The primary goal of testing for tuberculosis infection is to identify individuals who are at increased risk for the development of tuberculosis disease and who therefore would benefit from tuberculosis preventive treatment.
Testing for tuberculosis infection is NOT recommended in the following situations:
In persons who have a low risk of infection and if infected;
To support a tuberculosis disease diagnosis in adults and adolescents >12 years of age;
For routine mass screening of individuals outside of contact investigations or occupational screening programs; and
For the monitoring of tuberculosis disease treatment response.
Both the tuberculin skin test and interferon-gamma release assay are acceptable alternatives for tuberculosis infection diagnosis. Either test can be used for tuberculosis infection screening in any of the situations in which testing is indicated. However, there are preferences and exceptions:
An interferon-gamma release assay is the preferred test when:
children over two years of age and less than 10 years of age previously received a Bacille Calmette-Guérin (BCG) vaccine against tuberculosis;
persons at least 10 years of age received a BCG vaccine after infancy (older than one year of age), or received a BCG vaccine more than once and/or are uncertain about when they received a BCG vaccine;
adequate training and quality assessment and control are NOT available for tuberculin skin test administration and/or reading, but personnel and facilities to perform interferon-gamma release assays are available;
a person is unable or unlikely to return to have their tuberculin skin test read; or
tuberculin skin testing is contraindicated.
The tuberculin skin test is the preferred test when serial testing is planned to assess risk of new infection (ie, conversions). This includes repeat testing in a contact investigation, or serial testing of health care workers or other populations (eg, corrections staff or prison inmates) with potential for ongoing exposure. In these situations, interferon-gamma release assays are not acceptable.
Both tuberculosis infection diagnostic tests may be used sequentially in the following situations:
If either the tuberculin skin test or interferon-gamma release assay are negative, the other test may be used to increase sensitivity if the risk for infection is high, the risk for progression to tuberculosis disease is elevated, the risk for a poor outcome from tuberculosis disease is high and/or a person has conditions or habits that may reduce the sensitivity of the test.
If the initial tuberculin skin test is positive, but the likelihood of tuberculosis infection is low, or risk of a false positive result due to BCG is high, then an interferon-gamma release assay may be used to increase specificity.
When interpreting a tuberculosis infection diagnostic test result and considering whether someone is at risk of developing tuberculosis disease and would likely benefit from tuberculosis preventive treatment, the diagnostic test result should be considered in light of other factors, including the pretest probability for the person being truly infected, the individual risk of developing tuberculosis disease and the ability of the test to identify persons at risk of tuberculosis disease (ie, predictive value). Online tools exist to support this interpretation.
KEY POINTS
1. Introduction
While diagnosis and treatment of individuals with tuberculosis (TB) disease is the first priority for tuberculosis prevention and care, an important second priority is identification and treatment of individuals with TB infection, but without disease. In most individuals, Mycobacterium tuberculosis (M. tuberculosis) infection is contained initially by host defenses, and infection remains silent (latent).Citation1 However, TB infection has the potential to develop into TB disease at any time. Several risk factors, such as time since tuberculosis exposure, medical conditions, treatments or personal habits that affect host immunity, can affect an individual’s risk for progression from TB infection to TB disease.Citation2 Identification and treatment of TB infection can substantially reduce the risk of development of TB diseaseCitation3 (see Chapter Citation6: Tuberculosis Preventive Treatment in Adults) and thus has potential to protect the health of the individual from immediate and long-term health effects associated with TB disease, as well as protecting the public by reducing the number of potential sources of future transmission.Citation4,Citation5
There are 2 types of tests to identify TB infection: the tuberculin skin test (TST) and the interferon-gamma release assay (IGRA). The TST consists of an intradermal injection of a small amount of purified protein derivative (PPD) derived from a nonspecific mixture of antigens from M. tuberculosis bacteria.Citation6 In a person who has previously been infected and developed cell-mediated immunity to these tuberculin antigens, a delayed hypersensitivity reaction will occur within 48 to 72 hours. The reaction will cause localized swelling and will be manifest as induration of the skin at the injection site.Citation7 IGRAs are in vitro blood tests of cell-mediated immune response; they detect interferon-gamma released by a person’s T cells following stimulation by exogenous antigens specific to M. tuberculosis.Citation8,Citation9
2. Indications for TB infection testing and goal of testing
The primary goal of testing for TB infection is to identify individuals who are at increased risk for the development of TB disease and therefore may benefit from treatment of TB infection to prevent TB disease (often referred to as tuberculosis preventive treatment [TPT]). In some situations, testing for TB infection is performed among individuals at increased risk of TB exposure, those who regularly interact with vulnerable populations, or who may be at increased risk of adverse outcomes should TB disease develop.
Testing for TB infection among persons or groups who are healthy, have a low risk of exposure and/or have a low risk of progressing to TB disease is discouraged. The positive predictive value of any TB infection diagnostic test for progression to TB disease is low.Citation10 Since the risks of TPT may outweigh the potential benefits of averted TB disease (see Chapter Citation6: Tuberculosis Preventive Treatment in Adults) in populations at low risk of progression, routine mass screening of entire populations (e.g., all migrants) is discouragedCitation4 except in very specific circumstances (such as during contact investigations or occupational screening programs).
Neither the TST nor IGRA can distinguish between TB infection and TB disease,Citation8 as both tests measure host immune response, which is detectable in both TB infection and TB disease.Citation11 For this reason, when someone being screened for TB infection tests positive with a TST and/or IGRA, further testing is required to rule out TB disease (see Chapter Citation6: Tuberculosis Preventive Treatment in Adults for further discussion). Due to temporary anergy caused by TB disease, sensitivity of both tests is greatly reduced, and specificity is suboptimal for the diagnosis of TB disease.Citation12 Therefore, neither the TST nor IGRA should be used for diagnosis of TB disease in adults and adolescents (see Chapter Citation9: Pediatric Tuberculosis for discussion on children ≤12 years). Similarly, serial testing with TST or IGRA during TB disease treatment is not useful to monitor treatment response. Systematic reviews and studies evaluating both qualitative and quantitative changes in TST and IGRA response have shown low reversion rates (positive-to-negative) during and after successful treatment for TB disease.Citation13,Citation14 In addition, reversion of TST and IGRA may occur spontaneously in absence of treatment.Citation15
If testing for TB infection is performed, there must be an a priori commitment to providing TPT or active monitoring should test results be positive. In general, testing for TB infection should consider patient preferences toward treatment and is indicated when there is expected individual benefit from TPT.
Recommendation
We strongly recommend against testing for TB infection in the following situations: (1) in persons who have a low risk of infection and a low risk of progressing to TB disease if infected; (2) to support a TB disease diagnosis in adults and adolescents >12 years of age; (3) for routine mass screening of individuals outside of contact investigations or occupational screening programs; and (4) for the monitoring of TB disease treatment response (good evidence).
3. TB infection diagnostic tests
3.1. Tuberculin skin test
Five tuberculin units of Tubersol® PPD-Standard is recommended for use in Canada. New, more specific tuberculin testing products have been developed and recently recommended by the World Health Organization (WHO),Citation16 but as of writing, are not yet available in Canada. The only internationally recommended method of tuberculin skin testing is the Mantoux technique, which consists of intradermal injection of tuberculin material on the inner surface of the forearm.Citation7 An induration is measured on a second visit that occurs 48-72 hours after administration. As there are multiple visits to complete a TST, some persons may be unable or unlikely to return for reading—for these individuals, an IGRA should be considered (see next section on IGRAs).Citation16 Details on TST administration, contraindications, precautions and measurement of induration are contained in Appendix 1, Section A.1 at the end of this chapter. Administration and interpretation of TST requires adequate training and quality assurance measures. Methods and tools of quality assessment for TST placement and induration measurement have been described in mobile TST (mTST) protocols and are supported with accompanying videos.Citation17,Citation18 TST is appropriate for use at any age.
Persons with TB infection may display a wide range of induration sizes.Citation19–21 Setting a smaller induration size for a positive result will increase sensitivity (fewer false negatives) but will reduce specificity (more false positives); setting a larger induration size for a positive result will reduce sensitivity but increase specificity. The size of the induration is associated with risk of developing TB disease.Citation10 presents cutoff thresholds for TST interpretation.
Table 1. Interpretation of TST results and cutoff thresholds in various populations.Citation 7
3.2. Interferon-gamma release assays
IGRAs are in vitro blood tests of cell-mediated immune response. IGRAs measure T-cell release of interferon-gamma following stimulation by antigens specific to M. tuberculosis — specifically, early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10).Citation22 These antigens are more specific for M. tuberculosis than antigens contained in PPD because they are not shared with any BCG vaccine strains or most species of non-tuberculous mycobacteria (NTM) (other than M. marinum, M. kansasii, M. szulgai and M. flavescens).Citation8 Infection with any of these specifically listed NTM strains could create a false positive IGRA.Citation23
A key advantage of IGRA is that, as opposed to TST, they do not require a return visit by the patient to have their result interpreted and recorded. If a patient is unable to return to have a TST read or if there is a concern a patient will not return to have a TST read, an IGRA, therefore, offers logistical advantages.
