Abstract
Organic and inorganic substances released into the environment as a result of domestic, agricultural and industrial activities often lead to serious pollution. A number of primary and secondary treatment processes are normally used to remove easily settled materials and biodegradable components of the wastewater. The final result is a clear, apparently clean effluent which is discharged into natural water bodies. This secondary effluent is, loaded with inorganic compounds and causes eutrophication and long-term problems because of refractory organics and heavy metals that are being discharged. The aim of this review paper is to determine in some detail the wastewater treatment using algae plant energy. However, algal production raises a number of sustainability concerns regarding land use, net energy return, water use, and nutrient supply. Microalgae culture offers an interesting step for wastewater treatments, because they provide a tertiary treatment coupled with the production of potentially valuable biomass, which can be used for biofuels production. Microalgae cultures offer an elegant solution to tertiary treatments due to the ability of microalgae to use inorganic compounds for their growth. And also, for their capacity to remove heavy metals, as well as some toxic organic compounds, In the current review, the role of micro-algae in the treatment of wastewater and growth parameters to be affected for the cultivation. At the same time, algal cultivation has proven useful in waste treatment processes and thus this aspect is also treated in some detail.
1. Introduction
Pollution of surface water has become one of the most important environmental problems. There are many studies by various authors emphasizing the relationships of algae to clean water. Recently, algae have become significant organisms for biological purification of wastewater since they are able to accumulate plant nutrients, heavy metals, pesticides, organic and inorganic toxic substances and radioactive matters in their cells/bodies. Microalgae play a role in the definition of one planetary boundary, namely the “biochemical flows.” The flow of nutrients into water bodies stimulates algae growth. Microalgae are being pursued as a possible source of third generation biofuels by the trancesterification of algal-derived lipids.
In addition, at least under some conditions, their lipid content can be much higher than the oil seeds typically used at present in biodiesel production. However, there are still a number of technical challenges to be solved before commercial production of biofuels from algae becomes a reality, large scale production of microalgae would also pose a number of significant sustainability issues.
Microalgae biomass results mainly from photosynthesis, which utilizes inorganic compounds (including CO2). In simple terms biosynthesis can be described by the following chemical equations where ammonium and nitrate are the nitrogen sources, respectively:
In the above equations, the chemical formula C106H263 O110N16 represents algal biomass. In addition to nutrient availability, algal biomass production also depends on light energy. So the equation above is just showing us the role of algae in wastewater treatment by production biomass.
Practically, to treat wastewater we need to connect algae production with wastewater treatment plants by selecting good algae species compatible with the properties of the wastewater to be treated. Heavy metals treatment absorption which named Bioaccumulation of metals by algae; Alternative culture and treatment systems that combines algae and bacteria functions etc.
Careful selection of optimum, temperature, pH, treatment time, aeration and dosage of algae are important for favourable outcome.
The aim of this review paper is to determine in some detail the wastewater treatment using algae plant energy. An algae can use for absorption of many contaminants including heavy metals, Ammonium, nitrate, and BODs … It is important that treatment processes decrease the BOD in wastewater, because if water with a high BOD is discharged into a river, it could consume all oxygen in the water killing living organisms.
1.1. Microalgae description
In general algae can be referred to as plant-like organisms that are usually photosynthetic and aquatic, but do not have true roots, stems, leaves, vascular tissue and have simple reproductive structures. They are distributed worldwide in the sea, in freshwater and in most situations on land. Most are microscopic, but some are quite large, e.g., some marine seaweeds that can exceed 50 m in length. The algae have chlorophyll and can manufacture their own food through the process of photosynthesis. Microalgae represent an attractive feedstock for the production of higher energy density. Algae, in general, have the ability to produce a wide array of different chemical intermediates that can be converted into biofuels. Microalgae have the capability of producing hydrogen, lipids, hydrocarbons, and carbohydrates, which can be converted into a variety of fuels. In addition, the 3 and macroalgal biomass itself could be used to produce methane through anaerobic digestion, or syngas and bio-oil through various thermochemical conversion processes such as gasification and pyrolysis. Many species of microalgae are able to produce high levels of oil (up to 50% on a dry cell weight basis). Coupled with their rapid growth rate microalgae can produce 10–100 times more oil than terrestrial oilseed plants. They do not require the use of precious agricultural lands but instead can be cultivated on non-arable land which has little to no use. They are also capable of using a variety of different water sources including fresh, brackish, saline, and waste water, and can use waste CO2 sources as a critical nutrient. CO2 supply is a key issue in algae production. Current technology for algae production could yield a maximum of around 70 t/hectare per year of biomass and about 15,000 litres of algae oil/hectare per year.
1.2. Algae use
Algae are among the fastest growing plants in the world, and about 50% of their weight is oil. This lipid oil can be used to make biodiesel for cars, trucks, and airplanes. Microalgae have much faster growth rates than terrestrial crops. Algae can be grown almost anywhere.
Microalgae are used in many different sectors, like the food industry. It is astonishing from a physiological point of view that to date only approximately 30 microalgal species are economically used (Pulz & Gross, Citation2004). Despite the huge diversity of microalgae, only two cyanobacteria, one red algal species and primarily green algae and diatoms are cultivated on a large scale in different types of cultivation systems.
Microalgae are thought to enhance the nutritional content of conventional food and thus positively affect the health of humans and animals. Some microalgal species are established in the cosmetic industry and pharmacy.
1.3. Algae as a Monitor of water quality
During the last three decades several investigations have described the algal bioassays in response to environmental perturbations and their use as indicative organisms of water quality (Mohamed, Citation1994). In 1959, Palmer published a composite rating of organisms such as: Euglena, Oscillatoria, Chlamydomonas, Scenedesmus, Chlorella, Nitzschia and Navicula, which could be used as indicators of water pollution, whereas the presence of different organisms such as Lemanea, Stigeocloniumand certain species of Micrasterias, Staurastrum, Pinnularia, Meridion and Surirella would indicate that the water sample would be considered unpolluted.
2. Avialable methods
Algae culturing methods are usually divided into either suspension cultures; open ponds, closed reactors and hybrid systems, or immobilized cultures; matrix-immobilized systems and biofilms. The most widely used systems for wastewater treatment and biofuel production are based on suspension cultures. Algal culturing with suspension cultures using open pond systems, either natural water such as lagoons, lakes, and ponds, or artificial ponding systems such as raceway ponds, has received extensive interest. The method of choice for commercial microalgae production has been high rate algal ponds, but a variety of systems including; facultative ponds, maturation ponds, and high-rate algal ponds, have been widely used either separately or in combination in wastewater treatment.
Different processes are presently used for wastewater tertiary treatment Algal-based methods offer the possibility of coupling bioremediation with biofuels production.
Many species of microalgae are potentially able to grow in wastewater from different sources. Various contaminants are present in wastewater depending upon its source but are typically organic and inorganic nitrogen, phosphorus, pathogens, pharmaceuticals and inorganic particles. Extensive studies have been conducted to investigate the use of algae for nutrient removal, in particular nitrogen and phosphorus, from wastewater. In general, the efficiency of nutrient removal is variable, from rather poor to several studies announcing almost complete removal. Although, it is widely thought that environmental factors such as temperature and the amount of sunlight present challenges that restrict algal-based wastewater treatment to tropical countries where the temperature and sunlight are optimum. The major wastewater classes to be treated are municipal, agricultural (e.g., confined animal facilities including dairy, swine, and poultry), industrial (e.g., food processing including olive oil mill, textile, paper, etc.), and other eutrophic waters with high nutrient contents (e.g., agricultural drainage).
Municipal wastewater is one of the main sources of surface water pollution. As discussed above, ideal treatment includes three stages. Secondary treatment using microorganisms requires a constant supply of oxygen, which is expensive and requires intense operations, energy input, manpower, and expertise. Growing microalgae in the ponds and tanks where the treatment is carried out is a good alternative solution to this problem since algal growth and photosynthesis will release substantial amounts of oxygen. At the same time the microalgae will remove nutrients (nitrogen and phosphorus), incorporating them into biomass and thus carrying out tertiary treatment of the wastewater before it is released into the environment.
2.1. Selection of algae and Selection of wastewater
2.1.1. Selection of algae
For the present study, freshwater algae and marine algae was collected and washed thoroughly with tap water and placed in the respective set up for the experimental study. Algal species were identified at Botany laboratory, Thrissur.
2.1.1.1. Freshwater algae
Freshwater algae can be grouped into 10 major divisions (phyla) in relation to microscopical appearance (Table ). Some indication of the ecological and taxonomic diversity of these groups is given by the number of constituent species (Table ) for freshwater and terrestrial algae in in general (taken from John et al., Citation2002), with green algae and diatoms far outnumbering other groups – reflecting their widespread occurrence and ability to live in diverse habitats. Diatoms in particular (over 1600 species) are ecologically successful, both as planktonic and benthic organisms. In addition to the above groups, John et al. (Citation2002) also list other phyla – Raphidophyta (two species), Haptophyta (five species), Eustigmatophyta (three species), Prasinophyta (13 species) and Glaucophyta (two species). Although these minor phyla have taxonomic and phylogenetic interest, they have less impact in the freshwater environment.
