ABSTRACT
Background: Index hospital costs for transcatheter aortic valve replacement (TAVR) remain high. Next-day discharge (NDD) is a safe and feasible strategy in select patients. We sought to explore cost savings associated with NDD TAVR.
Methods: We conducted a retrospective observation cohort study of all fee-for-service Medicare beneficiaries who underwent elective, uncomplicated, transfemoral TAVR in 2016. We employed a cross-sectional regression analysis to estimate risk-adjusted hospital costs savings of NDD relative to longer length of stay (LOS), and logistic regression to determine differences in direct home discharge and readmission.
Results: Among 14,765 patients (59.2% of all TAVR), 2,169 (14.7%) were identified as NDD. They were younger (81.3 vs. 82.2, p < 0.01), more likely to be male (63% vs. 52%, p < 0.01), and had lower Charlson Comorbidity Index scores (2.73 vs. 2.98, p < 0.01). The adjusted cost for NDD was $7,499 lower compared to non-NDD (p < 0.001), and $5,188 when controlling for hospital fixed effects (p < 0.001); estimated total cost savings ranged from $6,522,183 to $16,265,331. NDD was associated with higher rates of discharged home (83% vs. 59%, p < 0.0001) without home health (16% vs. 28%, p < 0.0001), and lower incidence of readmission at 30 days [OR 0.72 (0.61–0.86), p = 0.0003], 60 days [0.71 (0.61–0.82), p < 0.0001] and 90 days [0.74 (0.65–0.85), p < 0.0001] than non-NDD.
Conclusions: There is significant heterogeneity in LOS after TAVR. Cost savings could be achieved with the implementation of clinical pathways and other strategies. Future research is needed to fully capture the multiple effects associated with LOS.
Introduction
Patients who have severe aortic stenosis and present with increased risk for morbidity and mortality with cardiac surgery are increasingly treated with transcatheter aortic valve replacement (TAVR). Clinical trials have demonstrated excellent outcomes with TAVR which has now surpassed surgical aortic valve replacement (SAVR) as the most common treatment for severe calcific aortic stenosis.Citation1–Citation5 Population aging, prevalence of aortic stenosis and widening indications to lower risk patients exert increased demand for access to care.Citation6
TAVR in the United States (US), index hospital costs have been estimated between $54,000 and $61,000 in recent clinical trials.Citation7 Based on current estimates and the impending indications for low-risk patients,Citation8 cost projections are poised to exert increasing pressure on health resources and funding agencies.
The chief drivers of costs include management of in-hospital complications, device price and duration of hospitalization.Citation9 Improved case selection, procedural approaches, technology, and expertise continue to decrease TAVR-related adverse events,Citation10,Citation11 while there is significant regional variation in negotiating device costs. Although the length of stay (LOS) has steadily decreased since the introduction of TAVR, median LOS in the US remains between 3 and 5 days.Citation12 The reduction of post-procedure LOS offers considerable opportunities for all programs, regardless of maturity and procedural volumes, to curb costs, decrease the intensity of health resource utilization, and continue to improve outcomes. Short LOS, including next-day discharge (NDD) has been shown to be feasible and safe in carefully selected patients in a large, multicenter prospective trial.Citation13 In order to better quantify the potential economic benefits of short LOS, we designed a study to compare the costs of care for elective out-patients who underwent next-day discharge (NDD) TAVR compared to those with longer lengths of stay.
Materials and methods
Study design, population and data sources
The aim of the current study was to explore the costs savings associated with NDD in patients who underwent elective, uncomplicated TF TAVR in the US in 2016. The study was designed as a retrospective, observational cohort study of contemporary transfemoral (TF) TAVR in the US. The population included all fee-for-service Medicare beneficiaries who underwent TF TAVR in 2016, identified from diagnosis-related groups (DRGs) 266 or 267 and analyzed by LOS. The primary cohort of interest was NDD, with patients with a LOS longer than 1 day constituting the comparative group (non-NDD). LOS was calculated by subtracting the day of discharge from the day of procedure. Individual consent was not required for the study. The authors had full access to the data; the first author takes responsibility for its integrity and the data analysis.
