Abstract
The number of acute caffeine poisoning cases have increased in Japan. We can use serum caffeine concentrations to evaluate the severity of caffeine poisoning and determine whether or not we should perform hemodialysis. In this study, we sought to develop a rapid method for measuring serum caffeine concentrations. We used liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the new method. We chose caffeine-d9 as the internal standard, and we used the standard addition method to quantify caffeine concentrations. We collected six blood samples from three patients with acute caffeine poisoning to measure serum caffeine concentrations. In our method, retention time for caffeine was 0.4 min, and the time required for the total LC-MS/MS analysis was 1 min per sample. We obtained accurate serum caffeine concentrations 7 min after injection into the LC-MS/MS instrument. Further, time-consuming sample pretreatment was not required because each sample was diluted 10,000-fold. As a result, we could obtain serum caffeine concentrations for each patient in a total of 40 min. Our findings suggest that rapid, accurate measurement of serum caffeine concentrations by LC-MS/MS could contribute to real-time evaluation of poisoning severity and determination of appropriate therapeutic strategies in acute clinical settings.
Introduction
Caffeine (1,3,7-trimethylxanthine), a natural alkaloid derived from tea leaves, coffee beans, cocoa beans, and kola nuts, has long been recognized as a habit-forming, mild stimulant [Citation1]. Aside from being present in tea, coffee, chocolate, and most soft drinks, caffeine is also present in various prescription drug mixtures and over-the-counter drugs, such as cold medicine, in doses ranging from 30 to 200 mg [Citation2]. In addition, supplements and energy drinks often contain caffeine in doses ranging from 50 to 505 mg [Citation2, Citation3]. According to Annual Report of the American Association of Poison Control Centers’ National Poison Data System, there were 6,178 exposures to caffeine containing products including stimulants (3,934) and various energy drinks (2,231) in 2019. In contrast, there were 140 theophylline exposures with only three death reports mentioning theophylline in 2019 [Citation4]. In Japan, a retrospective survey of 101 patients with acute caffeine poisoning from 38 emergency departments found that the number of acute caffeine poisoning cases markedly increased since April 2013. In that study, the median age of patients was 25 years, estimated caffeine doses ranged from 1.2 to 82.6 g (median, 7.2 g), and serum caffeine concentrations on admission ranged from 2.0 to 530.0 mg/L (median, 106.0 mg/L) [Citation5].
In a previous study of patients acutely poisoned with theophylline (1,3-dimethylxanthine), the decision whether to use hemodialysis depended upon timely serum theophylline concentrations [Citation6]. As with theophylline, timely caffeine concentrations would assist emergency physicians in the assessment of the poisoning and the need for emergency hemodialysis [Citation5]. However, serum caffeine concentrations are rarely available in acute clinical settings, even though caffeine concentrations for which hemodialysis is indicated are widely known. There is an urgent need for a rapid method for measuring serum caffeine concentrations.
The present study aimed to develop a rapid and accurate liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to measure serum caffeine concentrations for acute caffeine poisoning cases in order to achieve real-time evaluation of poisoning severity and aid in decision-making regarding therapeutic strategies in acute clinical settings.
Methods
Sample collection
We collected six blood samples from three patients who were treated in emergency departments of hospitals from different regions in Japan after consuming large amounts of caffeine anhydrous to measure serum caffeine concentrations.
Chemicals and reagents
We purchased standard caffeine (purity ≥98.5%), caffeine-d9, LC-MS-grade methanol, ammonium formate, and ultrapure water from Wako Pure Chemical Industries (Osaka, Japan). We purchased Captiva ND Lipid cartridges from Agilent Technologies (Santa Clara, CA, USA).
Liquid chromatography-tandem mass spectrometry instrument
We used an ExionLC system (SCIEX, Framingham, MA, USA) for LC. We performed chromatographic separation using a CAPCELL PAK ADME-HR S5 column (35 mm × 2.1 mm i.d., 5.0 μm particle size; Osaka Soda, Osaka, Japan) with a guard column (EXP® guard column, C18, 5.0 mm × 2.1 mm i.d.; Optimize Technologies, Oregon City, OR, USA). Mobile phase solvent A consisted of 0.1% (v/v) ammonium formate in water:methanol (95:5, v/v), and mobile phase solvent B consisted of 0.1% (v/v) ammonium formate in water:methanol (5:95, v/v). The mobile phase composition was isocratically 40% solvent A and 60% solvent B. The solvent flow rate was 0.5 mL/min, and the column temperature was maintained at 30 °C. The injection volume was 1.0 μL.
