Abstract
Acute poisoning may necessitate identification of the toxic agent; however, several acutely poisoned patients are treated with minimal laboratory assistance. We investigated whether focused reference to laboratory toxicology tests conducted during a pilot project for a subregional analytical toxicology service influences treatment decisions. Patients with acute poisoning presented to the level 1 regional emergency medical center from May 2018 to April 2019 were initially reviewed. Poison samples were referred to the subregional toxicological analytical service. In total, 253 substance samples were tested among 111 patients during the study. According to the reported drug levels, 3 (1.2%) samples contained lethal doses, 49 (19%) had toxic levels, and 28 (11%) contained detectable levels of a lethal toxin or pesticide. Disagreement between the clinical assessment and laboratory analyses was found for 62 patients (fair kappa = 0.24, 56%), and they often had lower Glasgow Coma Scale, higher severity scores, older age, and less likelihood of receiving gastrointestinal decontamination. The regional analytical toxicology services were helpful for diagnostic planning and therapeutic management of acute poisoning. For seriously poisoned patients with inconsistent histories, it is necessary to reevaluate the classic therapeutic process based on the medical history.
Introduction
Acute poisoning may necessitate identification of the toxic agent; however, several patients with acute poisoning are treated without any laboratory assistance other than general clinical chemistry and hematology [Citation1]. It is difficult for routine laboratories to provide a full spectrum of toxicological analyses, and clinicians must comprehend the reliability of clinical diagnoses for toxic agents. Detailed history-taking and exploring how the poisoning occurred by parents, relatives, witnesses, and paramedics are mandatory; however, reliable information provided and the personal history of intoxicated and/or psychiatric patients is often limited in the emergency department [Citation2].
Consequently, clinical impressions can be highly discrepant in acute poisoning, and toxicological laboratory reporting will be highly beneficial. However, this level of detailed toxicology laboratory work is unrealistic in poisoning centers and hospitals. Drug detection capabilities and the availability of toxicological laboratory tests vary among hospitals and facilities, and the available test items are highly limited. Therefore, the World Health Organization, the UK [Citation3,Citation4] and US [Citation5] guidelines specify testing availability for more than 10 essential substances (Group 1) with a 2-h turnaround time (TAT) for other major substances in regional centers.
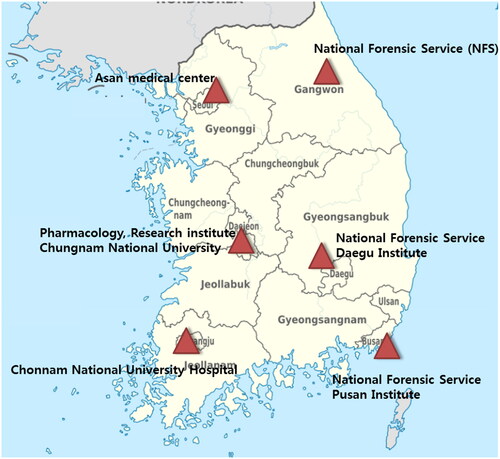
In South Korea, 24-h analytical toxicology services or Poison Control Centers (PCCs) have not been established. Most of the drug or poison concentration measurements, except for therapeutic drug monitoring, have TATs of approximately 1.5–3 days from the test request to the generation of results. Patients with poisoning are treated according to their clinical symptoms or medical history using the limited test items available at each hospital. Therefore, the Ministry of Health and Welfare has operated a regional toxicology laboratory testing service under a chemical disaster support project since 2017. As processing part of establishing the National PCC, six institutions (one university research center, two teaching hospitals, and three national scientific investigation laboratories) were selected as regional toxicological analytical centers in collaboration with the Korean Society of Clinical Toxicology, the National Emergency Medical Center, and the Ministry of Health and Welfare [Citation6,Citation7]. These centers provided analytical test results to the referral clinic, including blood and urine, for patients with suspected acute severe poisoning. After dividing the region into six subregions, six institutions are selected each year based on the TATs and quality control monitoring from the previous year (). This system enables the availability of results within 2–6 h for the initial screening and the 1st quantitative analysis report and in 12–24 h for the final report.
