Abstract
Ingestion of amatoxin-containing mushrooms can lead to fulminant hepatotoxicity and death. Despite recent interest in therapeutic options for amatoxin-exposed patients, there is no single, recommended treatment for amatoxin poisoning. Alpha-amanitin, the principal toxin in amatoxin-containing mushrooms, requires entry into hepatocytes via organic anion transporting polypeptide (OATP) 1B3 before the cascade of cellular events occurs leading to hepatocyte necrosis, liver failure, and in severe cases liver transplantation or death. Three patients managed through a single poison centre with confirmed amatoxin-containing mushrooms ingestions were treated with intravenous cyclosporine, a known potent OATP1B3 inhibitor, along with supportive care. All patients presented with classic delayed symptoms of nausea, vomiting and diarrhea. No patient progressed to fulminant hepatic failure, although two patients developed a transient rise in liver transaminases. All recovered and were discharged from hospital. No patient had an adverse effect from cyclosporine. In addition, we performed an in-vitro study of the role of cyclosporine in cultured HEK293T cells and human hepatoma Huh7 cells. Cyclosporine effectively inhibited OATP1B3-mediated uptake of alpha-amanitin, and improved cell viability of alpha-amanitin exposed cultured Huh7 cells.
We conclude that IV cyclosporine, a drug readily available in most hospitals, may be useful to reduce hepatotoxicity from amatoxin poisoning.
Introduction
Recent studies suggest that ingestion of amatoxin-containing mushrooms can lead to fulminant hepatotoxicity requiring liver transplant or death in 11% of cases (range between 2.5–44%) [Citation1]. Among the proposed treatments for amatoxin poisoning are activated charcoal, high-dose benzylpenicillin, prednisolone, silibinin, rifampin, N-acetylcysteine, and extracorporeal elimination. All have uncertain efficacy, and none is clearly the preferred treatment for these patients [Citation1–3].
Alpha-amanitin is the principal amatoxin causing human toxicity. Alpha-amanitin enters into hepatocytes via the organic anion transporting polypeptide (OATP) 1B3 transporter protein located on the sinusoidal membrane of hepatocytes [Citation4]. Upon cellular uptake, alpha-amanitin inhibits RNA Polymerase II, leading to cessation of intracellular protein synthesis, activation of apoptotic pathways, and eventual cell death [Citation2]. Since liver toxicity depends on amanitin hepatocyte uptake, pharmacological blockade of the OATP1B transporters has been investigated as a treatment strategy for mushroom poisoning [Citation4]. Indeed, the beta-lactam antibiotic, benzylpenicillin, the flavonolignan from the milk thistle plant, silibinin, and the antimycobacterial drug, rifampin, are OATP1B inhibitors that have been used clinically in amatoxin poisonings [Citation2–4]. Aside from limited evidence of efficacy of any of these agents, an important clinical challenge is availability. Silibinin and IV rifampin are not readily available in Canadian hospitals, leading to delays in initiation of these treatments beyond a window when they would have the greatest theoretical benefit in preventing toxin uptake.
Cyclosporine is a calcineurin inhibitor used in the treatment of organ transplant rejection and autoimmune disorders. Cyclosporine is a known highly potent inhibitor of the OATP1B3 transporter and is available in most hospitals in a parenteral formulation [Citation4]. Since inhibition of OATP alters the pharmacokinetics of OATP substrates [Citation5], we hypothesized that cyclosporine could reduce the uptake of amatoxin into hepatocytes, thereby limiting hepatotoxicity (defined as any elevation of aminotransferases above upper limit of normal) following amatoxin-containing mushroom ingestions.
This case series, presented in accord with the PROCESS guidelines [Citation6], illustrates the potential utility of cyclosporine as a treatment for amatoxin-containing mushrooms ingestions.