Two IGRAs are currently available in Canada: the QuantiFERON-TB Gold Plus (QFT-Plus) assay (Cellestis/Qiagen, Carnegie, Australia), and the T-SPOT.TB assay (Oxford Immunotec, Abingdon, United Kingdom). Both tests are approved by Health Canada. The WHO has recently concluded that Beijing Wantai’s TB-IGRA has comparable performance to other IGRAs and is an acceptable option, however, as of writing, this IGRA is not yet Health Canada-approved or available in Canada.Citation25
The QFT-Plus assay is an enzyme-linked immunosorbent assay (ELISA)-based, whole-blood test that uses ESAT-6 and CFP-10 antigens in an in-tube format (consisting of a TB1 and TB2 tube). The QFT-Plus assay is the fourth generation QuantiFERON assay, following initial generations QFT, QFT-Gold, and QFT-Gold-in-Tube. The major difference between QFT-Plus and the third generation QFT-Gold-in-Tube, is that QFT-Plus does not contain TB7.7 antigens and uses a second tube (TB2) that may stimulate CD8 T-cells (in addition to CD4 T-cells stimulated in the TB1 tube).Citation24,Citation25 The amount of interferon-gamma detected is reported quantitatively in international units (IU) per milliliter. A positive result for M. tuberculosis infection occurs if the TB1 and/or TB2 tube has an interferon-gamma response to TB antigens that is ≥0.35 IU above the negative control.Citation26
The T-SPOT.TB assay is an enzyme-linked immunospot (ELISPOT) assay performed on separated and counted peripheral blood mononuclear cells (PBMCs); it uses the same two peptides as the QFT-Plus assay. The result is reported as number of interferon-gamma-producing T cells (spot-forming cells). A positive result for M. tuberculosis infection occurs if the spot counts in the TB antigen wells exceed a specific threshold relative to the control wells, equal to 8 spots in Canada.Citation27,Citation28
Both commercial IGRAs (QFT-Plus and T-SPOT.TB) are acceptable to use; they differ, however, in terms of laboratory expertise required, cost, pre-analytical steps and ease of use. The decision on which commercial IGRA to offer is left to the discretion of provincial, commercial and hospital laboratories. In Canada, IGRAs should only be used among those older than 2 years of age (see Chapter Citation9: Pediatric Tuberculosis).
Indeterminate or invalid responses with IGRA occur when positive or negative control responses are too low or too high, respectively.Citation28,Citation29 Causes of indeterminate or invalid IGRA responses are varied and may be due to inappropriate storage, delays in analysis or technical or patient factors. In the event of an indeterminate response, the test should be repeated. In the rare event that responses are consistently indeterminate,Citation30 consider using an alternate IGRA (ie, if consecutively indeterminate with QFT-Plus use T-SPOT.TB) or a TST.
The manufacturer-recommended cut points for interpretation were chosen to maximize sensitivity and specificity. Unlike the TST, the manufacturer has not provided a gradient of interpretation for a positive test (such as those defined in ); instead, results are dichotomous. Interferon-gamma responses may be dynamic and results that are close to manufacturer-recommended cut points for a positive test should be interpreted cautiously. There is a manufacturer-defined “borderline” zone for T-SPOT.TB that is equal to 5-7 spots (a positive result according to the manufacturer is 8 spots or more), which recognizes this uncertainty. However, there is no such manufacturer-defined borderline zone for QFT. We suggest using a “borderline” zone for QFT that ranges from ≥0.2 to ≤1.0 IU/ml (a positive result according to the manufacturer is 0.35 IU/ml or greater).Citation31,Citation32
Recommendation
We strongly recommend both the tuberculin skin test and interferon-gamma release assay as acceptable alternatives for TB infection diagnosis. Either test can be used for TB infection screening in any of the situations in which testing is indicated. However, there are preferences and exceptions detailed in subsequent recommendations (good evidence).
Good practice statements
When the initial interferon-gamma release assay result is indeterminate or invalid, the interferon-gamma release assay should be repeated or a tuberculin skin test be performed.
When the initial interferon-gamma release assay result is borderline (equivalent to 5-7 spots with T-SPOT.TB or 0.2 to 1.0 IU/ml with QFT), the interferon-gamma release assay may be repeated or a tuberculin skin test used to help arrive at a diagnosis.
3.3. TST and IGRA accuracy for TB infection
There is no gold-standard test to confirm the presence or absence of TB infection. In lieu of a gold standard, surrogate populations are used to estimate the sensitivity and specificity of TB infection diagnostic tests. Typically, sensitivity is estimated among persons who have TB disease. There are limitations to this reference standard, as persons with TB disease may experience temporary anergy, causing a negative TST or IGRA. Specificity is estimated among persons who are at very low risk of exposure to M. tuberculosis and NTM. This too has limitations, as it is not possible to completely rule out exposure. However, in the absence of better reference standards, these surrogates have been utilized.Citation19,Citation22,Citation27
3.3.1. Tuberculin skin test
The sensitivity of the TST among persons with TB disease has been estimated in systematic reviews and meta-analyses.Citation11,Citation27,Citation33–35 One of the largest meta-analyses suggests that the TST at a threshold of ≥10 mm has a sensitivity of 77% (95% CI: 72 to 81%).Citation27 Among children, TST sensitivity is similar.Citation36
Among persons with immunocompromising conditions (such as HIV), sensitivity of the TST is significantly lower. In one meta-analysis, sensitivity of the TST at a threshold of ≥5 mm was estimated to be 60% (95% CI: 34 to 82%) among people living with HIV.Citation37 For other populations with immunocompromising conditions, similar reductions in sensitivity are found, such as among persons receiving dialysis, where sensitivity is 50-55%.Citation38–40
The specificity of the TST is primarily affected by the presence of NTM infection and/or previous BCG vaccination.Citation20 Among persons without a history of BCG vaccination, TST specificity was estimated in a meta-analysis to be 97% (95% CI: 95 to 99%).Citation27 Among BCG-vaccinated persons, TST specificity was estimated in the same meta-analysis to be 59% (95% CI: 46 to 73%).Citation27 However, studies conducted in Canada and several other countries show that if BCG was received in infancy (the first year of life), only 1% had a TST result of ≥10 mm if tested >10 years later.Citation20 Therefore, a history of BCG vaccination received in infancy can be ignored as the cause of a positive result in all persons aged 10 years and older when interpreting an initial TST reaction of 10 mm or greater.Citation20,Citation41–44
BCG vaccination should only be considered the likely cause of a positive TST if BCG vaccine was given after 12 months of age AND there has been no known exposure to TB disease or other risk factors for TB infection AND the person is either Canadian-born non-IndigenousCitation45 OR an immigrant/visitor from a low-TB-incidence country (<50 cases per 100,000 persons per year).Citation46 International TB incidence rates are available from the WHO TB data repository (https://www.who.int/teams/global-tuberculosis-programme/data).
In a prospective study in the USA of more than 20,000 persons, including both US-born persons and persons born abroad, TST and IGRA positivity varied.Citation30 Among US-born persons, the proportion testing positive with a TST and IGRA did not vary; however, among persons born abroad, IGRA positivity was substantially lower than TST positivity, likely due to the prevalence of BCG vaccination in the cohort (64.5%). In this study, timing of BCG vaccination was not collected, and longitudinal incidence of TB disease was not reported. In practice, when there is uncertainty about the timing of BCG vaccination, it is recommended to consult the BCG vaccination policies in different countries, as summarized in the recently updated BCG World Atlas (http://www.bcgatlas.org/ - see ).Citation47 However, if uncertainty remains regarding BCG vaccination and its timing, it is best to use an IGRA, if available, as the proportion of people testing false positive is expected to be lower.
3.3.2. Interferon-gamma release assay
A meta-analysis estimating the sensitivity and specificity of QFT-Plus suggests there is no significant difference in sensitivity (1.3% higher; 95% CI: 0.3% lower to 2.9% higher) and specificity (0.9% lower; 95% CI: 2.4% lower to 0.6% higher) compared to the previous generation QFT.Citation48 A previous meta-analysis of older QFT generations suggested sensitivity is 78% (95% CI: 73 to 81%), while among 13 studies of T-SPOT.TB, sensitivity was estimated to be 90% (95% CI: 86 to 93%).Citation27
Similar to the TST, IGRA sensitivity is reduced among people with immune-compromising conditions.Citation37,Citation49 IGRAs also have elevated rates of indeterminate results due to failure of positive controls in these populations. Sensitivity for T-SPOT.TB among people living with both HIV and TB disease was estimated to be 76% in one meta-analysis, while sensitivity for a previous generation QFT (QFT Gold) was estimated to be 69%.Citation50 A prospective study among people living with both HIV and TB disease tested with QFT-Plus exhibited similar sensitivity.Citation51
Both IGRAs are unaffected by BCG vaccination. For both QFT-Plus and T-SPOT.TB, specificity is high, around 98 and 93%, respectively.Citation27
There is evidence for previous QFT generations that the strength in response (higher IU/ml) may be associated with increased risk of developing TB disease in a dose-dependent manner (like magnitude of TST induration size).Citation52 Similar findings for T-SPOT.TB were found in a large prospective study in the United Kingdom.Citation53 A comparable analysis for the current generation of QFT (QFT-Plus) has not been performed.
Recommendation
We conditionally recommend that an interferon-gamma release assay is preferred over a tuberculin skin test in the following situations: (1) When children over 2 years of age and less than 10 years of age previously received a Bacille Calmette-Guérin vaccine (BCG); (2) when persons at least 10 years of age received a BCG vaccine after infancy (older than 1 year of age), received a BCG vaccine more than once and/or are uncertain about when they received a BCG; (3) when adequate training and quality assessment and control are NOT available for tuberculin skin test administration and/or reading, but personnel and facilities to perform interferon-gamma release assays are available; (4) when a person is unable or unlikely to return to have their tuberculin skin test read; and (5) when the tuberculin skin test is contraindicated (poor evidence).
3.4. TST and IGRA prediction of TB disease
Both the TST and IGRA exhibit low predictive value for the development of TB disease.Citation10 Although predictive value for TB disease is low, studies consistently show risk of TB disease is highest in the first 2 years following infection, then subsequently declines thereafter.Citation54
How well a positive TST and/or IGRA correlates with risk of developing TB disease is a key consideration when offering TPT, and thus may influence a decision to test. Knowing the annual risk of developing TB (i.e., the incidence rate) after a positive test is therefore especially useful in clinical settings. As both TST and IGRA sensitivity is imperfect, even people testing negative with either or both may still go on to develop TB disease. Knowing how much greater risk an individual has for developing TB disease with a positive vs negative test (ie, the incidence rate ratio [IRR]) is also useful in clinical settings to support decisions on which test to use, as well as to support TPT decisions once a test result is known.