Table 1. Major divisions of freshwater algae: microscopical appearanc.
2.1.1.2. Marine algae
Marine microalgae, the largest primary biomass, have been attracting attention as resources for new metabolites and biotechnologically useful genes. The diversified marine environment harbours a large variety of microalgae. There are at least 30,000 known species of microalgae. Microalgae are defined as photosynthetic cells mostly unicellular, although some complex associations give colonies with larger structures. This is a very heterogeneous group comprising prokaryotic organisms similar to bacteria (cyanobacteria, also called blue green algae) and eukaryotic organisms, such as diatoms. The number of blue-green species is very large and probably not fully explored.
2.2. Selection of wastewater
Wastewater chosen was rice mill wastewater which contains N and P. Experiments were performed under laboratory-based batch conditions since algae shows high growth rates over the batch growth period. The rice mill wastewater was collected from Nambiyattukudy agro mills, Perumbavoor, Ernakulam. Synthetic wastewater whose characteristics were similar to rice mill wastewater was used in the study for optimizing the parameters. The composition of this synthetic wastewater was given in Table . The optimum conditions obtained were applied for the treatment of natural wastewater (Table ).
Table 2. Composition of synthetic wastewater.
Table 3. Characteristics of wastewater.
2.2.1. Procedure
The synthetic wastewater of about 5 litres was fed into the feed tank and by gravity it was fed into the rectangular reactor of 35 cm × 25 cm × 18 cm size. The reactor was operated at room temperature. Each algae of initial dosage 60 g was fed to the reactor.
2.2.2. Optimization of number of days
The synthetic wastewater was fed to the reactor containing 60 g of each algae. No pH adjustment was made. Then at each day samples were collected and analysed for the various parameters like pH, TDS, Turbidity, BOD, COD, Ammonia Nitrogen, and Phosphate.
2.2.3. Optimization of pH
After optimizing number of days, the pH of synthetic wastewater was varied. The synthetic wastewater with different pH was fed to the reactor with 60 g of each algae. The selected pH were 4, 5, 6, 7, and 8. The samples collected after the optimized day were analysed for the various parameters.
2.2.4. Varying algal species
Oedogonium and Chara algae of 60 g each were taken separately for treating with synthetic wastewater. Combination of algal species of 60 g were also taken for the study simultaneously.
2.2.5. With aeration
After fixing optimum days and pH the synthetic wastewater was treated with 60 g of Oedogonium and Chara algae separately. Aeration of 9l/min has been provided. The samples were analysed after treatment. The same has been repeated with the combination of algal species.
2.2.6. Without aeration
After fixing optimum number of days and pH treatment of algae with synthetic wastewater has been carried out without aeration. Here also combination of algal species has been taken for the treatment. Similarly individual algal species also has been taken for the treatment. Samples after treatment have been taken for the analysis of the parameters.
2.2.7. Varying algal dosage
The synthetic wastewater was treated with algal species of varying dosage after optimizing number of days and pH. Aeration has been provided.20, 40, 60, 80, 120, and 140 g of each algal species were taken for the study. The samples collected after treatment were analysed for the various parameters.
2.3. Heavy metals removal from wastewater
Microalgae are known to sequester heavy metals (Rai, Gaur, & Kumar, Citation1981). Discharge of toxic pollutants to waste water collection systems has increased concurrently with society’s progressive industrialization.
Significant concentrations of heavy metals and toxic organic compounds have been measured in municipal wastewater. Consequently, the ability of wastewater treatment systems to tolerate and remove toxicity is of considerable importance. Microalgae are efficient absorbers of heavy metals. Bioaccumulation of metals by algae may create a feasible method for remediating wastewater contaminated with metals (Darnall et al., Citation1986; Nakajima, Horikoshi, & Sakagushi, Citation1981). On the other hand advantages of algae are that it may be grown in ponds with little nutritional input or maintenance.
Although the heavy metal contents in some drainage systems generally do not reach the proportions found in industrial effluents, certainly not those of metal processing industries, the problems caused by their presence, particularly in areas with dense population, are of public concern. It is well established that several marine and freshwater algae are able to take up various heavy metals selectively from aqueous media and to accumulate these metals within their cells (Afkar, Ababna, & Fathi, Citation2010; Chen, Chang, Kao, Pan, & Chang, Citation2012; Kumar & Gaur, Citation2011).
Several authors concluded that this method, including the separation of the metal-saturated algae from the medium, is an economic method for removing heavy metals from wastewater, resulting in high quality reusable effluent water (Bhat, Melo, Chaugule, & Souza, Citation2008; Filip, Peters, & Adams, Citation1979; Kiran, Kaushik, & Kaushik, Citation2007; Nasreen, Muhammad, Iqbal, & Javed, Citation2008; Pandi, Shashirekha, & Swamy, Citation2009; Shaaban et al., Citation2004). Numerous species of algae (living and non-living cells) are capable of sequestering significant quantities of toxic heavy metal ions from aqueous solutions. Algal metal sequestering processes occur by different mechanisms. This can be dependent on the alga, the metal ion species, the solution conditions and whether the algal cells are living or nonliving. In living algal cells trace nutrient metals (such as Co, Mo, Ca, Mg, Cu, Zn, Cr, Pb and Se) are accumulated intracellularly by active biological transport (Ajjabi & Chouba, Citation2009; Han, Wong, Wong, & Tam, Citation2007; Kiran, Sandberg, & Sebastian, Citation2011; Rajfur et al., (Citation2010); Singh, Pavankumar, & Lakshmanan, Citation2012; Tuzen & Sarı, Citation2010; Yüce, Nazır, & Dönmez, Citation2010; Yee, Benning, Phoenix, & Ferris, Citation2004).
Field experiments reported by Gale (Citation1986) indicated that, live photosynthetic microalgae have an effective role in metal detoxification of mine wastewater. By using cyanobacteria in a system of artificial pools and meanders, 99% of dissolved and particulate metals could be removed. Soeder, Payer, Runkel, Beine, and Briele (Citation1978) showed that Coelastrum proboscideum absorbs 100% of lead from 1.0 ppm solution with 20 h at 23 °C and about 90% after only 1.5 h at 30 °C.
Cadmium was absorbed a little less efficiently, with about 60% of the cadmium being absorbed from a 40 ppb solution after 24 h. McHardy and George (Citation1990), studied Cladophora glomerata in artificial freshwater channels and found that, the algae were excellent accumulators of zinc. There have also been reports of accumulation of Cu2+, Pb2+ and Cr3+ as well as Ni2+, Cd2+, Co2+, Fe2+and Mn2+ by algae (Chakraborty, Banerjee, & Pal, Citation2011; Chen, Ma, & Han, Citation2008; Gupta and Rastogi, Citation2008; Gupta, Rastogi, & Nayak, Citation2010; Lourie & Gjengedal, Citation2011; Kumar, Rai, & Gaur, Citation2012; Pahlavanzadeh, Keshtkar, Safdari, & Abadi, Citation2010; Piotrowska-Niczyporuk, Bajguz, Zambrzycka, & Godlewska-Żyłkiewicz, Citation2012; Sari and Tuzen, Citation2008; Tastan, Duygu, & Dönmez, Citation2012).
Algae in experimental rice paddles were found to accumulate and concentrate Cd2+ by a factor of about 1000 times when compared to the ambient (Liu, Cao, Luo, & Chen, Citation2009; Reiniger, Citation1977). Algae are also good accumulators of compounds such as organochlorides and tributyl tin (Payer & Runkel, Citation1978; Wright & Weber, Citation1991). They have also been reported to break down some of these compounds (Wu and Kosaric, Citation1991).
Baeza-Squiban, Bouaicha, Santa-Maria, and Marano (Citation1990) and Schimdt (Citation1991) have shown that the green alga Dunaliella bioculata produced an extracellular esterase which degrades the pyrethroid insecticide Deltamethrin. Algae have also been shown to degrade a range of hydrocarbons such as those found in oily wastes (Carpenter, Robertson, & Skierkowski, Citation1989; Cerniglia, Gibson, & Van Baalen, Citation1980).
2.4. Heavy metals removal by microalgae metabolism and microbe interactions
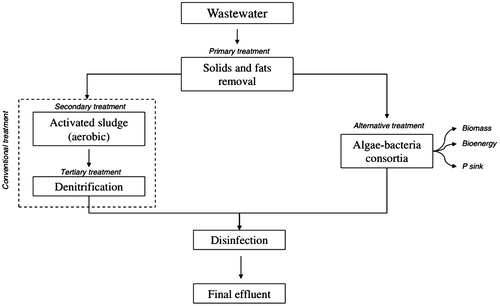
Heavy metals or suspended solids are allowed to settle in sedimentation tanks before they are incinerated or put into landfill. The process discharge and water treatment is referred to in (Figure ).