Case reports
Case 1
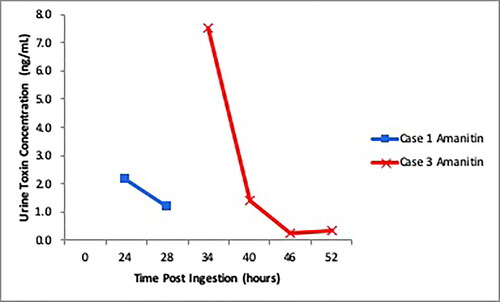
A 73-year-old man presented to hospital “A” with vomiting and diarrhea 12 h after consuming 2 large caps of white foraged mushrooms. The mushrooms were later identified by a mycologist as Amanita virosa. Aminotransferases, creatinine, and INR were normal on presentation: AST 33 IU/L, ALT 46 IU/L, creatinine 108 µmol/L (1.22 mg/dL), and INR 1.0. He was volume resuscitated, given an octreotide infusion (50 mcg/hour IV), and a cyclosporine infusion (5 mg/kg IV over 4–6 h every 24 h) started 28 h post ingestion for 48 h. His aminotransferases peaked at AST 144 and ALT 190 approximately 46 h post-ingestion with normalization of creatinine to 66 µmol/L (0.75 mg/dL). Alpha-amanitin was detected in his urine samples up to 28 h post-ingestion (). He had improvement in his symptoms and normalization of aminotransferases 6 days post-ingestion. INR remained normal at 1.1.
Case 2 and 3
A mother and daughter presented to hospital ‘B’ 22 h after ingesting a cooked meal made with foraged mushrooms, later identified as Lepiota spp. They were both symptomatic with vomiting and diarrhea. The 53-year-old mother (Case 2) presented with elevated aminotransferase (ALT 86 IU/L) and creatinine (100 µmol/L (1.13 mg/dL)). AST and ALT peaked after 78 h at 950 IU/L and 989 IU/L respectively, INR remained normal (1.2), with normalization of creatinine (51 µmol/L (0.58 mg/dL)). The 16-year-old daughter (Case 3), despite having severe diarrhea, had normal bloodwork (AST 17 IU/L, ALT 13 IU/L, creatinine 48 µmol/L (0.54 mg/dL), INR 1.1) throughout her admission. Alpha-amanitin was detected in urine samples up to 52 h post-ingestion for Case 3 (). Each patient received intravenous fluids, octreotide (50 mcg/hour IV), and cyclosporine (5 mg/kg IV over 4-6 h every 24 h) started around 24 h post ingestion until resolution of aminotransferases (96 h for Case 2), and 48 h (Case 3). Both patients had normal aminotransferases and resolution of symptoms by 5 days post-ingestion.
Detection of amatoxin
Urine and plasma samples were analyzed for alpha-amanitin using liquid chromatography-tandem mass spectrometry () [Citation7]. The limit of detection of the toxins was 0.2 ng/mL urine and 0.5 ng/mL plasma with a linear assay response from 0 − 10 ng/mL urine and plasma.
Inhibitors of OATP1B3-mediated alpha-amanitin transport and cellular toxicity in-vitro
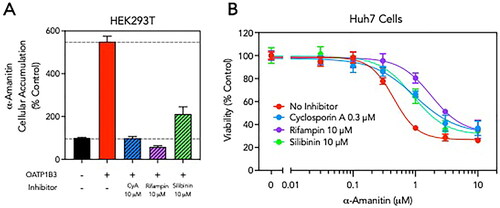
We evaluated the ability of cyclosporine, rifampin, and silibinin to inhibit OATP1B3 in cultured HEK293T cells. At equimolar concentrations (10 µM), both cyclosporine and rifampin completely inhibited OATP1B3-mediated cellular uptake of alpha-amanitin, while silibinin was less effective (). Next, we treated cultured human hepatoma Huh7 cells that natively express OATP1B3 and MRP2 with alpha-amanitin and monitored cell viability after 48 h using a mitochondrial methylthiotetrazole (MTT) reduction assay [Citation8]. Alpha-amanitin was toxic to Huh7 cells with an estimated TD50 of 0.4 µM (). Co-treatment of Huh7 cells with the OATP1B3 inhibitors, and specifically a clinically relevant concentration of cyclosporine, resulted in a right-shift in the alpha-amanitin toxicity curves, indicating protective properties ().
Figure 2. OATP1B3 inhibitors reduce α-amanitin cellular uptake and toxicity. (A) Cultured HEK293T cells were transfected with OATP1B3 expression plasmids and cellular accumulation of α-amanitin (5 µM) with and without co-incubation with cyclosporine A (CyA, 10 µM), rifampin (10 µM) or silibinin (10 µM) was determined by LC-MS/MS. Results are normalized to α-amanitin uptake in control cells not expressing OATP1B3. (B) Cultured human hepatoma Huh7 cells were treated with increasing concentrations of α-amanitin (0–10 µM) in the absence or presence of a constant concentration of the OATP1B3 inhibitor cyclosporine A (0.3 µM), rifampin (10 µM) or silibinin (10 µM). After 48 h, cell viability was assessed using spectrophotometric MTT assay. Results are normalized to cells not treated with α-amanitin. Data are shown as mean ± standard error.
