassays [Citation23] can detect PTX in either food scraps or collected coral specimens. No PTX confirmation from human body fluids is reported [Citation24]. Laboratory testing for PTX was not performed in the case presented here, and is not available in time to support clinical decision making. The patient’s presentation satisfies criteria for clinical diagnosis of PTX toxicity [Citation2], and he was treated accordingly. Apart from medical toxicologists, most physicians are likely not familiar with this unique toxicity, and consultation with the local poison center is encouraged.
Disclosure statement
The authors report no conflict of interest.
References
- Fountain H. Are aquariums getting too lifelike? New York Times [Internet]. 2010 Mar 23. May 11, 2018. Available from: https://www.nytimes.com/2010/03/23/science/23aquarium.html
- Tubaro A, Durando P, Del Favero G, et al. Case definitions for human poisonings postulated to palytoxins exposure. Toxicon. 2011;57(3):478–495.
- Rossini GP, Bigiani A. Palytoxin action on the Na(+), K(+)-ATPase and the disruption of ion equilibria in biological systems. Toxicon. 2011;57(3):429–439.
- Habermann E. Action and binding of palytoxin, as studied with brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1983;323(3):269–275.
- Alcala AC, Alcala LC, Garth JS, et al. Human fatality due to ingestion of the crab Demania reynaudii that contained a palytoxin-like toxin. Toxicon. 1988;26(1):105–107.
- Onuma Y, Satake M, Ukena T, et al. Identification of putative palytoxin as the cause of clupeotoxism. Toxicon. 1999;37(1):55–65.
- Hoffmann K, Hermanns-Clausen M, Buhl C, et al. A case of palytoxin poisoning due to contact with zoanthid corals through a skin injury. Toxicon. 2008;51(8):1535–1537.
- Nordt SP, Wu J, Zahller S, et al. Palytoxin poisoning after dermal contact with zoanthid coral. J Emerg Med. 2011;40(4):397–399.
- Ruiz Y, Fuchs J, Beuschel R, et al. Dangerous reef aquaristics: palytoxin of a brown encrusting anemone causes toxic corneal reactions. Toxicon. 2015;106:42–45.
- Chaudhry NL, Przybek J, Hamilton A, et al. Unique case of palytoxin-related keratitis. Clin Exp Ophthalmol. 2016;44(9):853–854.
- Farooq AV, Gibbons AG, Council MD, et al. Corneal toxicity associated with aquarium coral palytoxin. Am J Ophthalmol. 2017;174:119–125.
- Moshirfar M, Khalifa YM, Espandar L, Mifflin MD. Aquarium coral keratoconjunctivitis. Arch Ophthalmol. 2010;128(10):1360–1362.
- Durando P, Ansaldi F, Oreste P, et al. Ostreopsis ovata and human health: epidemiological and clinical features of respiratory syndrome outbreaks from a two-year syndromic surveillance, 2005-06, in north-west Italy. Euro Surveill. 2007;12(6):E070607.1.
- Louzao MC, Ares IR, Cagide E. Marine toxins and the cytoskeleton: a new view of palytoxin toxicity. FEBS J. 2008;275(24):6067–6074.
- Bernasconi M, Berger D, Tamm M, et al. Aquarism: an innocent leisure activity? Palytoxin-induced acute pneumonitis. Respiration. 2012;84(5):436–439.
- Thakur LK, Jha KK. Palytoxin-induced acute respiratory failure. Respir Med Case Rep. 2017;20:4–6.
- Sud P, Su MK, Greller HA, et al. Case series: inhaled coral vapor-toxicity in a tank. J Med Toxicol. 2013;9(3):282–286.
- Hall C, Levy D, Sattler S. A case of palytoxin poisoning in a home aquarium enthusiast and His family. Case Rep Emerg Med. 2015;2015(12):621815.
- Wieringa A, Bertholee D, Horst Ter P, et al. Respiratory impairment in four patients associated with exposure to palytoxin containing coral. Clin Toxicol. 2014;52(2):150–151.
- Gao S, Zheng X, Hu B, et al. Enzyme-linked, aptamer-based, competitive biolayer interferometry biosensor for palytoxin. Biosens Bioelectron. 2017;89(Part 2):952–958.
- Brovedani V, Sosa S, Poli M, et al. A revisited hemolytic assay for palytoxin detection: limitations for its quantitation in mussels. Toxicon. 2016;119:225–233.
- Ciminiello P, Dell’Aversano C, Iacovo Dello E, et al. Liquid chromatography-high-resolution mass spectrometry for palytoxins in mussels. Anal Bioanal Chem. 2015;407(5):1463–1473.
- Fraga M, Vilarino N, Louzao MC, et al. Detection of palytoxin-like compounds by a flow cytometry-based immunoassay supported by functional and analytical methods. Anal Chim Acta. 2016;903:1–12.
- Pelin M, Brovedani V, Sosa S, Tubaro A. Palytoxin-containing aquarium soft corals as an emerging sanitary problem. Mar Drugs. 2016;14(2):33.