Figure 1. Regional agencies and their coverage areas for toxicological analytic services in South Korea.
Therefore, we aimed to investigate whether focused use of laboratory toxicology tests obtained under a regional analytical toxicology service pilot project has an impact on clinical decisions. Furthermore, we reviewed the concordance between self-reported drug history and laboratory confirmation.
Methods
This retrospective observational study was performed on adults aged >15 years with acute poisoning after obtaining approval from the institutional review board (KNUH 2020-02018). Patients with acute poisoning who presented themselves to the emergency department of a level 1 regional emergency medical center (a 960-bed tertiary hospital) from May 2018 to April 2019 were initially reviewed (). This emergency center was selected by the Korean Ministry of Health and Welfare as one of the major regional facilities for providing chemical detoxification and stocking critical antidotes. Poison samples were referred to the subregional analytical toxicology service according to the following criteria ().
Figure 2. Flow chart for patient enrollment. ED = emergency department, PSS = poison severity score.
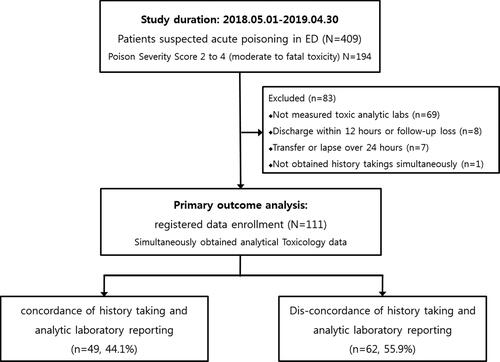
Table 1. Clinical characteristics of the study cohort.
Clinical and toxicological variables including gender, age, primary symptoms, vital signs, Glasgow Coma Scale (GCS) score, reason for exposure (intentional vs. unintentional), route of poisoning, QTc intervals from electrocardiograms, poison severity score (PSS) at hospital discharge, clinical decontamination and detoxification, suspicious poison substances obtained during history-taking, presumed poison from analytical toxicology reporting data, and clinical outcomes (e.g. occurrence of any complications and fatality within 1 month) were collected.
Blood and urine samples for toxicological screening were collected together with regular diagnostic blood samples on admission, and all laboratory tests were conducted simultaneously. The results were reported; the first qualitative test was a tox screening report or immunoassay testing. As the second analysis, high-resolution mass spectrometry was performed as a precise quantitative test. The second analysis describes five possible levels according to the drug analytical test results (undetectable, subtherapeutic, therapeutic, toxic, and lethal). Clinically positive laboratory tests represented positive laboratory values above the therapeutic level that were negative in the clinical evaluation or history-taking. In cases of known medicine ingestion, a positive laboratory test was considered as a positive laboratory value above the toxic level [Citation8]. For laboratory test comparisons, suspected primary and additional agents were combined in agent-specific groups to cover all suspected cases for each agent. Cohen’s kappa (κ) was calculated as an index of agreement. Poison severity score is intended to be an overall evaluation of the case, taking into account the most severe clinical features [Citation9].
The primary outcome variable was the clinical impact of laboratory toxicology tests obtained under a subregional analytical toxicology service pilot project. The clinical impact of the results on decision-making was defined according to their reliability and usefulness from the following list: (1) treatment decision (administration of a specific antidote), (2) diagnostic decision (administration of any other considered drug-specific treatment), (3) duration of monitoring, and (4) patient disposition (i.e. admission to a general ward if the test was positive vs psychiatric ward if negative) [Citation8]. The secondary outcome was the concordance between history-based substances and laboratory confirmation. All statistical analyses were conducted using SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA) and MedCalc (MedCalc Software, Mariakerke, Belgium).