Treatment uses some type of biological process to consume the large quantities of organic matter present in primary effluent. Traditional treatment processes use microorganisms in aeration tanks to carryout oxidation of the organic matter. Primary effluent is mixed with air in the presence of bacterial sludge and left for several hours; bacteria breaks down the organic matter into relatively harmless smaller and simpler molecules (CO2, PO4, NH3 etc.). Another sedimentation tank is used to remove excess bacteria and sludge. However, together primary and secondary treatments are not sufficient to completely remove inorganic nutrients from wastewater and the resulting secondary effluent can cause eutrophication of rivers and lakes due to a high content of nitrogen and phosphate. Tertiary treatment is designed to remove these nutrients and minimize ecological impacts on the environment. Physico-chemical methods, such as air stripping of ammonia, ion exchange and breakpoint chlorination, or biological methods can be used to remove nitrogen. The most common removal process is denitrification where nitrate is reduced first to nitrite and to nitrogen gas, easily released into the atmosphere. On the other hand, phosphorus is often removed by chemical precipitation using metal salts. While three forms of phosphorus are usually present in the initial wastewater, ortho-phosphate, polyphosphate and organic phosphate, the latter two forms are converted to ortho-phosphate during aerobic treatment.
Typically, microalgae grow autotrophically, i.e., they use sunlight as the energy source and inorganic carbon (CO2) along with inorganic nutrients (N, P, etc.) to form biochemical energy through photosynthesis In wastewater treatment, autotrophic metabolism is the most reported way for inorganic pollutants uptake and removal. As illustrated in Figure , microalgae can remove inorganic N and P from primary effluent, secondary effluent, or centrate from sludge digestion. However, the N- and P-removal efficiencies are dictated by the N/P ratio. Depending on the microalgae species, the N/P ratio can vary from 8 to 45 gN/gP. An interesting characteristic of some microalgae is that they can assimilate P in excess amounts (luxury uptake), which is stored within the cells in the form of polyphosphate granules, resulting in higher P removal capacities. However, P is frequently the growth-limiting factor during wastewater treatment. In case of N, and
are the main forms consumed by algae, followed by urea and nitrite (
).
The interactions between microalgae and bacteria can be positive for algal growth. When bacteria are present, oxygen is consumed to degrade organic matter, while carbon dioxide (CO2) is produced and later assimilated by microalgae. The synergy between microalgae and bacteria can lead to an increase in biomass production and a reduction in aeration requirements which represents N50% of the energy inputs and costs in biological treatment plants. Microalgae–bacteria interactions have been studied in order to understand the dominant microorganisms present in wastewater systems, together with the basic mechanisms by which wastewater components are adsorbed or degraded.
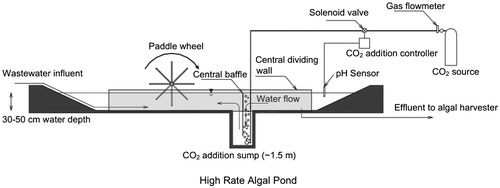
2.5. High rate algal ponds (HRAPs) for wastewater treatment
In practice, high rate algal ponds (HRAPs), also known as raceways ponds, are the most commonly used large scale production systems. HRAP is a technology developed by Oswald and colleagues for wastewater treatment, where it demonstrates a capability for a high rate removal of nutrients (N, P) and wastewater organic compounds, as well as a significant reduction in pathogens. Originally proposed as a method for combined wastewater treatment and biofuel production on a large scale more than fifty years ago, there has been a marked resurgence in interest in this field in the past decade. Structurally, HRAPs are open, relatively shallow ponds, gently mixed using paddle wheels. Thus, they are relatively cheap to construct and easy to operate. HRAPs are typically run at organic loading rates of 100 to 150 kg BOD5 ha−1 day−1, depths varying between 0.25 and 0.6 m, and hydraulic retention times, depending upon the season, from three to four days in the summer and 7–9 days in the winter. However, they can also suffer from several limitations that can affect any pond system, including; low productivity due to microbial and predator contamination, high evaporation rates, inefficient light distribution (dark zones), relatively poor mixing, large areal footprint, and inefficient CO2 absorption.
Algae growing in wastewater treatment HRAPs assimilate nutrients and thus subsequent harvest of the algal biomass recovers the nutrients from the wastewater (García et al., Citation2006; Park & Craggs, Citation2010; Powell, Shilton, Chisti, & Pratt, Citation2009). Wastewater treatment HRAPs are normally part of an Advanced Pond System which typically comprises advanced facultative ponds which incorporate anaerobic digestion pits, HRAP, algal settling ponds and maturation ponds in series (Craggs, Citation2005; Craggs, Green, & Oswald, Citation1999). Based on design for BOD removal, Advanced Pond Systems require approximately 50 times more land area than activated sludge systems (one of the most common wastewater treatment technologies), although this does not account for the land area needed to dispose of waste activated sludge. However, the capital costs for construction of an Advanced Pond System are less than half and operational costs are less than one fifth those of activated sludge systems (Craggs et al., Citationin press) (Figure ).
2.6. Photobioreactor
Photobioreactor optimization can potentially increase biomass production, as observed from improving only air bubbling in this study. Improved air delivery was achieved by changing from spherical to cylindrical ceramic diffusers, resulting in better mixing. Work by Richmond, Richmond, Zou and Qiang indicates that highly productive and efficient enclosed algal systems can be obtained by optimizing cell density and mixing rate in relation to photon flux density, particularly when nutrients are not limited. In addition, better aeration promotes increased mass transfer allowing for the removal of oxygen, which can become inhibiting at high concentrations.
However, there are limits to the photosynthetic conversion of sunlight energy into algal biomass in large-scale outdoor cultures. Under light-limited growth, there is an upper limit for light conversion efficiency of a large-scale culture. In practice, this usually translates to a maximum potential yield of 30–40 g dry wt m−2 day−1 under ideal outdoor sunlight conditions for short periods and considerably less for longer durations. This indicates that the non-optimized operation in this preliminary assessment was able to achieve 10% of the maximum. However, the cultures were grown under conditions of reduced light. It is possible to cultivate algae outdoor and improve light utilization through vertical reactor orientation, while keeping peak temperature down due to mutual shading of reactors. A Photobioreactor is a bioreactor that utilizes light to cultivate phototrophic microorganisms. These organisms use photosynthesis to generate biomass from light and carbon dioxide and include plants, mosses, macroalgae, microalgae, cyanobacteria, and purple bacteria (Figure ).
3. Alternative culture and treatment systems to biodiesel production
3.1. Hyperconcentrated cultures
Hyperconcentrated cultures are cultures with an algal biomass >1.5 g l−1. On a small-scale, experiments with hyperconcentrated cultures have shown that these can accelerate the removal of nutrients compared to normal cultures. Algae for such experiments are concentrated by flocculation and settling using a flocculent such as chitosan (Lavoie & De la Noüe, Citation1983; Morales, de la Noüe, & Picard, Citation1985). Cell concentrations of up to 1.9 g dry weight l−1 have been obtained for Oscillatoria sp. grown on sewage sludge (Hashimoto & Furukawa, Citation1989).
Working with Scenedesmus obliquus cultures have shown that great nitrogen removal was greatly accelerated for 1.9 g dry weight l−1 cultures compared to normal density cultures of 0.5 g dry weight l−1 (Lavoie & de la Noüe, Citation1985). They have also demonstrated that the rate of removal of ammonium and phosphorous in these hyperconcentrated cultures was proportional to algal concentration and independent of the obvious light limitation due to self-shading. Although this work has been carried out only on a small scale so far, the use of such hyperconcentrated cultures would require smaller pond areas, or would permit a reduced residence time, both of which have potential advantages. The engineering and economic feasibility of such systems on a large-scale remains to be determined.
3.2. Immobilized cell system
One of the major problems in the utilization of microalgae for the biological tertiary treatment of wastewater is their recovery from the treated effluent (Chevalier & de la Noüe, Citation1985). Among the ways of solving this problem which have been recently studied are immobilization techniques (De la Noüe and Proulx, Citation1988). Immobilization appears to offer several advantages in comparison with batch or continuous fermentation where free microorganisms are used (Hall and Rao, Citation1989). Chevalier and De la Noüe (Citation1985) found that k-carrageenan-immobilized Scenedesmus cells were able to take up nitrogen and phosphorus at rates similar to those of free microalgae. Immobilized living cells possess some advantages in comparison with suspended cells; for example, immobilized microalgae on a suitable support simplify the treatment of liquid substances because of the entrapment of living cells, which contributes to increasing the cells retention time in the reactor (Travieso, Benitez, & Dupeiron, Citation1992). It will be interesting to confirm the feasibility of using immobilized microalgae and cyanobacteria for removing nitrate, ammonium, and phosphate from high volume effluent discharges. It has been reported that Phormidium laminosum immobilized on polymer foam has the potential to remove nitrate in a continuous-flow system with uptake efficiencies above 90% (De la Noüe et al., Citation1990; Garbisu, Gil, Bazin, Hall, & Serra, Citation1991; Sawayama, Rao, & Hall, Citation1998; Travieso et al., Citation1992). Sawayama et al. (Citation1998) have reported that hollow fibre -immobilized cyanobacterial systems are easy to construct and immobilization does not take a long time. Also Markov, Bazin, and Hall (Citation1995) have reported that high rates of hydrogen production are made possible by using immobilized cyanobacteria on hollow-fibre immobilization systems could improve the removal efficiency of inorganic nutrients from treated wastewater. Direct generation of electricity has also been demonstrated by immobilizing the cyanobacterial species Mastigocladus (Ochiai, Shibata, Sawa, & Katoh, Citation1980) and Phormidium (Ochiai, Shibata, Sawa, Shoga, & Ohta, Citation1983) on SnO2 optically transparent electrodes.