Four systematic reviews published in 2020 evaluated the incidence rate of TB disease among different populations testing positive with a TST or IGRA who did not receive TPT.Citation2,Citation55–57 These reviews also evaluated the IRR of TB disease among different populations testing positive vs. negative with a TST or IGRA. The findings of these reviews are summarized in and categorized based on very high, high, moderate, or low risk of TB disease among persons testing positive. For populations thought to be at increased risk of TB disease where estimates of risk after a positive TST or IGRA were unavailable, risks were extrapolated from studies comparing risk of TB disease among persons with vs without a risk factor. The following sub-sections further describe the evidence base supporting .
Table 2. Risk of TB disease and the incidence rate ratio of TB disease among different populations stratified by risk.
3.4.1. General (adult) population
Contemporary estimates of TB disease risk in the general (adult) population suggest risks are low. Among three studiesCitation58–60 (from British Columbia, Saskatchewan and Florida) estimating the absolute risk of TB disease among 33,811 healthy, low-risk tuberculin reactors (TST ≥10 mm) followed for an average of 7.4 years, only 55 (0.2%) developed TB disease. This is equivalent to an annual risk of TB disease of approximately 0.03% per year.
3.4.2. TB contacts
Persons with recent tuberculosis exposure (tuberculosis contacts) are a priority group for TB infection screening and treatment, because of their high risk of progression to TB disease. Risk for progression to TB disease is highest among child and adolescent contacts (<18 years; highest among those <5 years).Citation2,Citation56,Citation57 TB infection diagnostic tests also have good ability to discriminate risk among adult contacts with IRRs ranging from 4.1 to 19.0.Citation2
3.4.3. People living with HIV
People living with HIV are another priority group for TB infection screening and treatment. Among people living with HIV, the use of antiretroviral therapy has been shown to reduce risk of progression to TB disease.Citation61 Provision of TPT further reduces this risk.Citation62 In a systematic review, both the TST and IGRA exhibited identical ability to discriminate between people living with HIV at high vs. low risk of progression, with an IRR of 11.0.Citation2 Among people living with HIV not receiving antiretroviral therapy and with a negative TST or IGRA, re-testing after antiretroviral therapy has been established may be warranted in certain clinical contexts, as high rates of conversion 6-12 months after initiation due to immune reconstitution have been observed; this effect may be modulated by CD4 levels.Citation63–68
3.4.4. People with non-HIV immunocompromising conditions
Risk of progression to TB disease among populations with non-HIV immunocompromising conditions is elevated, although the data are more limited and heterogeneous.Citation2,Citation56 In nearly all populations, the IRR of both TST and IGRA are diminished due to reduced immune function. Citation2
Risk of TB disease among persons with chronic kidney disease increases with worsening kidney function caused by uremia-induced immune impairment. One study estimated a 5% increase in TB disease risk for every 10 mL/min/1.73 m2 decrease in the estimated glomerular filtration rate.Citation69 TB disease risk among persons with a positive TST or IGRA is highest among those requiring dialysis, estimated to be 0.3 to 1.2% per year in a meta-analysis.Citation2 Among persons who had received a transplant, annual risk of TB disease among those with a positive TST or IGRA is 0.1 to 0.7%.Citation2,Citation56 Similarly, persons receiving immunosuppressant drugs for inflammatory diseases (such as tumor necrosis factor α inhibitors or steroids), have an annual risk of TB disease of 0.5% with a positive TST or IGRA.Citation2 Certain cancers have been implicated in risk of TB disease. A large retrospective study of migrants to British Columbia suggests risk of TB disease is highest among those with lung cancer, sarcoma, leukemia, lymphoma and gastrointestinal cancers, on the order of 3 to 11 times that of persons without these cancers after adjusting for several potential confounders.Citation70 This extrapolates to an annual risk of TB disease of approximately 0.1 to 0.4% among persons with these cancers. Systematic reviews have also found that cancers elevate the risk of TB.Citation71 Among people with diabetes, risk is likely increased by other health conditions (eg, chronic kidney disease) and is higher among those with poorly controlled diabetes, although data are mixed.Citation2,Citation72–74
3.4.5. Other populations
Primary data for the absolute risk of progression to TB disease among populations with certain habits or chest x-ray abnormalities and a positive TST or IGRA are scarce. However, many studies have evaluated the relative risk of TB disease based on these risk factors,Citation75–82 allowing approximations of absolute risk based on a known general population risk.Citation2
Good practice statement
Among people living with human immunodeficiency virus not receiving antiretroviral therapy and with a negative tuberculin skin test or interferon-gamma release assay, re-testing after antiretroviral therapy has been established may be warranted if the individual’s risk for TB infection is elevated (such as known previous contact).
3.5. Sequential testing
Sequential testing refers to the use of both a TST and an IGRA to help support a decision to proceed with TPT in someone with evidence of TB infection. There are two main situations to consider sequential testing: (1) to improve sensitivity; and (2) to improve specificity.
Improved test sensitivity may be desired in persons with a high suspicion of TB infection and/or who are at increased risk of developing TB disease. In this situation, if the initial test is negative (either TST or IGRA), using another test may improve overall sensitivity (while lowering specificity). In a meta-analysis, among contacts with an initial negative TST (<10 mm), the risk of incident tuberculosis was 5.1 times (95% CI: 2.4 to 10.8) higher among persons with a subsequent positive IGRA versus negative IGRA. In the opposite situation, among contacts with an initial negative IGRA, the risk of incident tuberculosis was 3.6 times (95% CI: 1.8 to 7.2) higher among persons with a subsequent TST ≥10 mm versus TST <10 mm.Citation2
Improved test specificity may be desired in persons with a low suspicion of TB infection and/or who received BCG vaccination post-infancy or at an unknown age and/or may be at increased risk of serious adverse events with TPT. In this situation, if the initial test is positive, using another test may improve overall specificity (while lowering sensitivity).Citation87 Current data suggest that sequential testing to enhance specificity should only be considered if the initial positive test is a TST. In a low-risk individual, such as a healthy adult receiving pre-employment screening, if the initial TST was positive (≥10 mm), the risk of incident tuberculosis is approximately 7.6 times (95% CI: 1.6 to 36.7) higher if the subsequent IGRA is positive. In an opposite scenario, if their initial test was a positive IGRA, the risk of incident tuberculosis is approximately 3.0 times higher (non-significant, with 95% CI: 0.2 to 40.7) if the subsequent TST is ≥10 mm.Citation2
Recommendation
We conditionally recommend sequential testing if:
Either the initial tuberculin skin test or interferon-gamma release assay is NEGATIVE (in which case the other test may be used to increase sensitivity), when:
the risk for infection is high;
the risk for progression to TB disease is elevated;
the risk for a poor outcome from TB disease is high; and/or
a person has conditions or habits that may reduce the sensitivity of the test.
or
The initial tuberculin skin test is POSITIVE (in which case an interferon-gamma release assay may be used to increase specificity), when:
the likelihood of TB infection is low; and/or
the risk of a false positive result due to Bacille Calmette-Guérin is high. (poor evidence).
3.6. Serial (repeated) testing
Both the TST and IGRA exhibit nonspecific variation when they are repeated for various reasons.Citation88–93 If serial testing is to be performed, an understanding of the causes and magnitude of test-to-test variability is important for overall interpretation. There are common and unique causes of variation with both the TST and IGRA (see Appendix 1, Section A.2, Table A1). These factors may result in both quantitative differences in measurement and qualitative differences in interpretation (ie, positive vs negative) for both tests.
Serial testing may occur among populations for two specific reasons: (1) to identify a new TB infection after a recent TB exposure, such as in the context of contact or outbreak investigations; and (2) to identify new TB infections in populations at increased risk of exposure to TB, such as workers in healthcare settings, homeless shelters or correctional facilities.
The TST is the preferred test when conducting serial testing, as conversions have been well described in multiple studies and are well understood.Citation85,Citation89,Citation94,Citation95 If the initial TST is at least 10 mm, there is no clinical utility in repeating it. Among persons with an initial TST <10 mm, a TST conversion occurs when a 6 mm or greater increase occurs between two TSTs or when a second test measures at least 10 mm. TST conversion occurs within 3-to-8 weeks of exposure.Citation89
With IGRAs, there remains no clear consensus on what constitutes a real “conversion” such that the change would indicate new TB infection. In multiple studies of serial testing, conversions and reversions occur frequently.Citation32,Citation96 A systematic review suggests that among health care workers, conversions occur 5 times more frequently with an IGRA than with a TST, with no supporting evidence in the form of observed increases in TB disease incidence.Citation97 Therefore, IGRAs are not recommended in serial testing situations.
Recommendation
We conditionally recommend use of tuberculin skin testing when serial testing is planned to assess risk of new infection (ie, conversions). This includes repeat testing in a contact investigation, or serial testing of health care workers or other populations (eg, corrections staff or prison inmates) with potential for ongoing exposure. In these situations, interferon-gamma release assays are not acceptable (poor evidence).
3.7. TB infection testing in select situations
3.7.1. Contact investigations
In the context of a contact investigation, to identify a true conversion (ie, new infection), a single TST should be performed as soon as possible after an exposure to TB is recognized and the contact is identified. If the first TST is negative and performed less than 8 weeks after contact with the index patient, then a second TST should be scheduled no sooner than 8 weeks after the contact was broken. This also means for contacts that are identified more than 8 weeks after contact with an index patient is broken (eg, casual contacts), a single TST will identify all those with new infection.Citation7,Citation89 For a complete discussion on TB infection testing in contact investigations see Chapter Citation11: Tuberculosis Contact Investigation and Outbreak Management.