The cohort was limited to patients admitted electively (<24 h prior to procedure) and discharged alive without major in-hospital postoperative complications (e.g., new permanent pacemaker, stroke, major vascular complications, delirium – See Supplementary Table 1) to make the NDD and non-NDD patients more similar for comparison. Patients, comorbidities, in-hospital complications, and LOS were identified using Medicare’s Provider Analysis and Review (MEDPAR). Comparisons were made among clinical characteristics, Charlson Comorbidity Index, and hospital costs, exclusive of physician remuneration. Cost data was defined as charges (by department) multiplied by the hospital and department-specific cost-to-charge ratios derived from Medicare cost reports. Cost data and saving estimates are reported in US dollars.
30-, 60- and 90-day hospital readmission rates were captured in the 2016 US Medicare Standard Analytic File (100% sample). We compared patients’ disposition at the time of discharge and all-cause hospital readmission to explore differences in the transition of care and early recovery after TAVR.
Statistical analysis
Categorical data are reported as frequencies, and continuous data are reported as means ± SD. Univariate differences in baseline demographic and clinical comorbidities between NDD and non-NDD patients were assessed using the χ2 test for categorical variables and the Student t test for continuous variables.
We employed a cross-sectional regression analysis to estimate risk-adjusted costs savings associated with NDD relative to longer stays among uncomplicated cases. The regression analysis exploring the impact of NDD on costs in uncomplicated, elective TAVR patients was adjusted for patient characteristics and comorbidities (see of the Supplement). An additional regression included fixed effects for hospitals in order to control for the likely impact of variation in hospital characteristics and local practices on the relationship between NDD and cost. This strategy enabled us to increase the robustness of the modeling and mitigate the potential artifact between hospital effects associated with local accounting practices and the clustering of NDDs in select sites.
Separately, we conducted a logistic regression analysis of the same uncomplicated, elective TAVR cohort to determine any impact of NDD on 30, 60, and 90-day readmission rates. We adjusted for the same patient demographics and comorbidities in this model (see of Supplement for a detailed list of covariates used). All analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
Study population
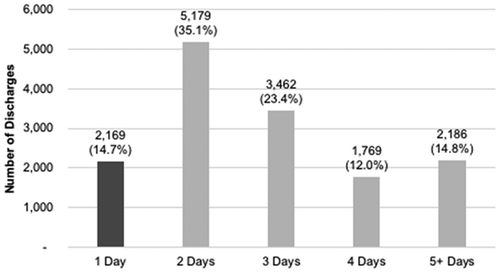
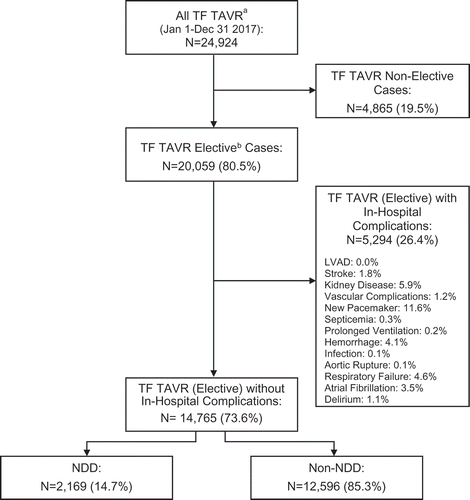
Between October 1, 2015 and September 30, 2016, 24,924 Medicare fee-for-service patients were discharged alive after TF TAVR. Of these, 14,765 (59.2%) were admitted electively and did not sustain one or more pre-specified in-hospital complications. In this cohort, 2,169 (14.7%) were identified as NDD [elective admission, without in-hospital complications] ().