3.3. Dialysis cultures
In dialysis culture the algae are separated from the nutrient-containing medium by a semi-permeable dialysis barrier. Low molecular weight compounds diffuse across this barrier in response to a concentration gradient (Jensen, Citation1976; Marsot, Cembella, & Houle, Citation1991). High cell density cultures can be maintained for prolonged periods in system with a high membrane surface area/culture volume ratio, and the algal cells show very efficient rates of nutrient utilization (Ney, Conary, & Chapman, Citation1981; Marsot et al., Citation1991). One advantage of dialysis culture is that they can serve to exclude inhibitory substances and it also allows the microbiologically pure culture of the algae. The latter is particularly important for the production of high quality large human consumption. Such a system has, as yet, not been applied to the use of wastewaters for algal culture, however, this type of system deserves critical evaluation.
3.4. Tubular photobioreactors
One of the most promising areas in the development of new reactor types is the tubular photobioreactors (Figure ). Basically, these reactors are a closed system consisting of a clear tube within which the algae grow. The algae are circulated by means of a pump and the system also has a gas exchange unit where CO2 can be added and photosynthetically produced O2 is stripped from the medium. If necessary, a heat exchanger is also added to either cool (in tropical areas) or heat (in temperate areas) the culture.
The concept of tubular reactors is not new. Simple reactors were already tested by Davis et al. (Citation1953), and many of the modern systems are derived from the work of Pirt et al. (Citation1983), although similar system had been used in Czechoslovakia at Trebon earlier to grow Chlorella.
Two basic kinds of systems are presently used consisting either of (a) straight tubes arranged flat on the ground or in long vertical rows (Pirt et al., Citation1983; Pirt, Citation1986; Torzillo et al., Citation1986; Bocci, Ttorzillo, Vincenzini, & Materassi, Citation1988; Chaumont et al., Citation1988), or (b) of tubes spirally wound around a central support (Robinson, Reeve, & Goulding, Citation1988; and Borowitzka, Citation1989b), or a similar helical structure (Lee & Bazin, Citation1990).
The tubes can be of glass, Perspex or PVC, and diameters range from about 24 cm to 24 mm. It is interesting to note that most systems are now tending to use the narrower diameter tubes, since these appear to have better hydrodynamic properties and result in improved productivity. Circulation of the algal culture is by means of diaphragm, peristaltic, lobe or centrifugal pumps or by an airlift. From an engineering point of view, the circular reactors are easier to construct, and occupy less land area per unit volume.
These photobioreactors have been used on a pilot scale to grow a wide variety of algae including Spirulina, Porphyridium, Chlorella, Dunaliella, Haematococcus, Tetraselmis and Phaeodactylum. These reactors also have the advantage of almost linear scale-up, unlike paddle wheel and similar ponds, where scale-up presents major difficulties (Borowitzka & Borowitzka, Citation1989a). Tubular reactors have several potential problems which affect algal productivity. These are temperature control, control of O2 and CO2, growth of the algae on the inner surface of the tubes and adequate circulation speeds without damage to the relatively fragile algal cells.
3.5. Stabilization ponds
Waste Stabilization Ponds (WSP) have proven to be effective alternatives for treating wastewater, and the construction of low energy-consuming ecosystems that use natural processes, in contrast to complex high-maintenance treatment systems, will hopefully lead to more ecologically sustainable wastewater treatment in future. CWs also have the capability of meeting the demand for a high percentage removal of pathogenic organisms, compared to conventional technologies. CWs combined, and joined with other technologies, may be important for even more improved performance of water cleaning systems. Many countries in tropical climates use WSPs for wastewater treatment (e.g., Tanzania, Kenya, Malawi, Uganda, Zambia, Botswana, Zimbabwe). Many of these systems have been performing below the required standards, due to lack of proper operation and maintenance (Kayombo et al., Citation1999).
Waste Stabilization Ponds (WSPs) are large, shallow basins in which raw sewage is treated entirely by natural processes involving both algae and bacteria. They are used for sewage treatment in temperate and tropical climates, and represent one of the most cost-effective, reliable and easily operated methods for treating domestic and industrial wastewater. Waste stabilization ponds are very effective in the removal of faecal coliform bacteria. Sunlight energy is the only requirement for its operation. Further, it requires minimum supervision for daily operation, by simply cleaning the outlets and inlet works. The temperature and duration of sunlight in tropical countries offer an excellent opportunity for high efficiency and satisfactory performance for this type of water-cleaning system. Further, the advantage of these systems, in terms of removal of pathogens, is one of the most important reasons for its use.
3.5.1. Types of waste stabilization ponds
WSP systems comprise a single string of anaerobic, facultative, and maturation ponds in series, or several such series in parallel. In essence, anaerobic and facultative ponds are designed for the removal of Biochemical Oxygen Demand (BOD), and maturation ponds for pathogen removal, although some BOD removal also occurs in maturation ponds and some pathogen removal in anaerobic and facultative ponds (Mara, Citation1987). In most cases, only anaerobic and facultative ponds will be needed for BOD removal when the effluent is to be used for restricted crop irrigation and fish pond fertilization, as well as when weak sewage is to be treated prior to its discharge to surface waters. Maturation ponds are only required when the effluent is to be used for unrestricted irrigation, thereby having to comply with the WHO guideline of >1000 faecal coliform bacteria/100 ml. The WSP does not require mechanical mixing, needing only sunlight to supply most of its oxygenation. Its performance may be measured in terms of its removal of BOD and faecal coliform bacteria.
3.5.1.1. Anaerobic ponds
Anaerobic ponds are (Figure ) commonly 2–5 m deep and receive wastewater with high organic loads (i.e., usually greater than 100 g BOD/m3 day, equivalent to more than 3000 kg/ha day for a depth of 3 m). They normally do not contain dissolved oxygen or algae. In anaerobic ponds, BOD removal is achieved by sedimentation of solids, and subsequent anaerobic digestion in the resulting sludge. The process of anaerobic digestion is more intense at temperatures above 15 °C. The anaerobic bacteria are usually sensitive to pH < 6.2. Thus, acidic wastewater must be neutralized prior to its treatment in anaerobic ponds. A properly designed anaerobic pond will achieve about a 40% removal of BOD at 10 °C, and more than 60% at 20 °C. A shorter retention time of 1.0–1.5 days is commonly used.
3.5.1.2. Facultative ponds
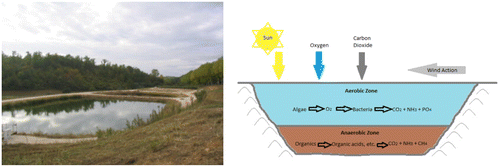
Facultative ponds (Figure ) (1–2 m deep) are of two types: Primary facultative ponds that receive raw wastewater, and secondary facultative ponds that receive particle-free wastewater (usually from anaerobic ponds, septic tanks, primary facultative ponds, and shallow sewerage systems). The process of oxidation of organic matter by aerobic bacteria is usually dominant in primary facultative ponds or secondary facultative ponds.
The processes in anaerobic and secondary facultative ponds occur simultaneously in primary facultative ponds, as shown in Figure . It is estimated that about 30% of the influent BOD leaves the primary facultative pond in the form of methane (Marais, Citation1970). A high proportion of the BOD that does not leave the pond as methane ends up in algae. This process requires more time, more land area, and possibly 2–3 weeks water retention time, rather than 2–3 days in the anaerobic pond. In the secondary facultative pond (and the upper layers of primary facultative ponds), sewage BOD is converted into “Algal BOD,” and has implications for effluent quality requirements. About 70–90% of the BOD of the final effluent from a series of well-designed WSPs is related to the algae they contain.
In secondary facultative ponds that receive particle-free sewage (anaerobic effluent), the remaining non-settleable BOD is oxidized by heterotrophic bacteria (Pseudomonas, Flavobacterium, Archromobacter and Alcaligenes spp). The oxygen required for oxidation of BOD is obtained from photosynthetic activity of the microalgae that grow naturally and profusely in facultative ponds.