3.7.2. Health care workers and other populations
Certain populations may be repeatedly tested for TB infection because they are at elevated risk of exposure. These persons should undergo two-step TSTs prior to any exposure to account for the booster effect.Citation89 This is because the initial TST may elicit an anamnestic immune response in persons with remote TB infection or prior BCG vaccination. This “boosted” immune response results in a greater response if a second TST is administered anytime from a week to more than a year later. In the absence of exposure, the likelihood that this greater reaction to a second test is indicative of true infection is low. This phenomenon is important to detect as it can be confused with new infection if the second test is only performed following exposure to a person with infectious pulmonary TB (see Appendix 1, Section A.3 for conduct and interpretation of two-step TST and Chapter Citation14: Prevention and Control of Tuberculosis Transmission in Healthcare Settings).
3.7.3. Nursing-home and long-term care residents
Performing universal TB infection testing among nursing-home and long-term care residents, prior to or shortly after entry, is discouraged. This is for three reasons. The most important is the high risk of age-related toxicity and the potential for drug-drug interactions in this population,Citation98 which results in low rates of being offered TPT and, if offered, low rates of treatment completion.Citation99–102 Second, the sensitivity of both the TST and IGRA are reduced in older age.Citation103–106 Third, available evidence suggests that TB disease risk is lowCitation101 and transmission is rare in residents of these facilities who develop TB disease.Citation101 In situations of exposure to a potentially infectious person with TB disease, testing for TB infection should be performed on a case-by-case basis, considering the balance of individual risks and benefits associated with treatment and the epidemiological context (see also Chapter Citation14: Prevention and Control of Tuberculosis Transmission in Healthcare Settings).
4. Interpreting and managing TST and/or IGRA results
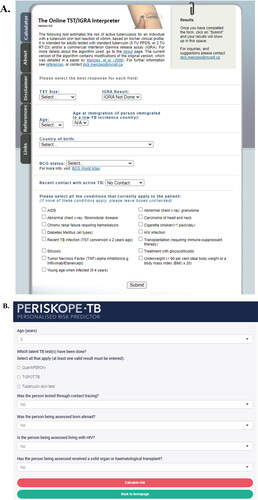
How to interpret a test result depends primarily on the clinical context. There are multiple dimensions to consider when faced with a positive or negative TST or IGRA to help decide whether someone is at risk of developing TB disease and would likely benefit from TPT. These include the pretest probability that the person is truly infected, the individual risk of TB disease and the ability of the test to identify persons at risk of disease (see Appendix 1, Section A.4 for a complete discussion). There are online tools that can help support decisions in interpreting TST or IGRA results among different populations that consider many of these dimensions (). These include The Online TST/IGRA Interpreter, Version 3.0 (http://tstin3d.com/; communicates absolute risk of TB disease and risk of adverse events with treatment; derived from systematic reviews)Citation107 and the PERISKOPE TB (http://www.periskope.org/; communicates absolute risk of TB disease; derived from an individual patient data meta-analysis).Citation56
Figure 2. Online tools that can support decision-making with respect to TST and IGRA interpretation and offering of TPT: The Online TST/IGRA Interpreter, Version 3.0 (A) and PERISKOPE TB (B). Abbreviations: TST, tuberculin skin test; IGRA, interferon-gamma release assay; TPT, tuberculosis preventive treatment.
If a health care provider decides that a TST or IGRA is truly positive, there is no clinical utility in performing a TST or IGRA in the future, so long as the test is properly performed, read and interpreted. All persons with a positive TST or IGRA should be referred for a medical evaluation to rule out TB disease prior to initiating TPT. After TB disease is ruled out, TPT should be started as soon as possible and according to guidance in Chapter Citation6: Tuberculosis Preventive Treatment in Adults.
Good practice statement
There are multiple dimensions to consider when faced with a positive or negative tuberculin skin test or interferon-gamma release assay to help decide whether someone is at risk of developing TB disease and would benefit from tuberculosis preventive treatment. These include the pretest probability that the person is truly infected, the individual risk of TB disease and the ability of the test to identify persons at risk of disease. There are online tools that can help support decisions in interpreting tuberculin skin test or interferon-gamma release assay results among different populations, which consider many of these dimensions.
5. Future research
Key areas of future research with respect to new TB infection diagnostic tests should address the major limitations of the TST and IGRA. Development of tests that don’t require lab infrastructure or a second visit for reading and that also overcome specificity limitations of the TST would make testing more accessible. Furthermore, tests or biomarker signatures that are more predictive for TB disease than the TST and IGRA are needed. Tests that identify those most likely to benefit from TPT would increase the benefit of treatment while substantially reducing the number of persons who would be exposed to the risks of treatment. As the two currently available TB infection diagnostics, the TST and IGRA, are not highly predictive for TB disease, more prospective research is required to improve decision-making surrounding which test to use, at which positive cut-point and in which population, to maximize individual benefits of treatment.
Disclosure statement
The CTS TB Standards editors and authors declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted on the CTS website.
Funding
The 8th edition Canadian Tuberculosis Standards are jointly funded by the Canadian Thoracic Society (CTS) and the Public Health Agency of Canada, edited by the CTS and published by the CTS in collaboration with AMMI Canada. However, it is important to note that the clinical recommendations in the Standards are those of the CTS. The CTS TB Standards editors and authors are accountable to the CTS CRGC and the CTS Board of Directors. The CTS TB Standards editors and authors are functionally and editorially independent from any funding sources and did not receive any direct funding from external sources. The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline and standards panels. No corporate funders played any role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the recommendations presented in this document.
References
- Schluger NW. The Pathogenesis of tuberculosis: the first one hundred (and twenty-three) years. Am J Respir Cell Mol Biol. 2005;32(4):251–256. doi:10.1165/rcmb.F293.
- Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ. 2020;368:m549. doi:10.1136/bmj.m549.
- International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bulletin of the World Health Organization. 1982;60(4):555–564.
- Campbell JR, Dowdy D, Schwartzman K. Treatment of latent infection to achieve tuberculosis elimination in low-incidence countries. PLoS Med. 2019;16(6):e1002824. doi:10.1371/journal.pmed.1002824.
- Marais BJ, Chakaya J, Swaminathan S, et al. Tackling long-term morbidity and mortality after successful tuberculosis treatment. Lancet Infect Dis. 2020;20(6):641–642. doi:10.1016/S1473-3099(20)30167-5.
- Huebner RE, Schein MF, John B, Bass J. The Tuberculin Skin Test. Clin Infect Dis. 1993;17(6):968–975. doi:10.1093/clinids/17.6.968.
- Reichman LB. Hershfield ES. Reichman and Hershfield’s Tuberculosis: A Comprehensive, International Approach. 1400. New York: Informa Healthcare USA; 2006.
- Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet (London, England). 2000;356(9235):1099–1104. doi:10.1016/S0140-6736(00)02742-2.
- Pai M, Riley LW, Colford JM. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4(12):761–776. doi:10.1016/S1473-3099(04)01206-X.
- Abubakar I, Drobniewski F, Southern J, et al. Prognostic value of interferon-gamma release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18(10):1077–1087. doi:10.1016/S1473-3099(18)30355-4.
- Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-γ Release Assays for Active Pulmonary Tuberculosis Diagnosis in Adults in Low- and Middle-Income Countries: Systematic Review and Meta-analysis. The Journal of Infectious Diseases. 2011;204(suppl_4):S1120–S1129. doi:10.1093/infdis/jir410.
- Toossi Z, Ellner JJ. Mechanisms of Anergy in Tuberculosis. In: Shinnick TM, ed. Tuberculosis. Berlin Heidelberg: Springer-Verlag; 1996. p. 221–238. Current Topics in Microbiology and Immunology.
- Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. Utility of Interferon-γ Release Assay Results to Monitor Anti-Tubercular Treatment in Adults and Children. Clin Ther. 2012;34(5):1041–1048. doi:10.1016/j.clinthera.2012.03.006.
- Clifford V, He Y, Zufferey C, Connell T, Curtis N. Interferon gamma release assays for monitoring the response to treatment for tuberculosis: A systematic review. Tuberculosis (Edinb)). 2015;95(6):639–650. doi:10.1016/j.tube.2015.07.002.
- Mancuso JD, Mody RM, Olsen CH, Harrison LH, Santosham M, Aronson NE. The Long-term Effect of Bacille Calmette-Guérin Vaccination on Tuberculin Skin Testing: A 55-Year Follow-Up Study. Chest. 2017;152(2):282–294. doi:10.1016/j.chest.2017.01.001.
- Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(11):1269–1278. doi:10.1016/S1473-3099(16)30216-X.
- Moayedi-Nia S, Barss L, Oxlade O, et al. The mTST – An mHealth approach for training and quality assurance of tuberculin skin test administration and reading. Plos One. 2019;14(4):e0215240. doi:10.1371/journal.pone.0215240.
- McGill TB Centre. TST Training Videos. 2021. https://www.mcgill.ca/tb/resources#aTools. Accessed December 16, 2021.
- Menzies D, Pai M, Comstock G. Meta-analysis: New Tests for the Diagnosis of Latent Tuberculosis Infection: Areas of Uncertainty and Recommendations for Research. Ann Intern Med. 2007;146(5):340–354. doi:10.7326/0003-4819-146-5-200703060-00006.
- Farhat M, Greenaway C, Pai M, Menzies D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis. 2006;10(11):1192–1204.