Figure 1. Study cohort drawn from all US Medicare fee-for-service TF TAVR performed in 2016 identified from diagnosis-related groups (DRGs) 266 or 267
NDD patients were younger (81.3 vs. 82.2, p < 0.01) and more likely to be male (63% vs. 52%, p < 0.01). On average, NDD patients had lower Charlson Comorbidity Index scores (2.73 vs. 2.98, p < 0.01) and a lower burden of atrial fibrillation, heart failure, hypertension, peripheral vascular disease and previous coronary artery bypass graft surgery than non-NDD (). 30-day mortality was 0.5% for NDD and 0.8% for non-NDD patients. These rates are unadjusted for risk.
Table 1. Patient characteristics at admission
Length of stay and disposition at discharge
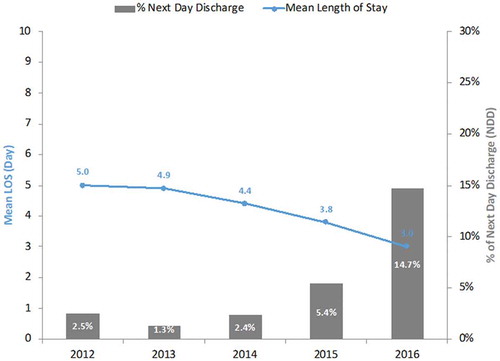
The mean LOS for uncomplicated, elective cases was 3.03 ± 2.13 days. While the NDD cohort totaled 14.7%, 50% (n = 7,348) were discharged within 48 h, and 27% (n = 3,955) had a LOS of 4 days or longer (). Temporal changes in mean LOS and proportion of NDD between 2012 and 2016 are depicted in .
Figure 3. Temporal change in the duration of in-hospital length of stay and proportion of next-day discharge after elective, uncomplicated TF TAVR compared to the longer length of stay (US Medicare, 2012–2016). LOS: Length of stay
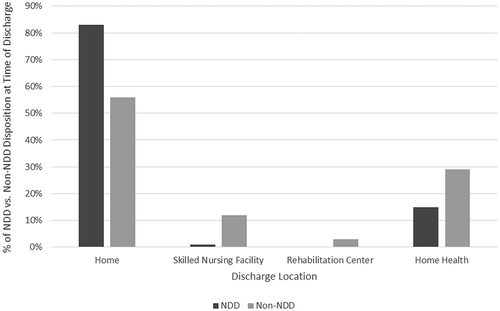
There were significant differences in patient disposition at the time of discharge, with 83% of NDD patients discharged home compared to 59% of non-NDD (p ≤ 0.0001), and lesser requirements for home health (16% vs. 28%, p < 0.0001), transfer to skilled nursing facility (1% vs. 10%, p < 0.0001) or rehabilitation center (0% vs. 2%, p < 0.0001) for the NDD group ().
Figure 4. Differences in elective, uncomplicated TF TAVR patient disposition at the time of discharge (home, skilled nursing facility, rehabilitation facility, home health services) after next-day discharge and longer length of stay (US Medicare, 2016). SNF: Skilled nursing facility; NDD: Next-day discharge
Cost savings
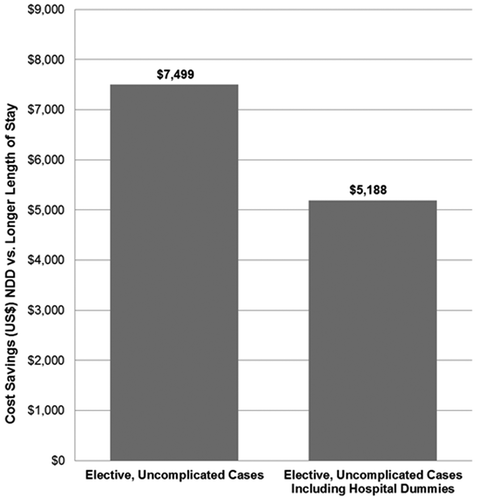
Overall, the adjusted cost of patient care for NDD was $7,499 lower compared to non-NDD (p < 0.001), and $5,188 when controlling for hospital fixed effects (p < 0.001) ().