We performed MS/MS detection using a 4500 QTRAP system (SCIEX, Framingham, MA, USA). We operated the ionization source in the positive mode set to an ion spray voltage of 4500 V, 60 psi for nebulizer gas 1, 70 psi for nebulizer gas 2, 20 psi for the curtain gas, and a temperature of 600 °C. We analyzed all molecules in the multiple reaction monitoring (MRM) mode setting Q1 and Q3 at unit resolution, the entrance potential at 10 eV, the collision cell exit potential at 16 eV, and the collision gas at 8 psi. The optimized collision energy was 27 V for caffeine and 31 V for caffeine-d9 (IS). We performed quantitation in the MRM mode. Product ion spectra were generated automatically when the MRM signal intensity at transition m/z 195 > 138 in caffeine and m/z 204 > 116 in caffeine-d9 (IS) were >2500 counts per second. The overall run time was 1 min.
Quantitative analysis by the standard addition method
We centrifuged whole blood samples at 2150 g for 5 min in order to separate serum. We transferred separated serum samples into 2 mL polypropylene tubes and stored at −80 °C until use.
We prepared a standard stock solution of caffeine (3000 ng/mL) by dissolving 3 mg of caffeine in 10 ml of ultrapure water, and further diluting 100-fold with ultrapure water. We prepared caffeine-d9 (30 ng/mL) as the IS by dissolving 2 mg in 2 mL of a 1:1 solution of methanol and ultrapure water, and further diluting 3 µL of the solution with a 1:1 solution of methanol and ultrapure water to a total of 100 mL. We prepared serum samples by diluting 200-fold with ultrapure water. In order to obtain quantitation values by the standard addition method, we mixed 10 µL of each serum sample with 190 µL of ultrapure water containing 10 µL of standard caffeine solutions at appropriate concentrations (for sample No. 1: 0, 5, 10, 25, 50, 100, and 150 ng/mL; for sample Nos. 2–6: 0, 50, 100, 250, 500, 1000, and 1500 ng/mL). We then mixed each standard addition sample with 300 µL of caffeine-d9. We vortexed thoroughly and centrifuged the mixture at 20400 g for 5 min. For extraction, we passed each standard addition sample through a Captiva ND Lipid cartridge which removes ion-suppressing phospholipids and proteins from serum samples. We then transferred each filtered solution to a 1.5 mL glass vial. We injected the solution into the LC-MS/MS system. We analyzed each point six times. We used the MRM transition at m/z 195 > 138 for quantitation and m/z 195 > 110 for qualifier ions. We used the MRM transition at m/z 204 > 116 for caffeine-d9 (IS). We set the total analysis time to within 1 min.
Ethics approval
This study was approved by the ethics committees of all participating hospitals, including the ethics committee of Saitama Medical University Hospital (notification number: 16096. 04).
Results
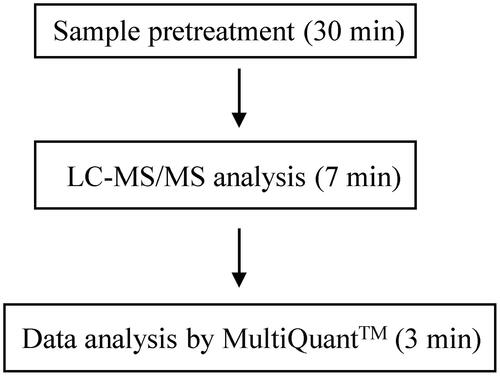
shows product ion spectra of the standard caffeine solution () and the standard caffeine-d9 solution (). Results of product ion spectra of caffeine obtained using serum samples were identical to that obtained using the standard caffeine solution. With the new method, the retention time for caffeine was 0.4 min, and the total LC-MS/MS analysis time required was 1 min per sample (). We evaluated carryover by preparing a caffeine concentration of 1000 mg/L using standard caffeine solution, which is above the maximum concentration that we would encounter and analyze in acute clinical settings.
Figure 1. Product ion spectra of caffeine and caffeine-d9. (A) Standard caffeine solution. (B) Standard caffeine-d9 solution (internal standard).
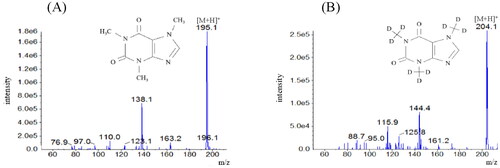
Figure 2. Representative multiple reaction monitoring data for the standard caffeine solution. Caffeine has a retention time of 0.4 min.