Facultative ponds are designed for BOD removal on the basis of a relatively low surface loading (100–400 kg BOD/ha day), in order to allow for the development of a healthy algal population, since the oxygen for BOD removal by the pond bacteria is generated primarily via algal photosynthesis. The facultative pond relies on naturally growing algae. The facultative ponds are usually dark-green in colour because of the algae they contain. Motile algae (Chlamydomonas and Euglena) tend to predominate the turbid water in facultative ponds, compared to none-motile algae (Chlorella).
The algal concentration in the pond depends on nutrient loading, temperature and sunlight, but is usually in the range of 500–2000 μg chlorophyll-a/litre (Mara, Citation1987). Because of the photosynthetic activities of pond algae, there is a diurnal variation in the dissolved oxygen concentration. The dissolved oxygen concentration in the water gradually rises after sunrise, in response to photosynthetic activity, to a maximum level in the mid-afternoon, after which it falls to a minimum during the night, when photosynthesis ceases and respiratory activities consume oxygen. At peak algal activity, carbonate and bicarbonate ions react to provide more carbon dioxide for the algae, leaving an excess of hydroxyl ions. As a result, the pH of the water can rise to above 9, which can kill faecal coliform. Good water mixing, which is usually facilitated by wind within the upper water layer, ensures a uniform distribution of BOD, dissolved oxygen, bacteria and algae, thereby leading to a better degree of waste stabilization.
3.5.1.3. Maturation ponds
The maturation ponds, usually 1–1.5 m deep, receive the effluent from the facultative ponds. Their primary function is to remove excreted pathogens. Although maturation ponds achieve only a small degree of BOD removal, their contribution to nutrient removal also can be significant. Maturation ponds usually show less vertical biological and physicochemical stratification, and are well-oxygenated throughout the day. The algal population in maturation ponds is much more diverse than that of the facultative ponds, with non-motile genera tending to be more common. The algal diversity generally increases from pond to pond along the series (Mara, Citation1989). Although faecal bacteria are partially removed in the facultative ponds, the size and numbers of the maturation ponds especially determine the numbers of faecal bacteria in the final effluent. There is some removal of solids-associated bacteria in anaerobic ponds, principally by sedimentation. The principal mechanisms for faecal bacteria removal in facultative and maturation ponds are now known to be:
| (a) | Time and temperature; | ||||
| (b) | High pH (>9); and | ||||
| (c) | High light intensity, combined with high dissolved oxygen concentration. | ||||
Time and temperature are the two principal parameters used in designing maturation ponds. Faecal bacteria die-off in ponds increases with both time and temperature (Feachem et al., Citation1983). High pH values (above 9) occur in ponds, due to rapid photosynthesis by pond algae, which consumes CO2 faster than can be replaced by bacterial respiration. As a result, carbonate and bicarbonate ions dissociate, as follows:
The resulting CO2 is fixed by the algae, and the hydroxyl ions accumulate, often raising the pH to values above 10. Faecal bacteria (with the notable exception of Vibrio cholerae) die very quickly at pH values higher than 9 (Pearson et al., Citation1987). The role of high light intensity and high dissolved oxygen concentration has recently been elucidated (Curtis, Mara, & Silva, Citation1992). Light of wavelengths between 425 and 700 nm can damage faecal bacteria by being absorbed by the humic substances ubiquitous in wastewater. They remain in an excited state sufficiently long to damage the cell. Light-mediated die-off is completely dependent on the presence of oxygen, as well as being enhanced at high pH values. Thus, the sun plays a threefold role in directly promoting faecal bacteria removal in WSP, and in increasing the pond temperature, and more indirectly by providing the energy for rapid algal photosynthesis. This not only raises the pond pH value above 9, but also results in high dissolved oxygen concentrations, which are necessary for its third role; namely, promoting photo-oxidative damage.
3.6. Algal mats
All the systems considered so far have used microalgae. An alternative system for nutrient removal from wastewaters is to use attached macroalgae or other aquatic plants. One such system is the algal mat system developed by Adey (Citation1982), and which is being used to remove nutrients from large tropical aquarium systems such as those at Reef World and at the James Cook University in Townsville. In this system, the algae (a range of turf-forming species such as Enteromorpha, Cladophora, Sphacelaria, Ectocarpus, Ceramium, Polysiphonia, Herposiphonia and Oscillatoria) are grown on a net or mesh and the nutrient-rich water is passed over them. The algae containing the nutrients are regularly removed by mechanically removing them from the mats. Although this system has proven very effective in controlling the nutrient levels in the aquarium water so that even corals, which are very sensitive to elevated nutrient levels, can grow, it does require a large surface area and is very labour intensive. In certain months of the year the natural daylight also has to be supplemented with artificial lighting to maintain an adequate rate of nutrient removal.
Other aquatic plant-based systems have also been proposed for nutrient removal using aquatic plants such as water hyacinth, Typha and Phragmites, however all of these systems have been shown to be less efficient than algal systems (Werblan, Smith, Van der Valk, & Davis, Citation1978; Wolverton, Citation1982; Finlayson & Chick, Citation1983; and Finlayson, Chick, von Oertzen, & Mitchell, Citation1987).
3.7. Utilization of harvested algae biomass in biofuels production
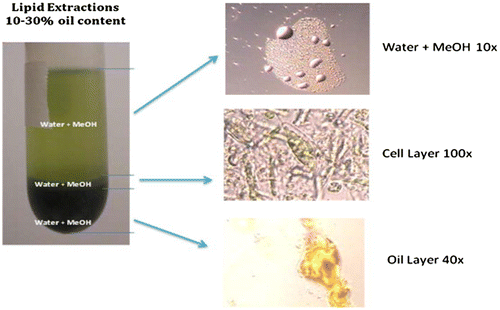
Waste-grown microalgae are a potentially important biomass for biofuel production. However, most of the wastewater treatment ponds systems do not use algae harvesting. Those that do, typically return the biomass to the ponds, where it decomposes on the pond floor, releasing methane to the atmosphere and degrading water quality (Chaiprasert, Citation2011). Instead, the algae biomass could be processed for lipid extraction to be used in transportation fuel, or it can be anaerobically digested to make biogas (US, DOE, Citation2009; Brune, Lundquist, & Benemann, Citation2009) (Figure ).
Waste-grown algae have widely varying lipid contents, and the technologies for lipid extraction are still under development (Woertz, Lundquist, Feffer, & Nelson, Citation2009). Thus, anaerobic digestion is likely to be the near term, appropriate use of algae biomass at wastewater treatment plants. However, algae typically yield less methane than wastewater sludge (∼0.3 vs. 0.4 L CH4/g volatile solids introduced). Ammonia toxicity and recalcitrant cell walls are commonly cited causes of the lower yields. Ammonia toxicity might be counteracted by co-digesting algae with high-carbon organic wastes. Carbon-rich feedstocks that are available near major wastewater pond systems include primary and secondary municipal sludge, sorted municipal organic solid waste, waste fats–oils greases (FOGs), food industry waste, waste paper, and various agricultural residues. Acclimation of the digester microbial community to algae digestion may also improve the yield.
Microalgae have two major advantages over higher plants with respect to biofuels production. First, biomass productivities are significantly greater for microalgae, with productivities projected at about 70 metric tons per hectare-year of ash-free dry weight (i.e., organic matter) in specialized growth reactors, such as high rate ponds (Sheehan et al. Citation1998). This productivity compares well with terrestrial temperate crops (e.g., 3 MT/ha yr for soybeans, 9 MT/ha yr for corn, and 10–13 MT/ha yr for switchgrass or hybrid poplars (Perlack et al., Citation2005). Second, the cultivation of microalgae does not require arable land or fresh water – it can be carried out in shallow ponds on hardpan soils, using saline or brackish water. Relatively few studies have been published on the anaerobic digestion of microalgae (reviewed recently by Sialve, Bernet, & Bernard, Citation2009). The earliest work compared digestion of domestic wastewater sludge and green microalgal biomass, Scenedesmus and Chlorella, harvested from wastewater ponds (Golueke, Oswald, & Gotaas, Citation1957). They found that these algae could yield as much as 0.25–0.50 L CH4/g VS input at an 11-day retention time when incubated at 35–50 °C. (Methane yield is typically expressed as litres of methane produced per gram of volatile solids introduced into a digester.) The lower value was 32% less than the yield from the wastewater sludge. In addition, the maximum VS destruction was about 45% for the algae, compared to 60% for the wastewater sludge. They suggested that the relatively low digestability and thus yield of microalgal biomass was the result of cell walls resisting bacterial degradation, but being more readily digested by bacteria at the higher temperature. Methane yield and productivity were doubled when equal masses of wastewater sludge and Spirulina biomass were co-digested (Samson & LeDuy, Citation1983). Similarly, Yen (Citation2004) and Yen and Brune (Citation2007) added waste paper (50% w/w) to aquacultural microalgal sludge to adjust the C:N ratio to around 20–25:1 which, in turn, doubled the methane production rate from 0.6 L/L day to 1.2 L/L day at 35 °C and with a hydraulic retention time of 10 days.