Figure 5. Cost savings estimates associated with next-day discharge compared to the longer length of stay after elective, uncomplicated TF TAVR (US Medicare, 2016)
As a sensitivity analysis, we estimated the cost savings for NDD compared to 2 days LOS, 2–3 days LOS, 2–4 days LOS. These analyses provide lower-end estimates for the average cost savings for next-day discharge ().
Table 2. Adjusted cost savings in elective uncomplicated cases (NDD vs. non-NDD)
Considering the population of 2,169 TF TAVR NDD patients (elective, uncomplicated) treated in 2016, the total annual cost savings in this cohort is estimated to be $16,265,331, relative to non-NDD cases, based on the adjusted savings of $7,499 per NDD patient determined in the first regression model. Using the $5,188 average savings determined in the hospital dummy regression, estimated annual costs are $11,252,772. NDD patients cost an average of $4,507 less than patients who have a 2 days LOS ($3,007 in the hospital dummy regression). This translated to an estimated cost savings $9,775,683 in 2016 ($6,522,183 in the hospital dummy regression) of NDD relative to 2 days LOS. Thus, the estimated total cost savings associated with NDD in 2016 among elective, uncomplicated Medicare patients ranges from $6,522,183 to $16,265,331.
Hospital readmission
The unadjusted rate of 30-, 60-, and 90-day hospital readmission in NDD patients was lower than for non-NDD (10%, 16% and 22.6% vs. 14.4%, 23.4%, and 31.9%) between January 1, 2016 and September 30, 2016 identified in the Medicare Standard Analytic File. In the risk-adjusted logistic regression, NDD was associated with significantly lower rates of readmission at 30 days [OR 0.72 (0.61–0.86), p = 0.0003], 60 days [0.71 (0.61–0.82), p < 0.0001] and 90 days [0.74 (0.65–0.85), p < 0.0001] than non-NDD ().
Table 3. Rates of hospital readmission in elective uncomplicated cases (NDD vs. non-NDD)
Discussion
The results of the retrospective observational cohort study can be summarized as follows: (1) There was variation in LOS: 15% were discharged the day after their procedure, 35% within 2 days, and 50% in 3 days and more; (2) NDD patients’ risk profile was more favorable; this group of patients was more likely to be discharged home without home health service requirements, experienced a lower rate of hospital readmission and had comparable rates of 30-day mortality than non-NDD patients; (3) in the risk-adjusted model constructed with available administrative variables, NDD was associated with $7,499 cost savings compared to non-NDD ($5,188 in hospital dummies model) while cost savings between NDD and 2 days, 2–3 days, and 2–4 days LOS were $4,507, $5,179 and $5,815, respectively ($3,007, $3,629 and $4,015 in hospital dummies model); and (4) among elective, uncomplicated TAVR patients, estimated cost savings with NDD represented up to $16,265,331 savings across the US in 2016.
Study context
To our knowledge, this is the first study to explore cost savings associated with NDD compared to longer LOS in a large contemporary population of patients treated in “real world” clinical practice in the current era of decreasing rates of complications.
To date, studies have focused on comparing TAVR and SAVR. Findings of high costs associated with a transcatheter approach have been echoed in multiple studiesCitation14–Citation16 including Ailawadi et al. who reported that TAVR in high and intermediate risk patients with a 6-day LOS incurred twice the median cost of SAVR in intermediate risk patients with a 7-day LOS ($81,638 ± 42,068 vs. $40,830 ± 33,474, p < 0.0001).Citation17 Others have argued that the shorter LOS, decreased the need for rehabilitation services at discharge, and early quality of life benefits associated with TAVR may render these incremental costs acceptable by current US and other standards.Citation11,Citation18–Citation20 Given the increasing acceptability of TAVR as a preferred treatment option, our study suggests that economic benefit comparisons may shift from contrasting TAVR and SAVR to exploring opportunities to quantify the cost savings achieved by fully optimized TAVR programs and help set a new standard of care.