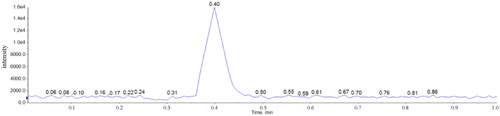
We determined serum caffeine concentrations in patients by the standard addition method. shows a representative standard addition plot for a poisoned patient who had a serum caffeine concentration of 120.6 mg/L. shows serum caffeine concentrations in all six samples, as well as the relative standard deviation (RSD%). We also measured serum caffeine concentrations in the six samples by using gas chromatography-mass spectrometry (GC-MS) to confirm the accuracy. The performance of the LC-MS/MS method was satisfactory for its intended purpose, as it was possible to obtain serum caffeine concentrations for each patient 7 min after injecting the respective samples into the LC-MS/MS instrument. The basic underlying advantages of the optimized method is that it utilizes only 0.35 µL serum sample and laborious and time-consuming pretreatment is not required because each serum sample is diluted 10,000-fold due to high selectivity and sensitivity to caffeine in LC-MS/MS. Since it was possible to substantially cut down pretreatment and LC-MS/MS analysis times, we could obtain serum caffeine concentrations in a total of 40 min for each patient ().
Figure 3. Representative standard addition plot for a patient sample with a serum caffeine concentration of 120.6 mg/L. We analyzed each point six times.
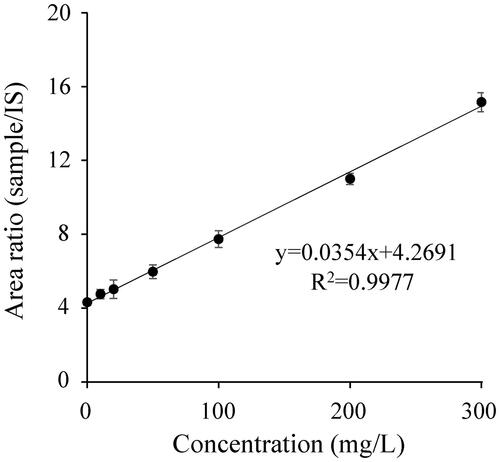
Table 1. Serum caffeine concentrations in six samples from three patients and relative standard deviation.
Discussion
Caffeine is the most widely consumed psychoactive compound worldwide. In general, its mechanism of action is dose-dependent, and clinical effects occur at serum concentrations of 15 mg/L or higher. Common signs and symptoms of acute caffeine poisoning include nausea, vomiting, excitement/agitation, depressed consciousness, tachypnea, and tachycardia. Severe caffeine poisoning can lead to death, primarily as a result of ventricular fibrillation. Serum caffeine concentrations exceeding 80–100 mg/L signify severe and potentially fatal poisoning [Citation5]. The main treatment for acute caffeine poisoning is supportive care. Severe cases require urgent hemodialysis [Citation7,Citation8]. On the other hand, surprisingly, there is an exceptional case report which describes a patient with severe caffeine poisoning (peak concentration: 258 mg/L) who successfully managed using only intravenous infusions of esmolol and norepinephrine [Citation9]. One of the most important information in that case is that “the caffeine concentration measurements occurred “days later” and not in real-time (Bruno Megarbane, personal communication)”. Timely caffeine concentrations would assist emergency physicians in determining the need for hemodialysis and may improve prognosis in severe cases.
Several analytical methods, including GC-MS, high-performance liquid chromatography with ultraviolet, liquid chromatography-mass spectrometry, and LC-MS/MS have been used to measure caffeine concentrations [Citation10–13]. Among these, LC-MS/MS is a powerful modality for quantifying caffeine concentrations in human serum due to its high selectivity and sensitivity [Citation14]. In sample pretreatment, several sample preparation procedures rely on traditional technologies such as protein precipitation (PPT) and liquid-liquid extraction (LLE). Although PPT is the simplest and fastest method, it often causes ion suppression because it does not lead to completely clean extracts. Further, LLE takes longer requiring multiple extraction steps to obtain cleaner extracts. Compared to these sample preparation methods, solid phase extraction offers reduced processing time and lower solvent consumption. Considering that finding caffeine-free human serum volunteers is challenging, it is not easy to use external calibration curves in measuring caffeine concentrations in human serum. For quantitation of caffeine in human serum, the standard addition method is the most straightforward technique [Citation15,Citation16]. In addition, we used a stable isotope internal standard (IS), which is essential since it improves reproducibility between injections, adjusts for the loss of sensitivity when running a batch of samples, and accounts for matrix effects that can occur during the ionization process.