- Cobelens FG, Egwaga SM, van Ginkel T, Muwinge H, Matee MI, Borgdorff MW. Tuberculin skin testing in patients with HIV infection: limited benefit of reduced cutoff values. Clin Infect Dis. 2006;43(5):634–639. doi:10.1086/506432.
- Pai M, Denkinger CM, Kik SV, et al. Gamma Interferon Release Assays for Detection of Mycobacterium tuberculosis Infection. Clin Microbiol Rev. 2014;27(1):3–20. doi:10.1128/CMR.00034-13.
- van Ingen J, de Zwaan R, Dekhuijzen R, Boeree M, van Soolingen D. Region of Difference 1 in Nontuberculous Mycobacterium Species Adds a Phylogenetic and Taxonomical Character. J Bacteriol. 2009;191(18):5865–5867. doi:10.1128/JB.00683-09.
- Hong JY, Park SY, Kim A, Cho S-N, Hur Y-G. Comparison of QFT-Plus and QFT-GIT tests for diagnosis of M. tuberculosis infection in immunocompetent Korean subjects. J Thorac Dis. 2019;11(12):5210–5217. doi:10.21037/jtd.2019.12.11.
- Shafeque A, Bigio J, Hogan CA, Pai M, Banaei N. Fourth-Generation QuantiFERON-TB Gold Plus: What Is the Evidence? J Clin Microbiol. 2020;58(9):e01950–19. doi:10.1128/JCM.01950-19.
- Moon H-W, Gaur RL, Tien SS-H, Spangler M, Pai M, Banaei N. Evaluation of QuantiFERON-TB Gold-Plus in Health Care Workers in a Low-Incidence Setting. J Clin Microbiol. 2017;55(6):1650–1657. doi:10.1128/JCM.02498-16.
- Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149(3):177–184. doi:10.7326/0003-4819-149-3-200808050-00241.
- Oxford Immunotec. T-SPOT.TB Package Insert. 2017. http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/Final-File-PI-TB-US-V6.pdf. Accessed December 16, 2021.
- QIAGEN. QFT-Plus Package Insert. 2019. https://www.quantiferon.com/us/wp-content/uploads/sites/13/2020/01/L1095849-R06-QFT-Plus-ELISA-IFU.pdf. Accessed December 16, 2021.
- Ho CS, Feng P-JI, Narita M, et al. Comparison of three tests for latent tuberculosis infection in high-risk people in the USA: an observational cohort study. The Lancet Infectious Diseases. 2021;0(0). 2022;22(1):85–96. doi:10.1016/S1473-3099(21)00145-6.
- Ringshausen FC, Nienhaus A, Schablon A, Schlösser S, Schultze-Werninghaus G, Rohde G. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis. 2010;10(1):220. doi:10.1186/1471-2334-10-220.
- Nemes E, Rozot V, Geldenhuys H, et al. Optimization and Interpretation of Serial QuantiFERON Testing to Measure Acquisition of Mycobacterium tuberculosis Infection. Am J Respir Crit Care Med. 2017;196(5):638–648. doi:10.1164/rccm.201704-0817OC.
- Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37(1):100–111. doi:10.1183/09031936.00114810.
- Ai L, Feng P, Chen D, Chen S, Xu H. Clinical value of interferon-γ release assay in the diagnosis of active tuberculosis. Exp Ther Med. 2019;18(2):1253–1257. doi:10.3892/etm.2019.7696.
- Laurenti P, Raponi M, de Waure C, Marino M, Ricciardi W, Damiani G. Performance of interferon-γ release assays in the diagnosis of confirmed active tuberculosis in immunocompetent children: a new systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):131. doi:10.1186/s12879-016-1461-y.
- Sollai S, Galli L, de Martino M, Chiappini E. Systematic review and meta-analysis on the utility of Interferon-gamma release assays for the diagnosis of Mycobacterium tuberculosis finfection in children: a 2013 update. BMC Infect Dis. 2014;14(S1):S6. doi:10.1186/1471-2334-14-S1-S6.
- Santin M, Muñoz L, Rigau D. Interferon-γ Release Assays for the Diagnosis of Tuberculosis and Tuberculosis Infection in HIV-Infected Adults: A Systematic Review and Meta-Analysis. PLOS One. 2012;7(3):e32482. doi:10.1371/journal.pone.0032482.
- Unsal A, Ahbap E, Basturk T, et al. Tuberculosis in dialysis patients: a nine-year retrospective analysis. J Infect Dev Ctries. 2013;7(3):208–213. doi:10.3855/jidc.2664.
- Chung WK, Zheng ZL, Sung JY, et al. Validity of interferon-γ-release assays for the diagnosis of latent tuberculosis in haemodialysis patients. Clin Microbiol Infect. 2010;16(7):960–965. doi:10.1111/j.1469-0691.2009.02949.x.
- Ates G, Yildiz T, Danis R, et al. Incidence of tuberculosis disease and latent tuberculosis infection in patients with end stage renal disease in an endemic region. Ren Fail. 2010;32(1):91–95. doi:10.3109/08860220903367528.
- Menzies R, Vissandjee B. Effect of bacille Calmette-Guérin vaccination on tuberculin reactivity. Am Rev Respir Dis. 1992;145(3):621–625. doi:10.1164/ajrccm/145.3.621.
- Karalliedde S, Katugaha LP, Uragoda CG. Tuberculin response of Sri Lankan children after BCG vaccination at birth. Tubercle. 1987;68(1):33–38. doi:10.1016/0041-3879(87)90005-5.
- Marcus JH, Khassis Y. The tuberculin sensitivity in BCG vaccinated infants and children in Israel. Acta Tuberc Pneumol Scand. 1965;46(2):113–122.
- Lifschitz M. The Value of the Tuberculin Skin Test as a Screening Test for Tuberculosis among BCG-Vaccinated Children. Pediatrics. 1965;36(4):624–627.
- Dawar M, Clark M, Deeks SL, Walop W, Ahmadipour N. A fresh look at an old vaccine: Does BCG have a role in 21st Century Canada? International Journal of Circumpolar Health. 2004;63(sup2):230–236. doi:10.3402/ijch.v63i0.17908.
- Campbell JR, Chen W, Johnston J, et al. Latent tuberculosis infection screening in immigrants to low-incidence countries: a meta-analysis. Mol Diagn Ther. 2015;19(2):107–117. doi:10.1007/s40291-015-0135-6.
- Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLoS Med. 2011;8(3):e1001012 doi:10.1371/journal.pmed.1001012.
- Oh CE, Ortiz-Brizuela E, Bastos ML, Menzies D. Comparing the Diagnostic Performance of QuantiFERON-TB Gold Plus to Other Tests of Latent Tuberculosis Infection: A Systematic Review and Meta-analysis. Clin Infect Dis. 2021;73(5):e1116–e1125. doi:10.1093/cid/ciaa1822.
- Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56(3):230–238. doi:10.1097/QAI.0b013e31820b07ab.
- Huo Z-y, Peng L. Accuracy of the interferon-γ release assay for the diagnosis of active tuberculosis among HIV-seropositive individuals: a systematic review and meta-analysis. BMC Infect Dis. 2016;16(1):350. doi:10.1186/s12879-016-1687-8.
- Petruccioli E, Chiacchio T, Navarra A, et al. Effect of HIV-infection on QuantiFERON-plus accuracy in patients with active tuberculosis and latent infection. J Infect. 2020;80(5):536–546. doi:10.1016/j.jinf.2020.02.009.
- Ledesma JR, Ma J, Zheng P, Ross JM, Vos T, Kyu HH. Interferon-gamma release assay levels and risk of progression to active tuberculosis: a systematic review and dose-response meta-regression analysis. BMC Infect Dis. 2021;21(1):467. doi:10.1186/s12879-021-06141-4.
- Gupta RK, Lipman M, Jackson C, et al. Quantitative IFN-γ Release Assay and Tuberculin Skin Test Results to Predict Incident Tuberculosis. A Prospective Cohort Study. Am J Respir Crit Care Med. 2020;201(8):984–991. doi:10.1164/rccm.201905-0969OC.
- Dale KD, Karmakar M, Snow KJ, Menzies D, Trauer JM, Denholm JT. Quantifying the rates of late reactivation tuberculosis: a systematic review. Lancet Infect Dis. 2021;21(10):e303–e317. doi:10.1016/S1473-3099(20)30728-3.
- Zhou G, Luo Q, Luo S, et al. Interferon-γ release assays or tuberculin skin test for detection and management of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(12):1457–1469. doi:10.1016/S1473-3099(20)30276-0.
- Gupta RK, Calderwood CJ, Yavlinsky A, et al. Discovery and validation of a personalized risk predictor for incident tuberculosis in low transmission settings. Nat Med. 2020;26(12):1941–1949. doi:10.1038/s41591-020-1076-0.
- Martinez L, Cords O, Horsburgh CR, et al. The Risk of Tuberculosis in Children After Close Exposure: An Individual-Participant Meta-analysis Including 137,647 Children from 46 Cohort Studies. Lancet (London, England). 2020;395(10228):973–984. doi:10.1016/S0140-6736(20)30166-5.
- Ward H, Stewart S, Al-Azem A, Reeder B, Hoeppner V. Quantifying local TB risk factors and determining the relative cost of treatment of latent TB infection to prevent tuberculosis. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2017;1(4):191–198. doi:10.1080/24745332.2017.1387879.
- Cook VJ, Hernández-Garduño E, Elwood RK. Risk of tuberculosis in screened subjects without known risk factors for active disease. The International Journal of Tuberculosis and Lung Disease. 2008;12(8):903–908.
- Horsburgh CR, O’Donnell M, Chamblee S, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. 2010;182(3):420–425. doi:10.1164/rccm.200909-1355OC.
- Lawn SD, Harries AD, Williams BG, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? The international journal of tuberculosis and lung disease: the official journal of the International Union against. Int J Tuberc Lung Dis. 2011;15(5):571–581. doi:10.5588/ijtld.10.0483.