LOS is a surrogate indicator of the cumulative effect of multiple factors, including patient characteristics, peri-procedure complications, post-procedure care, individual program experience and/or culture of care, and evolution of technology and procedural approach. It is exceedingly difficult to fully account for the totality of these variables in a national retrospective study. We recognize that the comorbidity profile of NDD patients was more favorable on average than non-NDD patients – the mean differences in age and Charlson Comorbidity Index scores were 9 months and 0.26 points, respectively. These significant differences are inherent to the treatment of a complex patient population and likely account for some of the findings of this study. It is more challenging to parse the effect of unstandardized care protocols on LOS, regardless of patient risk. For example, the hospital effect may indicate nesting of “rapid reconditioning” protocols – local anesthesia, avoidance of invasive lines, facilitated mobilization – in select centers that are facilitating NDD. There is emerging evidence that suggests that seemingly benign interventions, such as the use of a strategy of local anesthesia only or “light” conscious sedation,Citation21 the avoidance of urinary catheterization,Citation22 and the implementation of rapid mobilizationCitation23 can be implemented across various risks profiles, and may contribute to the avoidance of in-hospital deconditioning and help facilitate NDD. The study fails to answer if and how these non-modifiable variables (age, comorbid burden, frailty) and systems variables (peri-procedure approach, post-procedure protocols, discharge planning, and transitional care) impact the likelihood of NDD. There is a pressing need for further research to answer this important question.
Next-day discharge as the standard of care?
Reports of cost savings associated with the transition to various minimalist strategies, including the use of a cardiac catheterization laboratory, the avoidance of general anesthesia and/or a target of short LOS reflect this emerging interest.Citation24 Single site studies have reported savings of $10,000 to $16,000 for patients treated with a minimalist approach and discharged within shorter LOS without compromising patient safety.Citation25–Citation27
The recent publication of the Multimodality, Multidisciplinary but Minimalist TAVR (3M TAVR) study demonstrated the safety and reproducibility of the Vancouver TAVR Clinical PathwayCitation23 to facilitate NDD home in over 50% patients screened at 13 TAVR centers of varying procedural volume and program maturity while achieving excellent clinical outcomes.Citation13 Careful attention to all components of patients’ journey of care is essential to consistently achieve similar results. The practice of same-day admission for elective patients is associated with significantly lower hospitalization costs and length of stay, and similar mortality and complication rates compared to earlier admission.Citation28 The reported safety of local anesthesia and procedural sedation provides opportunities to transition away from the more resource intensive use of general anesthesia.Citation21,Citation29–Citation31 The avoidance of critical care in the post-procedure phase and a protocol of rapid mobilization and accelerated reconditioning are effective strategies to facilitate NDD. Lastly, a recent study from the STS/ACC TVT Registry and CMS reported that direct-home discharge accounted for 72.2% of all discharges after TAVR in the US in 2011–2015, and that there was no significant association between discharge home or transfer to a skilled nursing facility and rates of 30-day readmission.Citation32 Thus, a comprehensive clinical pathway that encompasses streamlined admission, minimalist peri-procedure approach, rapid reconditioning post-procedure care, and NDD home may operationalize significant cost savings while optimizing outcomes.