3.8. Biomass valorization
Once the biomass has been harvested and the extracellular water removed, the dry weight concentration is generally around 15–25%. The harvested biomass can be used in the agricultural sector, either as an animal feed or as a fertilizer. However, for these applications, microalgae biomass should not contain high concentration of persisting pollutants such as heavy metals or persisting organic pollutants that could be transferred into the animals or the soil. For those uses, drying would be required. Two techniques are particularly adapted since they do not denature the biomass: spray drying or solar drying. Spray-drying is very effective but is very energy-intensive due to the use of a hot gas (nitrogen or air) to dry the biomass. Solar drying is very efficient and has a very low energy demand but requires a large surface area. After drying the biomass can be used as animal feed or as a fertilizer. The wet biomass can also be used as a feedstock for composting. Composting has been successfully performed at the pilot-scale for macroalgae and is envisaged to be as equally successful with microalgal biomass. Indeed green seaweed compost could effectively increase the growth and water resistance of tomato plants. After drying, the biomass can also be used as feedstock for high-value molecules depending on the dominant microalgae strains in the wastewater grown biomass. As an example, cyanobacteria are a good source for pigment such as phycocyanin; this water-soluble pigment is easily extracted from the biomass. Other high-value molecules such as omega 3 or carotenoids are very interesting on an economical point of view. However, the productivity of these molecules in wastewater grown microalgal biomass is likely to be very low since it needs specific conditions to be optimized (axenic cultures, optimum temperature and medium, etc ...). Furthermore, strict regulations imposed by the food, pharmaceutical and cosmetic industries would probably impede the entry of wastewater-grown microalgal extracts on those markets.
Therefore, the most promising use of this biomass would be the energy market. For energy applications, drying should be avoided. Wet processes have to be used to convert the biomass into energy. Lipids can be extracted through wet extraction techniques and then converted through transesterification. Promising results are coming from recent screenings, for example, strains were found to grow on wastewater and accumulate lipids at the same time (up to 23.7 mg/L/day).
However, it is still difficult to adjust the microalgae metabolism to lipid accumulation in a microalgae culture growing on wastewater. High lipid productivities (at least over 200 mg/L/day) are needed for economic and energetic viability of microalgae to biofuel processes.
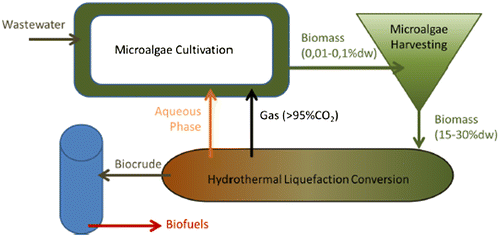
Direct wet conversion processes of the whole biomass such as anaerobic digestion or hydrothermal liquefaction (HTL) are therefore more adapted. Anaerobic digestion is the conversion of a biomass through dark fermentation into a biogas. It is efficient on microalgae with theoretical yields between 260 and 414 mL of CH4/g of volatile solids. Unfortunately, the economic value of biogas is too low at present (at most 1.33 /Nm3 of CH4 using the highest electricity buy-back rate of Electricité de France). Nowadays, anaerobic digestion is not an economically profitable solution for microalgae biomass valorization. HTL is a thermochemical process which converts wet biomass into a biocrude (heavy oil, yields between 20 and 87%, gas (>95% of CO2 that can be recycled to the cultivation step), some residual solids and an aqueous phase that contains large amount of nutrients. The potential to recycle the aqueous phase has been studied in order to reduce the cultivation costs and increase the overall sustainability of the process. Growth can be inhibited at first but after an adaptation period, higher biomass productivities have been observed probably due to mixotrophic growth.
The biocrude can be directly burned in a boiler or upgraded through hydrotreating into a biofuel (a mix of naphta, gasoline and jet fuel). HTL converts the whole biomass, therefore, there is no need for a monoclonal, monospecies, high lipid-producing microalgae in comparison to the lipid extraction and conversion pathway. The HTL biomass conversion process is agnostic to the type of feed and hence, broadens the range of biomass and mixtures of organic material (including the activated sludge (bacteria) and algal biomass, as well as zooplankton generated from wastewater treatment) that can be used. The concept is represented on Figure . Additionally, HTL pathways are getting closer to economic viability with estimated biofuel production cost around 2.5 €/L of biofuel .
4. Discussion
This study was conducted to assess the potential of cultivating algae using wastewater as a treatment. As we knew, microalgae are the effective for wastewater treatment. In this domain of wastewater treatment using algae plants biomass is very difficult to find algae species that have typically high ability to remove respective types of pollutants in water bodies. That is the limitation of all papers or articles viewed. We have not yet determined which species of algae can treat each kind of pollution.
After publication of this paper, the forthcoming research focuses on analysis of algae species, respectively, to their ability and adaptation for types of wastewater treatment in a given place. We also need to know the amount of pollutants removed by each algae species in a given times.
In large-scale algae cultivation, the biofuels production can use as bio-energies instead of fossils energies if we want to think about future generation.
5. Conclusion
The wastewater contributes enormous amounts of organic matters and plants nutrients, which give rise to eutrophication and pollution. The major wastewater classes to be treated are municipal, agricultural, industrial, and other eutrophic waters with high nutrient contents. Microalgae production is a promising way to treat and utilize wastewaters.
The urbanization and industrial development need for algae treatment plants became more important. Wastewater treatment which is applied to improve or upgrade the quality of a wastewater involves physical, chemical and biological processes in primary, secondary stages. More algae plants are designed to remove solids (primary process), followed by a secondary process to reduce the Biological Oxygen Demand (BOD).
Recently, algae have become significant organisms for biological purification of wastewater since they are able to accumulate plant nutrients, heavy metals, pesticides, organic and inorganic toxic substances, and radioactive matters in their cells/bodies with their bioaccumulation abilities. Particularly, biological wastewater treatment systems with microalgae have gained great importance in last 50 years and it is now widely accepted that algal wastewater treatment systems are as effective as conventional treatment systems.
Synthetic wastewater treatment using algal species has been done with varying conditions and it is optimized. Finding the optimal conditions for algae growth under the Presence of Carbon Dioxide is very important in this regard as maximum productivity and wastewater treatments.
Algae can be used in wastewater treatment for a range of purposes, including: reduction of BOD, removal of N and/or P, inhibition of coliforms, removal of heavy metals.
The basic aim of this paper is to improve knowledge about which algae are useful in wastewater treatment and algae biomass production to biogas or biofuels production.
We need to think about our future by algae biomass utilization, compared to these fossils energies algae biofuels energies are lowest cost and its natural process. It is hoped that with further research many of these challenges can be overcome.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adey, W.H. (1982 June). Algal turf scrubber. U.S. Patent No. 4333263. Washington, DC: U.S. Patent and Trademark Office.
- Afkar, A., Ababna, H., & Fathi, A.A. (2010). Toxicological response of the green alga Chlorella vulgaris to some heavy metals. American Journal of Environmental Sciences, 6, 230–237.10.3844/ajessp.2010.230.237
- Ajjabi, L.C., & Chouba, L. (2009). Biosorption of Cu+2 and Zn2+ from aqueous solutions by dried marine green macroalga Chaetomorpha linum. Journal of Environmental Management, 90, 3485–3489.
- Baeza-Squiban, A., Bouaicha, N., Santa-Maria, A., & Marano, F. (1990). Demonstration of the excretion by Dunaliella bioculata of esterases implicated in the metabolism of Deltamethrin, a pyrethroid insecticide. Bulletin of Environmental Contamination and Toxicology, 45, 39–45.10.1007/BF01701826
- Bhat, S.V., Melo, J.S., Chaugule, B.B., & Souza, S.F.D. (2008). Biosorption characteristics of uranium (VI) from aqueous medium onto Catenella repens, a red alga. Journal of Hazardous Materials, 158, 628–635.10.1016/j.jhazmat.2008.02.042
- Bocci, F., Ttorzillo, G., Vincenzini, M., Materassi, R. (1988). Growth physiology of Spirulina platensis in tubular photobioreactor under natural light. In T. Stadler, J. Mollion, M.C. Verdus, Y. Karamanos, H. Morvan, D. Christiaen (Eds.), Algal biotechnology (pp. 15–26). London: Eelsevier Applied Science.
- Borowitzka, L.J., & Borowitzka, M.A. (1989a). Carotene (Provitamin A) production with algae. In E.J. Vandamme (Ed.), Biotechnology of vitamins, pigments and growth factors, (pp. 15–26). London: Elsevier Applied Science.
- Borowitzka, L.J., & Borowitzka, M.A. (1989b). Industrial production: methods and economics. In R. C. Cresswell, T. A. V. Rees, & N. Shah (Eds.), Algal and cyanobacterial biotechnology (pp. 244–316). London: Longman Scientific.