Results
For 111 patients (median age, 60 years) referred for toxicology laboratory analysis, the therapeutic interventions after admission was as follows: 21 patients (18.9%) received mechanical ventilator treatment, 19 (17.1%) were administered activated charcoal for gastrointestinal decontamination, 17 (15.3%) were treated with gastric lavage, and 7 (6.3%) were treated with hemodialysis, hemoperfusion during hospitalization (). Among the indications reported to the regional toxicology analytical service, unknown causes for a comatose state (n = 52, 46.8%) were the most common, followed by discrepancy between clinical evaluation and history-taking (n = 36, 32.4%), pesticides and lethal toxin poisoning (n = 15, 13.5%), and unknown causes for cardiac arrest or others (n = 8, 7.2%). The median toxic analytical TAT allows for results within 40 min for the initial screening test, 2.3 h for the 1st quantitative analysis report, and 6.8 h for the final report.
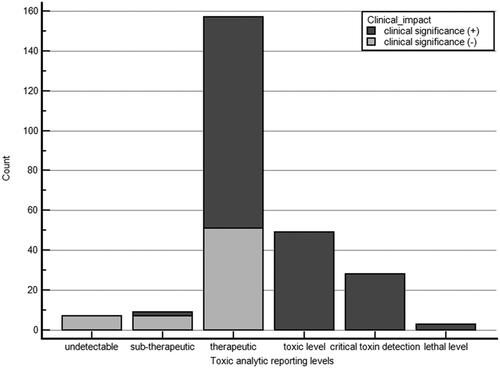
In total, 253 substance samples were tested for 111 patients during the study period. Of these 253 analytical substances, 91 (36.0%) were above the significantly toxic or lethal concentration (). For non-intoxicated patients determined from history-taking (n = 58), 177 toxicological analytical substances and toxins were measured. In either poisoning or non-poisoning group according to history-taking, zolpidem was the most commonly detected drug in all patients. The agreement between the clinical assessment and laboratory analyses was fair (κ = 0.24, fair 0.2–0.5). Discrepancies between the medical history and toxic analytical results were found in 192 cases (75.9%).
Figure 3. Clinical usefulness according to toxicology analytical level of the 253 toxic samples reported.
Among 111 patients, testing resulted in drug-specific additional diagnostic test for 69 patients (62.1%), with special treatment for 26 (23.4%) patients, prolonged monitoring for 17 (15.3%) toxicity-positive patients, and disposition changes for 35 (31.5%) patients. Overall, 64.9% of the tests had an impact on patient management (). Disagreement between the clinical assessment and toxicological analytical data was found in 62 patients (55.9%). Patients with discordant medical history and toxic analytical findings often had lower GCS scores, higher PSS values, older age, and a lower likelihood of receiving gastrointestinal decontamination ().
Figure 4. Clinical impact of toxicological laboratory analysis. Data presented as response numbers (%) based on multiple response analysis for all 111 patients.
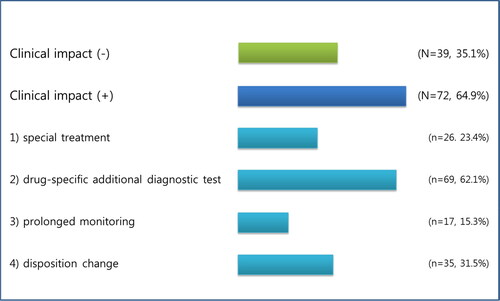
Table 2. Clinical characteristics of the study patients according to the agreement between history-taking and analytical laboratory reporting.
Discussion
To our knowledge, this is the first pilot study to explore how the operation of a region-based toxicology laboratory service influences physicians and clinical toxicologists. The results demonstrated an extremely low degree of concordance between the identified substance and the medical history, with fair agreement between the two (κ = 0.24). Patients with discrepancies had more severe PSS and a lower chance of receiving gastrointestinal decontamination on time. The toxicology laboratory test results revealed 75% clinical usefulness, such as changes in the disposition strategy and diagnosis in severe poisoning cases.