Clinical and policy implications
Our study identified a significant opportunity to potentially achieve $6.5M to $16.2M annual national cost savings by transitioning to an NDD strategy for elective patients who don’t sustain in-hospital complications. This strategy can help answer the call to arms of policy-makers and funders to address the pressing need to reduce the economic impact of TAVR to sustain access to treatment and ensure value for treatment. This is particularly pertinent given current discussions about widening indications to low-risk patients and ongoing discussions about closing the gap between the cost of TAVR and SAVR. The avoidance of in-hospital complications, reduced health service utilization, decreased requirements for critical care and shorter LOS are recognized drivers of cost savings and program efficiency.
Decreased LOS, safe transition home and reduced post-acute rehabilitation services are the major cost offsets of TAVR compared to SAVR.Citation33 Costs associated with SAVR range from $35,000 to $51,000, and are primarily driven by preoperative risk and postoperative complications.Citation34 The approximate $25,000 cost differences between transcatheter and surgical devices requires TAVR programs to consistently achieve short LOS target, excellent outcomes and the avoidance of Medicare Post-Acute Care Transfer (PACT) penalties to realize the required cost savings. In addition, the ongoing examination and advocacy to address the barriers associated with device cost will continue to exert pressure to close the gap between TAVR and SAVR. In this context, TF TAVR can provide immediate high cost-effectiveness value by accelerating recovery, facilitating safe discharge home, avoiding hospital readmission, and improving short-term quality of life while conforming to US public policies aimed to incentivize value in cardiac care.Citation33,Citation35
Limitations
The findings must be interpreted in the context of the study design. The limitations of this study are those inherent in retrospective administrative database analyses, including clinical outcomes being imputed from data not prepared for research purposes, and the under-reporting of certain patient characteristics, events, and diagnoses. The data may fail to adequately capture the multiple effects associated with LOS. In particular, the differences noted in age, sex, and co-morbidities between the NDD and non-NDD patients underline the caution warranted in the interpretation of the findings. The absence of a propensity analysis, the potential confounding effect of variables not appropriately accounted for in the model, and the risk of selection bias highlighted in the discussion present additional important limitations that should be considered in this exploratory study. However, the exclusion of in-patients and a comprehensive list of post-TAVR in-hospital complications reflect our attempt to study a relatively homogenous population, and remove known strong predictors of longer LOS.
The use of Medicare data to assess cost savings has distinct advantages because it includes all charges and services. Actual hospital charges for all procedures were used to examine the financial impact of transfemoral TAVR. These were obtained from Medicare’s MEDPAR files, which represent completely adjudicated claims on individual hospitalizations.
Conclusion
In a large contemporary national US cohort of patients who had elective and uncomplicated TF TAVR, there was an estimated $5,200-$7,500 cost savings associated with NDD compared to non-NDD in a retrospective risk-adjusted analysis, a higher rate of direct-home discharge, and a lower rate of hospital readmission. This exploratory study highlights opportunities for significant savings by leveraging rapidly evolving evidence supporting the transformation of processes of care, setting of new clinical expectations and standards of care, and developing increased program capacity in anticipation of broadening indications and increased patient need.
Supplemental Material
Download Zip (12.9 KB)Disclosure statement
SB Lauck has been a consultant for Edwards; SJ Baron has been a consultant for Abbott and Edwards; C Thompson and S Clancy are employees of Edwards; P Genereux has been a consultant for Cardiovascular Systems and has received research grants from Boston Scientific and Cardiovascular Systems; JG Webb has been a consultant for Abbott, Edwards, and Medtronic; S Kodali has been a consultant for Edwards, Merrill Lifesciences, and Claret Medical M Reynolds has been a consultant for Edwards and Medtronic DA Wood has been a consultant for Abbott and Edwards, and has received research grants from Abbott and Edwards; DJ Cohen has received research grants support from Edwards, Medtronic, Boston Scientific, Abbott and has been a consultant for Medtronic and Edwards.
Supplemental data
Supplemental data for this article can be accessed here.
References
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi:10.1016/S0140-6736(15)60308-7.
- Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66(2):113–121. doi:10.1016/j.jacc.2015.05.017.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616.