- Brune, D.E., Lundquist, T.J., & Benemann, J.R. (2009). Microalgal biomass for greenhouse gas reductions: Potential for replacement of fossil fuels and animal feeds. Journal of Environmental Engineering, 135, 1136–1144.10.1061/(ASCE)EE.1943-7870.0000100
- Carpenter, M., Robertson, J., & Skierkowski, P. (1989). Biodegradation of an oily bilge waste using algae. Biotreatment. The use of microorganisms in the treatment of hazardous materials and hazardous wastes. O (pp. 141–150). Odisha, India: The Hazardous Materials Control Research Institute.
- Cerniglia, C.E., Gibson, D.T., & Van Baalen, C. (1980). Oxidation of napthalene by Cyanobacteria and microalgae. Microbiology, 116, 495–500.
- Chaiprasert, P. (2011). Biogas production from agricultural wastes in Thailand. Journal of Sustainable Energy & Environment Special Issue, 63–65.
- Chakraborty, N., Banerjee, A., & Pal, R. (2011). Accumulation of lead by free and immobilized cyanobacteria with special reference to accumulation factor and recovery. Bioresource Technology, 102, 4191–4195.10.1016/j.biortech.2010.12.028
- Chaumont, D., Thepenier, C., Gudin, C., & Junjias, C. 1988. Scaling up a tubular photoreactor for continuous culture of Porphyridium cruentum from laboratory to pilot plant (1981–1987). In T. Stadler, J. Mollion, M.Cc. Verdus, Y. Karamanos, H. Morvan, D. Cchristiaen (Eds.), Algal biotechnology (pp. 199–208). London: Elsevier Applied Science.
- Chen, C., Chang, H., Kao, P., Pan, J., & Chang, J. (2012). Biosorption of cadmium by CO2-fixing microalga Scenedesmus obliquus CNW-N. Bioresource Technology, 105, 74–80.10.1016/j.biortech.2011.11.124
- Chen, Z., Ma, W., & Han, M. (2008). Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): Application of isotherm and kinetic models. Journal of Hazardous Materials, 155, 327–333.10.1016/j.jhazmat.2007.11.064
- Chevalier, P., & de la Noüe, J. (1985). Wastewater nutrient removal with microalgae immobilized in carrageenan. Enzyme and Microbial Technology, 7, 621–624.10.1016/0141-0229(85)90032-8
- Craggs, R.J. (2005). Advanced integrated wastewater ponds. In A. Shilton (Ed.), Pond treatment technology (pp. 282–310). London: IWA Scientific and Technical Report Series, IWA.
- Craggs, R.J., Green, F.B., & Oswald, W.J. (1999). Economic and energy requirements of advanced integrated wastewater pond systems (AIWPS). Proceedings of the NZWWA Annual Conference, pp. 1–7.
- Curtis, T.P., Mara, D.D., & Silva, S.A. (1992). Influence of pH, oxygen and humic substances on ability of sunlight to damage faecal coliforms in waste stabilization pond water. Applied and Environmental Microbiology, 58, 1335–1343.
- Darnall, D.W., Greene, B., Henzl, M.T., Hosea, J.M., McPherson, R.A., Sneddon, J., & Alexander, M.D. (1986). Selective recovery of gold and other metal ions from an algal biomass. Environmental Science & Technology, 20, 206–208.10.1021/es00144a018
- Davis, E.A., Dedrick, J., French, C.S., Milner, H.W., Myers, J., Smith, J.H.C., & Spoehr, H.A. (1953). Laboratory experiments on Chlorella culture at the Carnegie Institution of Washington Department of Plant Biology. In J. S. Burlew (Ed.), Algal culture. From laboratory to pilot plant (pp. 105–153). Washington, DC: Ccarnegie Institution of Washington.
- De la Noüe, J., & Proulx, D. 1988. Tertiary treatment of urban wastewater by chitosan-immobilized Phormidium sp. In T. Stadler, J. Mollion, M.C. Verdus, Y. Kamaranos, H. Morvan, D. Christaien (Eds.), Algal biotechnology (pp. 159–168). New York, NY: Elsevier Applied Science.
- De la Noüe, J., Chevalier, P., & Proulx, D. 1990. Effluent treatment with immobilized microalgae and cyanobacteria: a critical assessment. In T. Tyagi, Vembuk (Eds.), Wastewater treatment by immobilized cells (pp. 143–152). Boca Raton, FL: CRC Press.
- Feachem, R.G., Bradley, D.J., Garelick, H., & Mara, D.D. 1983. Sanitation and disease: Health aspects of excreta and wastewater management. Chichester: Wiley
- Filip, S.D., Peters, T., & Adams, V.D. (1979). Middle brooks E.J. Residual heavy metal removal by an algae-intermittent sand filtration system. Water Research, 13, 305–313.10.1016/0043-1354(79)90211-2
- Finlayson, C.M., & Chick, A.J. (1983). Testing the potential of aquatic plants to treat abattoir effluent. Water Research, 17, 415–422.10.1016/0043-1354(83)90138-0
- Finlayson, M., Chick, A., von Oertzen, I., & Mitchell, D. (1987). Treatment of piggery effluent by an aquatic plant filter. Biological Wastes, 19, 179–196.10.1016/0269-7483(87)90051-6
- Gale, N. L. (1986). The role of algae and other microorganisms in metal detoxification and environmental clean-up. Biotechnology and Bioengineering Symposium, 16, 171–180.
- Garbisu, C., Gil, J.M., Bazin, M.J., Hall, D.O., & Serra, J.L. (1991). Removal of nitrate from water by foam-immobilized Phormidium laminosum in batch and continuous-flow bioreactors. Journal of Applied Phycology, 3, 1–14.
- García, J., Green, B.F., Lundquist, T., Mujeriego, R., Hernández-Mariné, M., & Oswald, W.J. (2006). Long term diurnal variations in contaminant removal in high rateponds treating urban wastewater. Bioresource Technology, 97, 1709–1715.10.1016/j.biortech.2005.07.019
- Golueke, C.G., Oswald, W.J., & Gotaas, H.B. (1957). Anaerobic digestion of algae. Applied Microbiology, 5, 47–55.
- Gupta, V.K., & Rastogi, A. 2008. Biosorption of lead (II) from aqueous solution by non-living algal biomass Oedogonium sp. and Nostoc sp.- A comparative study. Colloids and Surfaces B: Biointerfaces, 64, 170–178.
- Gupta, V.K., Rastogi, A., & Nayak, A. (2010). Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. Journal of Colloid and Interface Science, 342, 533–539.10.1016/j.jcis.2009.10.074
- Hall, D.O., & Rao, K.K. (1989). Immobilized photosynthetic membranes and cells for the production of fuel and chemicals. Chimicaoggi, 3, 40–47.
- Han, X., Wong, Y.S., Wong, M.H., & Tam, N.F.Y. (2007). Biosorption and bioremedation of Cr(VI) by a microalgal isolate. Journal of Hazardous Materials, 146, 65–72.10.1016/j.jhazmat.2006.11.053
- Hashimoto, S., & Furukawa, K. (1989). Nutrient removal from secondary effluent by filamentous algae. Journal of Fermentation and Bioengineering, 67, 62–69.10.1016/0922-338X(89)90088-3
- Jensen, A. (1976). Dialysis culture in integrated aquaculture. In O. Devik (Ed.), Harvesting polluted waters (pp. 143–149). New York, NY: Plenum Publishing Corp.10.1007/978-1-4613-4328-8
- John et al., 2002. A Biodiversity: number of species of freshwater and terrestrial algae; adapted from Sigee et al, (2004). Freshwater algae: identification and use as bioindicators, pp. 5–7.
- Kayombo, S., Mbwette, T.S.A., Mayo, A.W., Katima, J.H.Y., & Jorgensen, S.E. (1999). Modelling diurnal variation of dissolved oxygen in waste stabilization ponds. Proceedings of the 4th International Specialist Conference on Waste Stabilization Ponds: Technology and Environment; Poster Session, IAWQ, Marrakech, Morocco.
- Kiran, B., Kaushik, A., & Kaushik, C.P. (2007). Biosorption of Cr(VI) by native isolate of Lyngbya putealis (HH-15) in the presence of salts. Journal of Hazardous Materials, 141, 662–667.10.1016/j.jhazmat.2006.07.026
- Kiran, S., Sandberg, C., & Sebastian, R. (2011). Treatment of category generation and retrieval in aphasia: effect of typicality of category items. Journal of Speech Language Hearing Research., 54, 1101–1117. doi:10.1044/1092-4388(2010/10-0117)
- Kumar, D., & Gaur, J.P. (2011). Metal biosorption by two cyanobacterial mats in relation to pH, biomass concentration, pretreatment and reuse. Bioresource Technology, 102, 2529–2535.10.1016/j.biortech.2010.11.061
- Kumar, D., Rai, J., & Gaur, J.P. (2012). Removal of metal ions by Phormidium bigranulatum (Cyanobacteria)-dominated mat in batch and continuous flow systems. Bioresource Technology, 104, 202–207.10.1016/j.biortech.2011.11.002
- Lavoie, A., & De la Noüe, J. (1983). Harvesting microalgae with chitosan. Journal of the World Mariculture Society, 14, 685–694.