Toxicological laboratory services are an essential component of poison control programs [Citation1,Citation3,Citation4]. In most countries, these services are not commonly available at hospitals. Emergency toxicological analyses (24-h availability) can affect immediate patient management and have a relatively limited number of analytical items. More complex clinical toxicological assays that are less frequently needed and can often be offered on a less-urgent basis are generally provided by regional or national centers due to the need for efficient use of resources [Citation3–5]. Recommendations regarding assays that should be provided at regional centers are available for the UK and US and are generally applicable.
The usefulness of laboratory analysis for poisoning patients has been evaluated using point-care test, urine Tox Screen or qualitative screening test [Citation8–13], or kappa analysis of the laboratory findings and medical history for some substances [Citation14]. The agreement between the clinical assessment and laboratory analyses was found to be good for ethanol and paracetamol (κ = 0.70 for both) but was only moderate or fair for other agents (κ = 0.22–0.51). The sensitivity of the clinical assessments compared with the laboratory results was better for common agents than for rare agents and better for higher than lower serum concentrations. The four most common agents (ethanol, benzodiazepines, paracetamol, and opiates) had an overall sensitivity of 82% for higher-than-median serum concentrations, whereas other agents had sensitivities of 14–71%. Most previous studies evaluated the usefulness of screening tests or the concordance between medical history and the identified poisoning agents. A few studies examined how the designation of a local toxicological laboratory affects the treatment process for the hospital staff. In our study, these laboratory results were found to be clinically useful for 65% of referral patients, and >31.2% of cases with toxic to lethal levels were detected. Accordingly, the final clinical disposition decision was modified for these patients.
The actual impact of drug screening has been questioned as to whether it rarely changes patient management [Citation2,Citation15,Citation16]. Kellerman et al. evaluated 405 adult drug overdose patients and reported a change in the clinical management after drug tests for only 4.4% of the patients [Citation11,Citation17]. Therefore, they recommended that drug screening should be considered only when the anticipated results might affect patient disposition. However, our study revealed a higher rate of clinical usefulness than that in previous studies. This difference might be because target referral patients of the present study were primarily those exhibiting disagreements between medical history and unknown causes of mental changes. Although there is evidence indicating that drug testing may not always be necessary or cost-effective, there has been little research comparing self-reported drug use and analytical toxicology data. A correlation assessment between self-reported drug use and laboratory confirmation of the drug use would help determine whether this test is necessary in future evaluations.
Pohjola-Sintonen et al. [Citation18] reported that the information obtained at admission was fully consistent with the laboratory findings in only 27% of previous acute poisoning cases. Minor discrepancies between the history and the results from drug analyses identifying the drugs taken were found in 55% of the cases. In 18% of the cases, the discrepancies were considered clinically important. Serious symptoms occurred in approximately 20% of the patients, but all of them survived. Such assays would enable optimal treatment of several patients with acute poisoning by reducing the need for supervision and expensive treatments and facilitating the identification of cases requiring prompt drug-specific treatment.
Here, 55.9% of the patients with severe poisoning showed inconsistencies between their medical history and test results. This implies that patients with such discrepancies experience increased severity without adequate treatment. Failure to confirm accurate intoxication information while obtaining the patient’s history may lead to the patient losing their opportunity to receive gastrointestinal decontamination or use the antidote within the critical golden time. For patients with severe acute intoxication, it may be necessary to overview only usable history-based supportive care without backup of toxic laboratory analysis.
Currently, this project continues at the government’s cost, so continuous quality management (e.g. communication, reporting publication) and feedback are essential for the project to continue.
This study has some limitations. First, it was conducted at a single regional emergency facility that receives only patients referred for toxicological tests, and hence good overall patient representation is lacking. Second, the study included cases of severe and unknown coma, which can cause overestimation of sedative concentrations and mismatched medical history. For patients with severe acute intoxication, it may be necessary to overview only usable history-based supportive care without backup of toxic laboratory analysis. Moreover, this study evaluated a pilot project and did not consider the cost-effectiveness. Finally, the regional toxicological laboratory center is operated such that only participating hospitals that were approved by the appropriate research ethics committee can use the complete data set. We would recommend adding in details about what drugs are being tested for, how the tests are done, what the actual outcome changes were in detail.