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376(14):1321–1331. doi:10.1056/NEJMoa1700456.
- Thonghong T, De Backer O, Sondergaard L. Comprehensive update on the new indications for transcatheter aortic valve replacement in the latest 2017 European guidelines for the management of valvular heart disease. Open Heart. 2018;5(1):e000753. doi:10.1136/openhrt-2017-000753.
- Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi:10.1016/j.jacc.2013.05.015.
- Cohen DJ. Cost-effectiveness of transcatheter vs. surgical aortic valve replacement in intermediate risk patients. Results from the PARTNER 2A and SAPIEN 3 intermediate risk trials. TCT; October 31, 2017, Denver, CO.
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019. doi:10.1056/NEJMoa1814052.
- Iannaccone A, Marwick TH. Cost effectiveness of transcatheter aortic valve replacement compared with medical management or surgery for patients with aortic stenosis. Appl Health Econ Health Policy. 2015;13(1):29–45. doi:10.1007/s40258-014-0141-6.
- Gupta T, Khera S, Kolte D, et al. Transcatheter versus surgical aortic valve replacement in patients with prior coronary artery bypass grafting: trends in utilization and propensity-matched analysis of in-hospital outcomes. Circ Cardiovasc Interv. 2018;11(4):e006179. doi:10.1161/CIRCINTERVENTIONS.118.006388.
- Wassef AWA, Alnasser S, Rodes-Cabau J, et al. Institutional experience and outcomes of transcatheter aortic valve replacement: results from an international multicentre registry. Int J Cardiol. 2017;245:222–227. doi:10.1016/j.ijcard.2017.07.079.
- Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy registry. J Am Coll Cardiol. 2017;69(10):1215–30.
- Wood DA, Lauck SB, Cairns JA, Humphries KH, Cook R, Welsh R, Leipsic J, Genereux P, Moss R, Jue J, Blanke P. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: the 3M TAVR study. JACC: Cardiovasc Interv. 2019;12(5):459–69.
- Hawkins RB, Downs EA, Johnston LE, et al. Impact of transcatheter technology on surgical aortic valve replacement volume, outcomes, and cost. Ann Thorac Surg. 2017;103(6):1815–1823. doi:10.1016/j.athoracsur.2017.02.039.
- McCarthy FH, Savino DC, Brown CR, et al. Cost and contribution margin of transcatheter versus surgical aortic valve replacement. J Thorac Cardiovasc Surg. 2017;154(6):1872–1880.e1871. doi:10.1016/j.jtcvs.2017.06.020.
- Brown JW, Boyd JH, Patel PM, et al. Transcatheter aortic valve replacement versus aortic valve bypass: a comparison of outcomes and economics. Ann Thorac Surg. 2016;101(1):49–54; discussion 54–45. doi:10.1016/j.athoracsur.2015.05.125.
- Ailawadi G, LaPar DJ, Speir AM, et al. Contemporary costs associated with transcatheter aortic valve replacement: a propensity-matched cost analysis. Ann Thorac Surg. 2016;101(1):154–160; discussion 160. doi:10.1016/j.athoracsur.2015.05.120.
- Reynolds MR, Lei Y, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement with a self-expanding prosthesis versus surgical aortic valve replacement. J Am Coll Cardiol. 2016;67(1):29–38. doi:10.1016/j.jacc.2015.10.046.
- Sud M, Tam DY, Wijeysundera HC. The economics of transcatheter valve interventions. Can J Cardiol. 2017;33(9):1091–1098. doi:10.1016/j.cjca.2017.03.015.
- Tam DY, Hughes A, Fremes SE, et al. A cost-utility analysis of transcatheter versus surgical aortic valve replacement for the treatment of aortic stenosis in the population with intermediate surgical risk. J Thorac Cardiovasc Surg. 2018;155(5):1978–1988.e1971. doi:10.1016/j.jtcvs.2017.11.112.