Discussion
We report the first case series of patients treated with cyclosporine for confirmed symptomatic amatoxin ingestions. Our in vitro data illustrate that cyclosporine inhibits OATP1B3-mediated alpha-amanitin uptake and improves viability of alpha-amanitin exposed cultured human hepatoma Huh7 cells, similar to previously published results [Citation4]. This validates that in-vitro inhibition of hepatocyte uptake of amatoxin via a potent OATP1B3 inhibitor such as cyclosporine can decrease the degree of hepatotoxicity of alpha-amanitin.
In our small case series, all patients presented in a delayed fashion with characteristic symptoms of amatoxin exposure, including severe gastrointestinal symptoms starting >6 h after ingestion. We were able to confirm exposure to amatoxin both by mycological identification of the consumed mushrooms and detection of alpha-amanitin in urine samples of Case 1 and 3 patients. While alpha-amanitin was not detected in the urine of the patient in Case 2, this patient prepared and shared the meal with the patient in Case 3 suggesting that both were exposed to the same mushrooms. We did not detect alpha-amanitin in any of the plasma samples in keeping with the known short plasma half-life of alpha-amanitin [Citation9]. All three patients received standard anti-rejection doses (5 mg/kg IV over 4–6 h every 24 h) of cyclosporine as soon as possible after presentation once a high clinical suspicion of amatoxin exposure was confirmed. Cyclosporine has an established safety profile with renal dysfunction and hypertension being chronic adverse effects that would not be expected with short-term use such as for amatoxin poisoning. All three patients tolerated the cyclosporine administration with no adverse effects identified. Two patients (Cases 1 and 2) developed a transient rise in aminotransferases. Both had complete clinical recovery and normalization of liver enzymes after several days. Additional treatments such as N-acetylcysteine were not administered in any of these cases.
We hypothesize that inhibition of OATP1B3 is the main mechanism of action for cyclosporine to limit hepatotoxicity as demonstrated by our in vitro data. There may be an additional beneficial effect of cyclosporine. Other research suggests that cyclosporine may have a role in rescue treatment of patients with acute liver injury by inhibiting hepatic cell apoptosis via the p53 pathway [Citation10]. This suggests that cyclosporine may have some potential benefit in treating hepatotoxicity from mushroom exposures even when given late, after alpha-amanitin has been taken up by the hepatocyte.
Treatment of amatoxin-containing mushroom poisoning through inhibition of the OATP1B3 transporter has been attempted previously with other xenobiotics. High dose, intravenous benzylpenicillin has modest biochemical effect but unfortunately does not improve mortality [Citation11]. Silibinin, as an intravenous formulation (Legalon®), has been used in Europe for almost 40 years (not readily available in North America) for treatment of mushroom poisonings [Citation12]. A 2012 retrospective review of case series authored by employees of the company marketing Legalon®, concluded that silibinin reduces mortality by 50% if you compare death rates prior to its European availability [Citation12]. In 2010, a prospective, Phase II, multicenter, open-label study of intravenous silibinin for amatoxin poisonings in the U.S. was initiated (NCT00915681) [Citation13]. However, in 2020, the sponsor terminated the trial for undisclosed reasons [Citation13], raising doubts about the ultimate final reporting of study outcomes. Additionally, challenges in acquisition of silibinin would often lead to a delay in administration, a less-than ideal scenario when the goal is prevention of toxin uptake into end-organs [Citation2]. Our in vitro studies corroborate that cyclosporine is a more potent inhibitor of the OATP1B3 than silibinin [Citation4]. For rifampin, the experience in amatoxin ingestions is restricted to a single report of two cases in Australia [Citation3]. While intravenous rifampin is available in Canada, it requires import with delayed accessibility through the Health Canada Special Access authorization process. Typically, oral medications are difficult to administer in this patient population due to the severe gastrointestinal symptoms associated with the toxicity, limiting the utility of oral rifampin. IV cyclosporine on the other hand is readily accessible and often stocked in hospital pharmacies which circumvents the issues with delay to administration.