Conclusions
The toxicology analytical results provided by the regional analytical toxicology service model were helpful in managing poisoning cases. For several serious cases without consistent history-taking, the opportunity to perform gastrointestinal decontamination or administer life-saving antidotes was limited. For patients with severe acute intoxication, it may be necessary to reevaluate the classic therapeutic process of treatment based on only the medical history or toxidrome without backup from toxic laboratory analysis.
| Abbreviations | ||
| ED | = | emergency department |
| GCS | = | Glasgow Coma Scale |
| ICU | = | intensive care unit |
| PCC | = | Poison Control Centers |
| PSS | = | poison severity score |
| TAT | = | turnaround time. |
Disclosure statement
The authors declare that they have no conflicts of interest.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Flanagan RJ. Developing an analytical toxicology service: principles and guidance. Toxicol Rev. 2004;23(4):251–263.
- Camilleri R. A meta-analysis of the reliability of the history in suspected poisoning. J Emerg Med. 2015;48(6):679–684.
- National Poisons Information Service; Association of Clinical Biochemists. Laboratory analyses for poisoned patients: joint position paper. Ann Clin Biochem. 2002;39(Pt 4):328–339.
- Thompson JP, Watson ID, Thanacoody HK, et al. Guidelines for laboratory analyses for poisoned patients in the United Kingdom. Ann Clin Biochem. 2014;51(Pt 3):312–325.
- Wu AH, McKay C, Broussard LA, et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem. 2003;49(3):357–379.
- Dart RC, Goldfrank LR, Erstad BL, et al. Expert consensus guidelines for stocking of antidotes in hospitals that provide emergency care. Ann Emerg Med. 2018;71(3):314–325.e1.
- Sohn CH, Ryoo SM, Lim KS, et al. Kind and estimated stocking amount of antidotes for initial treatment for acute poisoning at emergency medical centers in Korea. J Korean Med Sci. 2014;29(11):1562–1571.
- Erdmann A, Werner D, Hugli O, et al. Focused use of drug screening in overdose patients increases impact on management. Swiss Med Wkly. 2015;145:w14242.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score. Grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213.
- Wu AH. Limitations of point-of-care testing in the ED or ICU: a role for regional centralized toxicology laboratories. Clin Pharmacol Ther. 2010;88(3):295–298.
- Perrone J, De Roos F, Jayaraman S, et al. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med. 2001;19(1):49–51.
- Osterloh JD. Utility and reliability of emergency toxicologic testing. Emerg Med Clin North Am. 1990;8(3):693–723.
- Moreno JL, Duprey MS, Hayes BD, et al. Agreement between self-reported psychoactive substance use and urine toxicology results for adults with opioid use disorder admitted to hospital. Toxicol Commun. 2019;3(1):94–101.
- Bentur Y, Lurie Y, Tamir A, et al. Reliability of history of acetaminophen ingestion in intentional drug overdose patients. Hum Exp Toxicol. 2011;30(1):44–50.
- Bjornaas MA, Hovda KE, Mikalsen H, et al. Clinical vs. laboratory identification of drugs of abuse in patients admitted for acute poisoning. Clin Toxicol (Phila). 2006;44(2):127–134.
- Tenenbein M. Do you really need that emergency drug screen?Clin Toxicol (Phila). 2009;47(4):286–291.
- Kellermann AL, Fihn SD, LoGerfo JP, et al. Impact of drug screening in suspected overdose. Ann Emerg Med. 1987;16(11):1206–1216.
- Pohjola-Sintonen S, Kivisto KT, Vuori E, et al. Identification of drugs ingested in acute poisoning: correlation of patient history with drug analyses. Ther Drug Monit. 2000;22(6):749–752.