- Ross JM, Badje A, Rangaka MX, et al. Isoniazid preventive therapy plus antiretroviral therapy for the prevention of tuberculosis: a systematic review and meta-analysis of individual participant data. Lancet Hiv. 2021;8(1):e8–e15. doi:10.1016/S2352-3018(20)30299-X.
- Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122(2):597–602. doi:10.1378/chest.122.2.597.
- Van Ginderdeuren E, Bassett J, Hanrahan CF, Mutunga L, Van Rie A. High conversion of tuberculin skin tests during the first year of antiretroviral treatment among South African adults in primary care. AIDS. 2021;35(11):1775–1784. doi:10.1097/QAD.0000000000002952.
- Kirenga BJ, Worodria W, Massinga-Loembe M, et al. Tuberculin skin test conversion among HIV patients on antiretroviral therapy in Uganda. Int j Tuberc Lung Dis. 2013;17(3):336–341. doi:10.5588/ijtld.12.0298.
- Talebi-Taher M, Abbasian L, Alavi-Niakou SN, Javad-Moosavi SA, Pahlavani S. Tuberculin Skin Test Conversion among Individuals with Human Immunodeficiency Virus Infection on Antiretroviral Therapy in a Referral Teaching Hospital, Tehran, Iran. Tanaffos. 2017;16(3):201–206.
- Girardi E, Palmieri F, Zaccarelli M, et al. High incidence of tuberculin skin test conversion among HIV-infected individuals who have a favourable immunological response to highly active antiretroviral therapy. AIDS. 2002;16(14):1976–1979. doi:10.1097/00002030-200209270-00021.
- Doshi S, Chen TF, Zapata J, et al. Risk factors for tuberculin skin test conversion among HIV-infected patients in New York City. Int J Infect Dis. 2012;16(7):e518–e521. doi:10.1016/j.ijid.2012.03.002.
- Cho PJ-Y, Wu C-Y, Johnston J, Wu M-Y, Shu C-C, Lin H-H. Progression of chronic kidney disease and the risk of tuberculosis: an observational cohort study. Int J Tuberc Lung Dis. 2019;23(5):555–562. doi:10.5588/ijtld.18.0225.
- Kumar DS, Ronald LA, Romanowski K, et al. Risk of active tuberculosis in migrants diagnosed with cancer: a retrospective cohort study in British Columbia, Canada. BMJ Open. 2021;11(3):e037827 doi:10.1136/bmjopen-2020-037827.
- Cheng MP, Abou Chakra CN, Yansouni CP, et al. Risk of Active Tuberculosis in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2017;64(5):635–644. doi:10.1093/cid/ciw838.
- Leegaard A, Riis A, Kornum JB, et al. Diabetes, Glycemic Control, and Risk of Tuberculosis: A population-based case-control study. Diabetes Care. 2011;34(12):2530–2535. doi:10.2337/dc11-0902.
- Piette JD, Kerr EA. The Impact of Comorbid Chronic Conditions on Diabetes Care. Diabetes Care. 2006;29(3):725–731. doi:10.2337/diacare.29.03.06.dc05-2078.
- Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in Poorly Controlled Type 2 Diabetes: Altered Cytokine Expression in Peripheral White Blood Cells. Clin Infect Dis. 2008;47(5):634–641. doi:10.1086/590565.
- Nolan CM, Elarth AM. Tuberculosis in a cohort of Southeast Asian Refugees. A five-year surveillance study. Am Rev Respir Dis. 1988;137(4):805–809. doi:10.1164/ajrccm/137.4.805.
- Grzybowski S, McKinnon NE, Tuters L, Pinkus G, Philipps R. Reactivations in Inactive Pulmonary Tuberculosis. American Review of Respiratory Disease. 1966;93(3P1):352–361. doi:10.1164/arrd.1966.93.3P1.352.
- Grzybowski S, Fishaut H, Rowe J, Brown A. Tuberculosis among patients with various radiologic abnormalities, followed by the chest clinic service. Am Rev Respir Dis. 1971;104(4):605–608. doi:10.1164/arrd.1971.104.4.605.
- Horwitz O, Wilbek E, Erickson PA. Epidemiological basis of tuberculosis eradication. 10. Longitudinal studies on the risk of tuberculosis in the general population of a low-prevalence area. Bull World Health Organ. 1969;41(1):95–113.
- Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public Health. 2008;8(1):289 doi:10.1186/1471-2458-8-289.
- Maurya V, Vijayan VK, Shah A. Smoking and tuberculosis: an association overlooked. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union against Tuberculosis and Lung Disease. 2002;6(11):942–951.
- Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335–342. doi:10.1001/archinte.167.4.335.
- Lin H-H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. doi:10.1371/journal.pmed.0040020.
- Jeon CY, Murray MB. Diabetes Mellitus Increases the Risk of Active Tuberculosis: A Systematic Review of 13 Observational Studies. PLoS Med. 2008;5(7):e152. doi:10.1371/journal.pmed.0050152.
- Comstock GW, Edwards LB, Livesay VT. Tuberculosis morbidity in the U.S. Navy: its distribution and decline. Am Rev Respir Dis. 1974;110(5):572–580. doi:10.1164/arrd.1974.110.5.572.
- Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106.
- Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax. 2015;70(7):677–682. doi:10.1136/thoraxjnl-2014-206470.
- Muñoz L, Santin M, Alcaide F, OPTIMIST Study Team, et al. QuantiFERON-TB Gold In-Tube as a Confirmatory Test for Tuberculin Skin Test in Tuberculosis Contact Tracing: A Noninferiority Clinical Trial. Clin Infect Dis. 2018;66(3):396–403. doi:10.1093/cid/cix745.
- Tagmouti S, Slater M, Benedetti A, et al. Reproducibility of Interferon Gamma (IFN-γ) Release Assays. A Systematic Review. Ann Am Thorac Soc. 2014;11(8):1267–1276. doi:10.1513/AnnalsATS.201405-188OC.
- Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999;159(1):15–21. doi:10.1164/ajrccm.159.1.9801120.
- Whitworth WC, Hamilton LR, Goodwin DJ, et al. Within-Subject Interlaboratory Variability of QuantiFERON-TB Gold In-Tube Tests. PLOS One. 2012;7(9):e43790. doi:10.1371/journal.pone.0043790.
- Banaei N, Gaur RL, Pai M. Interferon Gamma Release Assays for Latent Tuberculosis: What Are the Sources of Variability? J Clin Microbiol. 2016;54(4):845–850. doi:10.1128/JCM.02803-15.
- Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med. 2013;187(2):206–211. doi:10.1164/rccm.201203-0430OC.
- Banaei N, Pai M. Detecting New Mycobacterium tuberculosis Infection. Time for a More Nuanced Interpretation of QuantiFERON Conversions. Am J Respir Crit Care Med. 2017;196(5):546–547. doi:10.1164/rccm.201707-1543ED.
- Sepulveda RL, Ferrer X, Latrach C, Sorensen RU. The influence of Calmette-Guérin bacillus immunization on the booster effect of tuberculin testing in healthy young adults. Am Rev Respir Dis. 1990;142(1):24–28. doi:10.1164/ajrccm/142.1.24.
- Feld R, Bodey GP, Gröschel D. Mycobacteriosis in Patients With Malignant Disease. Arch Intern Med. 1976;136(1):67–70. doi:10.1001/archinte.1976.03630010051009
- Dorman SE, Belknap R, Graviss EA, for the Tuberculosis Epidemiologic Studies Consortium, et al. Interferon-γ Release Assays and Tuberculin Skin Testing for Diagnosis of Latent Tuberculosis Infection in Healthcare Workers in the United States. Am J Respir Crit Care Med. 2013;189(1):131203133239003–131203133239087. doi:10.1164/rccm.201302-0365OC.
- Sosa LE. Tuberculosis Screening, Testing, and Treatment of U.S. Health Care Personnel: Recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR Morbidity and Mortality Weekly Report. 2019;68(19):439–443. doi:10.15585/mmwr.mm6819a3.
- Campbell JR, Trajman A, Cook VJ, et al. Adverse events in adults with latent tuberculosis infection receiving daily rifampicin or isoniazid: post-hoc safety analysis of two randomised controlled trials. Lancet Infect Dis. 2020;20(3):318–329. doi:10.1016/S1473-3099(19)30575-4.
- Verma G, Chuck AW, Jacobs P. Tuberculosis screening for long-term care: a cost-effectiveness analysis. Int J Tuberc Lung Dis. 2013;17(9):1170–1177. doi:10.5588/ijtld.12.0934.
- Reddy D, Walker J, White LF, et al. Latent Tuberculosis Infection Testing Practices in Long-Term Care Facilities, Boston, Massachusetts. J Am Geriatr Soc. 2017;65(6):1145–1151. doi:10.1111/jgs.14696.
- Cadieux G, Fung C, Fitzgerald-Husek A, et al. Tuberculosis Screening on Admission to Long-Term Care Homes in Ontario. 2019.
- Khalil NJ, Kryzanowski JA, Mercer NJ, Ellis E, Jamieson F. Tuberculosis Outbreak in a Long-term Care Facility. Can J Public Health. 2013;104(1):e28–e32. doi:10.1007/BF03405650.
- Dorken E, Grzybowski S, Allen EA. Significance of the Tuberculin Test in the Elderly. Chest. 1987;92(2):237–240. doi:10.1378/chest.92.2.237.
- de Visser V, Sotgiu G, Lange C, TBNET, et al. False-negative interferon-γ release assay results in active tuberculosis: a TBNET study. Eur Respir J. 2015;45(1):279–283. doi:10.1183/09031936.00120214.