The major limitation of this case series is small sample size. Further prospective studies are underway at our centre to study the safety and efficacy of cyclosporine as a therapeutic for amatoxin-containing mushroom exposures. At the time these case series patients were treated, a research treatment protocol [Citation14] of intravenous silibinin for amatoxin poisonings in the U.S. included IV octreotide (to reduce gallbladder contractility and theoretically reduce enterohepatic recirculation of the toxin). Based upon this, our three patients received IV octreotide in their treatment. Although we did not identify any adverse effects of this treatment, no further evidence has emerged since then to support the use of octreotide for this indication. Furthermore, we considered that octreotide is also a known OATP inhibitor [Citation15], with a further theoretical role in altering the pharmacokinetics of amatoxin. On further analysis, measured serum octreotide levels in our case series patients (unpublished results) were not sufficiently high to act as an OATP inhibitor making the role of octreotide for this indication uncertain.
Conclusion
Cyclosporine is a readily available, potent inhibitor of the hepatocyte OATP1B3 transporter and may be useful as a treatment to decrease uptake of amatoxin into human hepatocytes and potentially reduce hepatotoxicity in amatoxin-poisoned patients. Further work to elucidate the role of cyclosporine in the prevention of amatoxin toxicity is required.
Disclosure statement
No potential competing interest reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Refere nces
- Liu J, Chen Y, Gao Y, et al. N-acetylcysteine as a treatment for amatoxin poisoning: a systematic review. Clin Toxicol (Phila). 2020 Nov;58(11):1015–1022. PMID: 32609548.
- Le Daré B, Ferron PJ, Gicquel T. Toxic effects of amanitins: repurposing toxicities toward new therapeutics. Toxins (Basel). 2021 Jun;13(6):417. PMID: 34208167; PMCID: PMC8230822.
- Zuker-Herman R, Tong R, Wong A. Intravenous rifampicin use in the management of amanita phalloides toxicity. Clin Toxicol (Phila). 2021 Sep;59(9):843–845. PMID: 33605821.
- Letschert K, Faulstich H, Keller D, et al. Molecular characterization and inhibition of amanitin uptake into human hepatocytes. Toxicol Sci. 2006 May;91(1):140–149. Epub 2006 Feb 22. PMID: 16495352.
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009 Oct;158(3):693–705. PMID: 19785645; PMCID: PMC2765590.
- Agha RA, Sohrabi C, Mathew G, ROCESS Group, et al. The PROCESS 2020 guideline: updating consensus preferred reporting of CasESeries in Surgery (PROCESS) Guidelines. Int J Surg. 2020;84:231–235.
- Zhang S, Zhao Y, Li H, et al. A simple and high-throughput analysis of amatoxins and phallotoxins in human plasma, serum and urine using UPLC-MS/MS combined with PRiME HLB μElution platform. Toxins (Basel). 2016 May;48(5):128. PMID: 27153089; PMCID: PMC4885043.
- Jouan E, Le Vee M, Denizot C, et al. Drug transporter expression and activity in human hepatoma HuH-7 cells. Pharmaceutics. 2016;9(4):3.
- Sun J, Niu YM, Zhang YT, et al. Toxicity and toxicokinetics of amanita exitialis in beagle dogs. Toxicon. 2018;143:59–67.
- Yu W, Zhang X, Liu J, et al. Cyclosporine a suppressed glucose oxidase induced P53 mitochondrial translocation and hepatic cell apoptosis through blocking mitochondrial permeability transition. Int J Biol Sci. 2016;12(2):198–209.
- Enjalbert F, Rapior S, Nouguier-Soule J, et al. Treatment of amatoxin poisoning: 20-year retrospective analysis. J Toxicol Clin Toxicol. 2002;40(6):715–757.
- Mengs U, Pohl RT, Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012 Aug;13(10):1964–1970. PMID: 22352731; PMCID: PMC3414726.
- https://clinicaltrials.gov/ct2/history/NCT00915681?V_14=View#StudyPageTop.
- Goupil RC, Davis M, Kaufman A, et al. Clinical recovery of 5 dogs from amatoxin mushroom poisoning using an adapted Santa Cruz protocol for people. J Vet Emerg Crit Care (San Antonio). 2021 May;31(3):414–427. PMID: 33458945.
- Visentin M, Stieger B, Merz M, et al. Octreotide inhibits the bilirubin carriers organic anion transporting polypeptides 1B1 and 1B3 and the multidrug resistance-associated protein 2. J Pharmacol Exp Ther. 2015 Nov; 355(2):145–151. PMID: 26330539.