Previously published studies have described methods to rapidly measure caffeine simultaneously with other drugs and poisons [Citation17,Citation18]. To our knowledge, however, there have never been described the successful rapid measurement of serum caffeine concentrations by LC-MS/MS for acute clinical settings. For our previous method using GC-MS, it takes approximately 3 h to obtain serum caffeine concentrations due to time-consuming pretreatment and GC-MS analysis times. On the other hand, the total time required with the present method is 40 min, which is sufficiently rapid for emergency physicians to determine whether hemodialysis is necessary. Furthermore, the method gives reliable results across the range from nontoxic to toxic concentrations. We have not fully validated these modalities by this study and require further examination. Nonetheless, we can use the method in acute clinical settings and expect to aid in decision-making regarding therapeutic strategies for acute caffeine poisoning cases.
Conclusion
The present method, which rapidly and accurately measures serum caffeine concentrations in patients with acute caffeine poisoning by LC-MS/MS, allows for real-time evaluation of poisoning severity and determination of appropriate therapeutic strategies in acute clinical settings.
Acknowledgments
We thank the emergency departments of participating hospitals for providing samples. This work was supported by a Grant-in-Aid for studies regarding the development of methods for rapid detection of addictive substances acting on the central nervous system (Principle Investigator, Masahiko Funada) from the Japan Agency for Medical Research and Development (AMED).
Disclosure statement
The authors report no conflict of interest.
References
- Rogers PJ, Richardson NJ, Elliman NA. Overnight caffeine abstinence and negative reinforcement of preference for caffeine-containing drinks. Psychopharmacol. 1995;120(4):457–462.
- Banerjee P, Ali Z, Levine B, et al. Fatal caffeine intoxication: a series of eight cases from 1999 to 2009. J Forensic Sci. 2014;59(3):865–868.
- Nowak D, Jasionowski A. Analysis of the consumption of caffeinated energy drinks among Polish adolescents. Int J Environ Res Public Health. 2015;12(7):7910–7912.
- Gummin DD, Mowry JB, Beuhler MC, et al. Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin Toxicol. 2020;58(12):1360–1541.
- Kamijo Y, Takai M, Fujita Y, et al. A retrospective study on the epidemiological and clinical features of emergency patients with large or massive consumption of caffeinated supplements or energy drinks in Japan. Intern Med. 2018;57(15):2141–2146.
- Ghannoum M, Weigand TJ, Liu KD, et al. Extracorporeal treatment for theophylline poisoning: systemic review and recommendations from the EXTRIP workgroup. Clin Toxicol. 2015;53(4):215–229.
- Yoshizawa T, Kamijo Y, Hanazawa T, et al. Which of hemodialysis and direct hemoperfusion is more recommended for treating severe caffeine poisoning?Am J Emerg Med. 2019;37(9):1801–1802.
- Yoshizawa T, Kamijo Y, Hanazawa T, et al. Criterion for initiating hemodialysis based on serum caffeine concentration in treating severe caffeine poisoning. Am J Emerg Med. 2021;46:70–73.
- Grémain V, Chevillard L, Saussereau E, et al. Massive suicidal ingestion of caffeine: a case report with investigation of the cardiovascular effect/concentration relationships. Clin Toxicol. 2021.
- Neves DBDJ, Caldas ED. Determination of caffeine and identification of undeclared substances in dietary supplements and caffeine dietary exposure assessment. Food Chem Toxicol. 2017;105:194–202.
- Cunha RR, Chaves SC, Ribeiro MMAC, et al. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J Sep Sci. 2015;38(10):1657–1662.
- White JR, Jr, Padowski JM, Zhong Y, et al. Pharmacokinetic analysis and comparison of caffeine administered rapidly or slowly in coffee chilled or hot versus chilled energy drink in healthy young adults. Clin Toxicol (Phila). 2016;54(4):308–312.
- Yu T, Campbell SC, Stockmann C, et al. Pregnancy-induced changes in the pharmacokinetics of caffeine and its metabolites. Clin Pharmacol. 2016;56(5):590–596.
- Mendes VM, Coelho M, Tomé AR, et al. Validation of an LC-MS/MS Method for the quantification of caffeine and theobromine using non-matched matrix calibration curve. Molecules. 2019;24(16):2863.
- Eeckhaut AV, Lanckmans K, Sarre S, et al. Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(23):2198–2207.
- Krautbauer S, Büchler C, Liebisch G. Relevance in the use of appropriate internal standards for accurate quantification using LC-MS/MS: Tauro-conjugated bile acids as an example. Anal Chem. 2016;88(22):10957–10961.
- Feng S, Tian Y, Zhang Z, et al. Rapid simultaneous determination of paracetamol, amantadine hydrochloride, caffeine and chlorpheniramine maleate in human plasma by liquid chromatography/tandem mass spectrometry. Arzneimittel-Forschung. 2009;59(2):86–95.
- Li H, Zhang C, Wang J, et al. Simultaneous quantitation of paracetamol, caffeine, pseudoephedrine, chlorpheniramine and cloperastine in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;51(3):716–722.