- Bae W, Park KU, Song EY, et al. Comparison of the Sensitivity of QuantiFERON-TB Gold In-Tube and T-SPOT.TB According to Patient Age. Plos One. 2016;11(6):e0156917. doi:10.1371/journal.pone.0156917.
- Khan A, Rebhan A, Seminara D, Szerszen A. Enduring Challenge of Latent Tuberculosis in Older Nursing Home Residents: A Brief Review. J Clin Med Res. 2019;11(6):385–390. doi:10.14740/jocmr3763.
- Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union against Tuberculosis and Lung Disease. 2008;12(5):498–505.
- Youssef E, Wooltorton E. Serious allergic reactions following tuberculin skin tests. CMAJ. 2005;173(1):34–34. doi:10.1503/cmaj.050710.
- Schatz M, Patterson R, Kloner R, Falk J. The prevalence of tuberculosis and positive tuberculin skin tests in a steroid-treated asthmatic population. Ann Intern Med. 1976;84(3):261–265. doi:10.7326/0003-4819-84-3-261.
- Bovornkitti S, Kangsadal P, Sathirapat P, Oonsombatti P. Reversion and reconversion rate of tuberculin skin reactions in correction with the use of prednisone. Dis Chest. 1960;38:51–55. doi:10.1378/chest.38.1.51.
- Brody JA, Overfield T, Hammes LM. Depression of the Tuberculin Reaction by Viral Vaccines. N Engl J Med. 1964;271:1294–1296. doi:10.1056/NEJM196412172712505.
- Howard TP, Solomon DA. Reading the Tuberculin Skin Test: Who, When, and How? Arch Intern Med. 1988;148(11):2457–2459. doi:10.1001/archinte.1988.00380110093020
- Duboczy BO, Brown BT. Multiple Readings and Determination of Maximal Intensity of Tuberculin Reaction. Am Rev Respir Dis. 1961;84(1):60–68. doi:10.1164/arrd.1961.84.1.60.
- Tarlo SM, Day JH, Mann P, Day MP. Immediate hypersensitivity to tuberculin. In vivo and in vitro studies. Chest. 1977;71(1):33–37. doi:10.1378/chest.71.1.33.
- Rose W, Kitai I, Kakkar F, Read SE, Behr MA, Bitnun A. Quantiferon Gold-in-tube assay for TB screening in HIV infected children: influence of quantitative values. BMC Infect Dis. 2014;14:516. doi:10.1186/1471-2334-14-516.
- Menzies R, Vissandjee B, Rocher I, St Germain Y. The booster effect in two-step tuberculin testing among young adults in Montreal. Ann Intern Med. 1994;120(3):190–198. doi:10.7326/0003-4819-120-3-199402010-00003.
- Stead WW, To T. The significance of the tuberculin skin test in elderly persons. Ann Intern Med. 1987;107(6):837–842. doi:10.7326/0003-4819-107-6-837.
- Salles CG, Ruffino-Netto A, Lapa-e-Silva JR, et al. The presence of a booster phenomenon among contacts of active pulmonary tuberculosis cases: a retrospective cohort. BMC Public Health. 2007;7(1):38. doi:10.1186/1471-2458-7-38.
- Dogan E, Erkoc R, Sayarlioglu H, Uzun K. Tuberculin Skin Test Results and the Booster Phenomenon in Two-Step Tuberculin Skin Testing in Hemodialysis Patients. Ren Fail. 2005;27(4):425–428. doi:10.1081/JDI-65379.
- WHO. In press. Use of novel tuberculin skin tests for the diagnosis of TB infection. Geneva, Switzerland: World Health Organization.
- WHO. Use of alternative interferon-gamma release assays for the diagnosis of TB infection: WHO policy statement. 2022; Geneva, Switzerland: World Health Organization. https://www.who.int/publications/i/item/9789240042346. Accessed February 1, 2022.
Appendix 1
A.1. Tuberculin skin test (TST) administration and interpretation
A.1.1. Administration of TST
The only internationally recommended method of tuberculin skin testing is the Mantoux technique, which consists of intradermal injection of tuberculin material on the inner surface of the forearm. The instructions on how to perform this technique have been reproduced and adapted,Citation7,Citation17 with supporting videos available.Citation18
A.1.1.1. Handling the solution
The purified protein derivative (PPD) should be stored between 2° and 8 °C and never frozen. Discard the solution if it freezes.
Remove the tuberculin solution from the vial under aseptic conditions. A little more than 0.1 mL of PPD solution should be drawn into the TB syringe. Hold the syringe upright and lightly tap out the air, then expel one drop. Check that a full 0.1 mL remains in the syringe.
Do not transfer the solution from one container to another, as the potency of the PPD may be diminished.
Draw up the solution just before injecting it. Do not preload syringes for later use as the potency of the PPD may be diminished.
The solution can be adversely affected by exposure to light. PPD should be stored in the dark except when doses are actually being withdrawn from the vial.
Use the solution within one month after opening, as the potency of the solution may be diminished. Label each bottle with the discard date when it is opened.
A.1.1.2. Preparing the person to be tested
Seat the person comfortably and explain the procedure.
Use the inner aspect of the forearm, preferably the nondominant arm (where administration and reading of the reaction is easiest), about 10 cm (4 inches) below the elbow; avoid areas with abrasions, swelling, visible veins or lesions. If there is a localized rash, a burn or localized eczema, avoid this area.
If neither forearm is suitable, use the outside of the forearm or the upper arm. In this case mark the location clearly in the record.
Cleanse the area to be injected with an alcohol swab and let the area dry.
Do not use EMLA® cream (or similar local anesthetic cream), as application of this cream has been reported to cause localized edema, which could easily be confused with a positive TST result.
A.1.1.3. Injecting the PPD tuberculin solution
Use a 0.6 to 1.3 cm (¼ to ½ inch), 26- or 27-gauge needle with a disposable plastic tuberculin syringe.
Position the bevel of the needle so that it opens facing up.
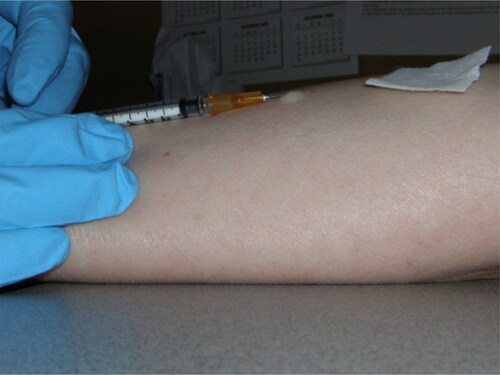
While holding the skin of the inner aspect of the forearm taut, insert the needle at a 5°-15° angle to the skin without aspirating. The tip of the needle will be visible just below the surface of the skin. The needle is inserted until the entire bevel is covered (see Appendix ).
Administer the PPD by the slow intradermal injection of 0.1 mL (5 tuberculin units).
A discrete, pale elevation of the skin (a wheal) 6-10 mm in diameter should appear. The wheal will typically disappear in 10-15 minutes. The size of the wheal is not completely reliable, but if a lot of liquid runs out at the time of injection and there is no wheal, then repeat the injection on the opposite forearm, or on the same forearm as before, but at least 5 cm from the previous injection site.
A drop of blood may be seen — this is normal. The person tested should be offered gauze to remove the blood but should be advised not to massage the site in order to avoid squeezing out the PPD and disrupting the test.
Do not cover the site with a bandage.
Tell the patient that they should not scratch the site but may perform all normal activities, including showering or bathing.
Place uncapped disposable needles and syringes in appropriate puncture-resistant containers immediately after use.
If the TST is accidentally given as a subcutaneous or an intramuscular injection, this should not pose a serious risk of harm. It is possible that tuberculin-sensitive persons may have localized inflammation, which should be self-limited. It would not be possible to take a measurement of, or clinically interpret, any such reaction, so the TST should be administered again immediately using proper intradermal technique on the volar surface of the forearm.
After administration, record the following:
Date of injection
Dose of PPD (5 tuberculin units, 0.1 mL)
PPD manufacturer
PPD lot number
Expiration date of the PPD reagent
Site of injection
Person administering the TST.
In settings where TST administration may be unsupervised or performed by persons with minimal experience, the quality of TST administration may be assessed by following mobile TST (mTST) protocols,Citation17,Citation18 whereby photos of the wheal created after administration are taken and evaluated by an experienced reviewer.
A.1.2. Precautions
Acute allergic reactions, including anaphylaxis, angioedema, urticaria and/or dyspnea, have been rarely reported as temporally (not necessarily causally) associated with administration of Tubersol®.Citation108 The events have been reported in Canada at a rate of less than 1 per million doses; some were reported in persons without a prior history of TST.
Epinephrine hydrochloride solution (1:1000) and other appropriate agents should be routinely available for immediate use in case an anaphylactic or other acute hypersensitivity reaction occurs. Health care providers should be familiar with the current recommendations of the National Advisory Committee on Immunization for monitoring of the patient for immediate reactions over a period of at least 15 minutes after inoculation and with the initial management of anaphylaxis in non-hospital settings.
Those with a history of receiving BCG vaccination(s).
Those with a common cold.
Those who are pregnant or are breast-feeding.
Those immunized within the previous four weeks with vaccines other than those listed below (live-virus vaccines).
Those with a previous positive TST.
Those taking low doses of systemic corticosteroids. A steroid dose equivalent to ≥15 mg prednisone daily for 2-4 weeks is required to suppress tuberculin reactivity.Citation109,Citation110
The following persons should NOT receive a TST:
Those with positive, severe blistering TST reactions in the past or with extensive burns or eczema present over TST testing sites, because of the greater likelihood of adverse or severe reactions.
Those with documented TB disease or a well-documented history of adequate treatment for TB infection or disease in the past. In such patients, the test is of no clinical utility.