- Hyman MC, Vemulapalli S, Szeto WY, et al. Conscious sedation versus general anesthesia for transcatheter aortic valve replacement: insights from the national cardiovascular data registry Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy registry. Circulation. 2017;136(22):2132–2140. doi:10.1161/CIRCULATIONAHA.116.026656.
- Lauck SB, Kwon JY, Wood DA, Baumbusch J, Norekvål TM, Htun N, Stephenson L, Webb JG. Avoidance of urinary catheterization to minimize in-hospital complications after transcatheter aortic valve implantation: An observational study. Eur J Cardiovasc Nurs. 2018;17(1):66–74.
- LauckSB, Wood DA, Baumbusch J, et al. Vancouver transcatheter aortic valve replacement clinical pathway: minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes. 2016;9(3):312–321. doi:10.1161/CIRCOUTCOMES.115.002541.
- Lauck SB, Wood DA, Achtem L, et al. Risk stratification and clinical pathways to optimize length of stay after transcatheter aortic valve replacement. Can J Cardiol. 2014;30(12):1583–1587. doi:10.1016/j.cjca.2014.07.012.
- Babaliaros V, Devireddy C, Lerakis S, et al. Comparison of transfemoral transcatheter aortic valve replacement performed in the catheterization laboratory (minimalist approach) versus hybrid operating room (standard approach): outcomes and cost analysis. JACC Cardiovasc Interv. 2014;7(8):898–904. doi:10.1016/j.jcin.2014.04.005.
- Attizzani GF, Alkhalil A, Padaliya B, et al. Comparison of outcomes of transfemoral transcatheter aortic valve implantation using a minimally invasive versus conventional strategy. Am J Cardiol. 2015;116(11):1731–1736. doi:10.1016/j.amjcard.2015.08.044.
- Mallikethi-Reddy S, Akintoye E, Telila T, et al. Transcatheter aortic valve implantation in the United States: predictors of early hospital discharge. J Interv Cardiol. 2017;30(2):149–155. doi:10.1111/joic.12373.
- Patel SV, Jhamnani S, Patel P, et al. Influence of same-day admission on outcomes following transcatheter aortic valve replacement. J Card Surg. 2016;31(10):608–616. doi:10.1111/jocs.12819.
- D’Errigo P, Ranucci M, Covello RD, et al. Outcome after general anesthesia versus monitored anesthesia care in transfemoral transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth. 2016;30(5):1238–1243. doi:10.1053/j.jvca.2016.05.034.
- Frohlich GM, Lansky AJ, Webb J, et al. Local versus general anesthesia for transcatheter aortic valve implantation (TAVR)–systematic review and meta-analysis. BMC Med. 2014;12:41. doi:10.1186/s12916-014-0141-2.
- Maas EH, Pieters BM, Van de Velde M, Rex S. General or local anesthesia for TAVI? A systematic review of the literature and meta-analysis. Curr Pharm Des. 2016;22:1868–1878.
- Dodson JA, Williams MR, Cohen DJ, et al. Hospital practice of direct-home discharge and 30-day readmission after transcatheter aortic valve replacement in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) registry. J Am Heart Assoc. 2017;6(8). doi:10.1161/JAHA.117.006127.
- Reynolds MR, Baron SJ, Cohen DJ. Economic implications of transcatheter aortic valve replacement in patients at intermediate surgical risk. Circulation. 2016;134(19):1416–1418. doi:10.1161/CIRCULATIONAHA.116.021962.
- Osnabrugge RL, Speir AM, Head SJ, et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg. 2013;96(2):500–506. doi:10.1016/j.athoracsur.2013.04.038.
- Farmer SA, Darling ML, George M, Casale PN, Hagan E, McClellan MB. Existing and emerging payment and delivery reforms in cardiology. JAMA Cardiol. 2017;2(2):210–217. doi:10.1001/jamacardio.2016.3965.