Those with current major viral infections (eg, measles, mumps, varicella).
Those who have received live virus immunization within the past 4 weeks, as this has been shown to increase the likelihood of false-negative TST results.Citation111 Note that only measles vaccination has been shown to cause false-negative TST results, but it would seem prudent to follow the same 4-week guideline for other live-virus immunizations, including mumps, rubella, varicella (chickenpox) and yellow fever. However, if the opportunity to perform the TST might be missed, the TST should not be delayed for live-virus vaccines since these are theoretical considerations.
Note: A TST may be administered before or on the same day as the immunizations but at a different site.
A.1.3. Measuring induration
The TST should be read by a trained health professional. Individuals without experience in reading a TST may not feel slight induration, and the TST would be mistakenly recorded as 0 mm.
Self-reading is very inaccurate and is strongly discouraged.Citation112
Reading should be performed 48 to 72 hours after administration, as maximum induration can take up to 48 hours to develop, but after 72 hours it is difficult to interpret a reaction. Reactions may persist for up to one week, but for as many as 21% of individuals with a positive reaction at 48 to 72 hours, the reaction will be negative after 1 week.Citation113 If the TST cannot be read within 72 hours, it should be repeated at a location far enough from the previous test that the reactions do not overlap. There is no minimum wait time and the test can be readministered immediately.
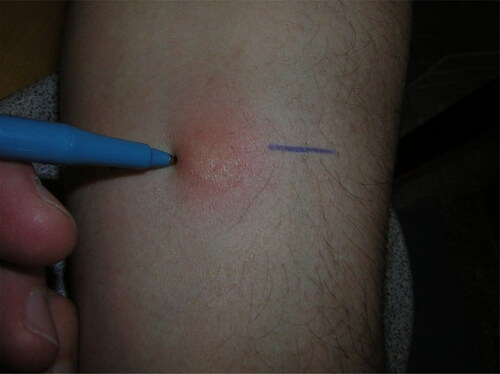
The forearm should be supported on a firm surface and slightly flexed at the elbow. Induration is not always visible. Palpate with fingertips to check if induration is present. If there is induration, mark the border of induration by moving the tip of a pen at a 45° angle laterally toward the site of the injection (Appendix ). The tip will stop at the edge of the induration, if present. Repeat the process on the opposite side of the induration. This pen method has advantages of being as reliable as the traditional palpation method (which relies entirely on fingertips) among experienced readers, and of being easier for new readers to learn and use.Citation7
Using a caliper, measure the distance between the pen marks, which reflects the diameter of the induration at its widest transverse diameter (at a right angle to the long axis of the forearm). A caliper is recommended because readings will be more precise and setting the caliper may reduce rounding error. If a caliper cannot be found a flexible ruler could be used.
Do not record erythema (redness). Approximately 2-3% of persons tested will have localized redness or rash (without induration) that occurs within the first 12 hours. These minor allergic reactions do not indicate TB infection and they are not a contraindication to future TSTs.Citation114
Blistering, which can occur in 3 to 4% of subjects with positive tests, should be recorded.
Record the result in millimeters (mm). Record no induration as “0 mm.” Recordings of positive, negative, doubtful, significant and non-significant are not recommended.
Do not round off the diameter of the induration to the nearest 5 mm, as this can interfere with determining whether TST conversion has occurred in the event of a future TST. If the measurement falls between demarcations on the ruler, the smaller of the two numbers should be recorded.
After measuring, record the following and provide a record of the result to the patient:
Date the induration was read
Measurement of the induration, if any, in mm
Any adverse reactions (eg, blistering)
Name of the individual reading the test
In settings where TST measurement may be unsupervised or performed by persons with minimal experience, the quality of TST measurement may be assessed by following mTST protocols,Citation17,Citation18 whereby photos of the induration are taken and evaluated by an experienced reviewer. This method is most accurate when applied to no or large indurations. In all settings, routine quality control and quality assessment measures of TST induration measurement should be employed to maximize accuracy.
A.2. Reproducibility and causes of TST and IGRA variation
Table A1. Causes of variation with TST or IGRA.Citation 88–90 , Citation 92 , Citation 115
A.3. Serial TST: Booster effect and conversions
A.3.1. TST booster effect
A single TST may elicit little response yet stimulate an anamnestic immune response, such that a second TST at any time from 1 week to one year later will elicit a much greater response.Citation89 This phenomenon is important to detect, as it represents a false positive, not a new TB infection. The booster effect was first described in older persons in whom it was felt to show TB infection acquired many years before (remotely) with subsequent waning of immunity.Citation95 It has also been described in persons with prior BCG vaccination or sensitivity to nontuberculous mycobacterial antigens.Citation94,Citation116
A two-step TST should be performed if subsequent TSTs will be conducted at regular intervals (eg, among health care or correctional workers).Citation89 This is to reduce the chance of a false-positive TST conversion when the TST is repeated. Please refer to Chapter Citation13: Tuberculosis Surveillance and Tuberculosis Infection Testing and Treatment in Migrants for recommendations on use of two-step TST in specific travelers.
The two-step protocol needs to be performed and documented ONCE. Any subsequent TST should be 1 step, regardless of how long it has been since the last TST.Citation7
The same material and techniques of administration and reading should be used as with any other TST. The second test should be performed one to 4 weeks later. Less than one week does not allow enough time to elicit the booster phenomenon, while more than 4 weeks increases the possibility of a true TST conversion. Both tests should be read and recorded at 48 to 72 hours after administration. Expanding the interval to read the first TST after 1 week (and therefore immediately before a second TST) is less accurate and is not recommended.
Longitudinal studies of the risk of TB following a booster reaction defined the reaction simply as a second TST result of 10 mm or more induration.Citation85,Citation117–119 Therefore, a second TST result of 10 mm or more should be considered significant and the patient referred for medical evaluation and chest radiography. Citation7,Citation89
All subjects with a reaction of 10 mm or more on the second TST of a two-step TST do not need a TST in the future. There is no clinical utility.Citation7 They should be referred for medical evaluation, as performed for those with a positive first TST. In longitudinal studies of the elderly, subjects with a second TST response of 10 mm or more had a risk of TB that was approximately half that of subjects whose first TST response was 10 mm or more. Similar findings were shown in a small cohort of hemodialysis patients. Since the risk of TB is about half that of patients from the same population group whose initial TST result is positive, the decision to provide TPT should be individualized.
A common question is how to manage a person whose first TST measured 5-9 mm and the second test measured 10 + mm but increased by less than 6 mm from the first test. As previously mentioned, this should be managed as a “positive TST,” meaning referral for medical evaluation and no further TSTs. While appropriate epidemiologic data are lacking, it seems reasonable to suggest that the risk of TB disease would be lower than in persons whose second TST increased by at least 6 mm. The decision to provide TPT should be individualized.
A.3.2. TST conversions
If there has been recent exposure, such as close contact with a person with TB disease or occupational TB disease exposure, then TST conversion will be more likely than when there has been no exposure. Conversion is defined as a TST of 10 mm or greater when an earlier test resulted in a reaction of less than 5 mm. If the earlier result was between 5 and 9 mm, the definition of conversion is more controversial. Increases of 6 or 10 mm have been proposed, but there is weak evidence supporting both.Citation89 In general, the larger the increase, the more likely it is due to a true conversion. However, like consideration of a “booster,” any second TST result of 10 mm or greater should be considered a “positive” and the patient evaluated for possible TPT.
All available experimental and epidemiologic evidence consistently shows that TST conversion occurs within 3-8 weeks of exposure.Citation89 Therefore, to identify a true conversion (ie, new infection), a single TST should be performed as soon as possible after an exposure to tuberculosis is recognized and the contact is identified. If the first TST is negative and performed less than 8 weeks after contact with the index patient, then a second TST should be scheduled no sooner than 8 weeks after the contact was broken. This also means for contacts that are identified more than 8 weeks after contact with an index patient is broken (eg, casual contacts), a single TST can be performed, and the result acted upon.Citation7
A.4. Dimensions to consider when interpreting TB infection diagnostic test results
A.4.1. Pretest probability of TB infection and predictive value
The pretest probability of TB infection refers to the probability a person truly has infection. The positive predictive value therefore reflects the likelihood that a positive result truly represents infection and the negative predictive value the likelihood a negative result truly represents absence of infection. The positive predictive value of the TST may be significantly reduced by BCG vaccination in populations with low pretest probability of infection; similarly, in populations with significant immune impairment, the negative predictive value of both TST and IGRA may be significantly impacted in populations with high pretest probability of infection.
A.4.2. Discriminatory ability of the test to identify individuals at increased risk of TB disease
The discriminatory ability of the test to identify individuals who will develop TB disease refers to the IRR, that is, the likelihood of developing disease among persons testing positive vs. negative. In populations where false negatives with a TST or IGRA are expected to be common (eg, due to immune impairment or other biologic reasons), the IRR of a test may fall substantially, even with very high specificity. Similarly, in populations where false positives are expected to be common, the IRR of a test may be substantially impacted, even if sensitivity is very high. Therefore, the discriminatory ability of a test is likely to be severely impacted in populations where sensitivity and/or specificity are expected to be reduced.
A.4.3. Risk of TB disease
The risk of TB disease is elevated among persons with medical conditions that affect immunity, recent infection or certain habits, and is described for various populations among persons with a positive test in of the main text. Using estimates of IRR, risk of TB disease can be estimated among persons with a negative test. Risk of disease is the most paramount consideration when faced with a test result, as TB disease may result in long-term patient morbidity or even death. Risk of disease at the time the test is done should not be the only consideration; it is important to also consider future risk of disease. This is especially important among certain persons, such as those tested prior to initiating immunosuppressants, prior to transplantation, or early in chronic kidney disease, as risk of disease is likely to increase in the future.