- Lavoie, A., & de la Noüe, J. (1985). Hyperconcentrated cultures of Scenedesmus obliquus. A new approach for wastewater biological tertiary treatment. Water Research, 19, 1437–1442.10.1016/0043-1354(85)90311-2
- Lee, E.T.Y., & Bazin, M.J. (1990). A laboratory scale air-lift helical photobioreactor to increase biomass output rate of photosynthetic algal cultures. New Phytologist, 116, 331–335.10.1111/nph.1990.116.issue-2
- Liu, Y., Cao, Q., Luo, F., & Chen, J. (2009). Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. Journal of Hazardous Materials, 163, 931–938.10.1016/j.jhazmat.2008.07.046
- Lourie, E., & Gjengedal, E. (2011). Metal sorption by peat and algae treated peat: Kinetics and factors affecting the process. Chemosphere, 85, 759–764.10.1016/j.chemosphere.2011.06.055
- McHardy, B.M., & George, J.J. (1990). Bioaccumulation and toxicity of zinc in green alga Cladophora glomerata. Environmental Pollution, 66, 55–66.10.1016/0269-7491(90)90198-L
- Mara, D.D. (1987). Waste stabilization ponds: Problems and controversies. Water Quality International, 1, 20–22.
- Mara, D.D. 1989. The conservation of drinking-water supplies: Techniques for lowincome settlements. Nairobi: United Nations Centre for Human Settlements.
- Marais, G.V.R. (1970). Dynamic behaviour of oxidation ponds. In R.E. McKinney (Ed.), Proceedings of the Second International Symposium on Waste Treatment Lagoons (pp. 15–46). Laurence, KS: University of Kansas.
- Markov, S.A., Bazin, M.J., & Hall, D.O. (1995). Hydrogen, photoproduction and carbon dioxide uptake by immobilized Anabaena variabilis in a hollow-fiber photobioreactor. Enzyme and Microbial Technology, 17, 306–310.10.1016/0141-0229(94)00010-7
- Marsot, P., Cembella, A., & Houle, L. (1991). Growth kinetics and nitrogen-nutrition of the marine diatom Phaeodactylum tricomutum in continuous dialysis culture. Journal of Applied Phycology, 3, 1–10.10.1007/BF00003914
- Mohamed, N.A. (1994). Application of algal ponds for wastewater treatment and algal production. ( M.Sc. thesis). Fac.of Sci. (Cairo Univ.) Bani-Sweef Branch.
- Morales, J., de la Noüe, J., & Picard, G. (1985). Harvesting marine microalgae species by chitosan flocculation. Aquacultural Engineering, 4, 257–270.10.1016/0144-8609(85)90018-4
- Nakajima, A., Horikoshi, T., & Sakagushi, T. (1981). Recovery of varnlum by immobilized microorganisms. European Journal of Applied Microbiology and Biotechnology, 16, 88–91.
- Nasreen, A., Muhammad, I., Iqbal, Z.S., & Javed, I. (2008). Biosorption characteristics of unicellular green alga Chlorella sorokiniana immobilized in loofa sponge for removal of Cr(III). Journal of Environmental Sciences, 20, 231–239.
- Ney, J.M., Conary, C.L., & Chapman, S.R. (1981). High density diatom production utilizing dialysis techniques. Aquaculture, 24, 363–369.10.1016/0044-8486(81)90070-3
- Ochiai, H., Shibata, H., Sawa, Y., & Katoh, T. (1980). “Living electrode” as a long-lived photoconverter for biophotolysis of water. Proceedings of the National Academy of Sciences USA, 77, 2442–2444.10.1073/pnas.77.5.2442
- Ochiai, H., Shibata, H., Sawa, Y., Shoga, M., & Ohta, S. (1983). Properties of semiconductor electrodes coated with living films of cyanobacteria. Applied Biochemistry and Biotechnology, 8, 289–303.10.1007/BF02779496
- Pahlavanzadeh, H., Keshtkar, A.R., Safdari, J., & Abadi, Z. (2010). Biosorption of nickel (II) from aqueous solution by brown algae: Equilibrium, dynamic and thermodynamic studies. Journal of Hazardous Materials, 175, 304–310.10.1016/j.jhazmat.2009.10.004
- Pandi, M., Shashirekha, V., & Swamy, M. (2009). Biosorption of chromium from retan chrome liquor by cyanobacteria. Microbiological Research, 164, 420–428.10.1016/j.micres.2007.02.009
- Palmer, C.M. 1959. Algae in water supplies US Dept. of Health, Edu. and Wel, Pub Health Service, Cincinnati.
- Park, J.B.K., & Craggs, R.J. (2010). Wastewater treatment and algal production in highrate algal ponds with carbon dioxide addition. Water Science and Technology, 61, 633–639.10.2166/wst.2010.951
- Payer, H.D., & Runkel, K.H. (1978). Environmental pollutants in freshwater algae from open-air mass cultures. Arch. Hydrobiol. Beih., 11, 184–198.
- Pearson, H.W., Mara, D.D., Konig, A., De Oliveira, R., Mills, S.W., Smallman, D.J., & Silva, S.A. (1987). Water column sampling as a rapid and efficient method of determining effluent quality and performance of waste stabilization ponds. Water Science and Technology, 19, 100–119.
- Perlack, R.D., Wright, L.L., Turhollow, A.F., Graham, R.L., Stokes, B.J., & Erbach, D.C. (2005). Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply (p. 78). US Department of Energy and US Department of Agriculture.
- Piotrowska-Niczyporuk, A., Bajguz, A., Zambrzycka, E., & Godlewska-Żyłkiewicz, B. (2012). Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiology and Biochemistry, 52, 52–65.10.1016/j.plaphy.2011.11.009
- Pirt, S.J., Lee, Y.K., Walach, M.R., Pirt, M.W., Balyuzi, H.H.M., & Bazin, M.J. (1983). A tubular bioreactor for photosynthetic production of biomass from carbon dioxide: design and performance. Journal of Chemical Technology and Biotechnology Biotechnology, 33B, 35–58.
- Pirt, S. J. (1986). Culture growth and apparatus therefore. UK Patent No. 2118572.
- Powell, N., Shilton, A., Chisti, Y., & Pratt, S. (2009). Towards a luxury uptake process via microalgae – Defining the polyphosphate dynamics. Water Research, 43, 4207–4213.10.1016/j.watres.2009.06.011
- Pulz, O., & Gross, W. (2004). Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65, 635–648. doi:10.1007/s00253-004-1647-x
- Rai, L.C., Gaur, J.P., & Kumar, H.D. (1981). Phycology and heavy-metal pollution. Biological Reviews, 56, 99–151.10.1111/brv.1981.56.issue-2
- Rajfur, M., Klos, A., & Waclawek, M. (2010). Sorption of copper (II) ions in the biomass of alga Spirogyra sp. Bioelectrochemistry, 80, 81–86.
- Reiniger, P. (1977). Concentration of cadmium in aquatic plants and algal mass in flooded rice culture. Environmental Pollution (1970), 14, 297–301.10.1016/0013-9327(77)90141-0
- Robinson, P.K., Reeve, J.O., & Goulding, K.H. (1988). Phosphorous uptake kinetics of immobilized Chlorella in batch and continuous culture. Enzyme and Microbial Technology, 11, 590–596.
- Samson, R., & LeDuy, A. (1983). Improved performance of anaerobic digestion of Spirulina maxima algal biomass by addition of carbon-rich wastes. Biotechnology Letters, 5, 677–682.10.1007/BF01386361
- Sari, A., & Tuzen, M. (2008). Biosorption of Pb(II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. Journal of Hazardous Materials, 152, 302–308.
- Sawayama, S., Rao, K.K., & Hall, D.O. (1998). Nitrate and phosphate ions removal from water by Phormidium laminosum immobilized on hollow fibres in a photobioreactor. Applied Microbiology and Biotechnology, 49, 463–468.10.1007/s002530051199
- Shaaban, A.M., Haroun, B.M., Ibraheem, I.B.M. 2004. Assessment of impact of Microcystis aeruginosa and Chlorella vulgaris in the uptake of some heavy metals from culture media. In Proc. 3rd Int. Conf. Biol. Sci. Fac. Sci., Tanta Univ., 28–29 April, vol. 3, pp. 433–450.
- Schimdt, K. (1991). Antioxidant vitamins and beta-carotene-effects on immunocompetence. The American Journal of Clinical Nutrition, 53, S383–S385.
- Sheehan, J., Dunahay, T., Benemann, J., Roessler, P. (1998). A look back at the U.S. department of energy’s aquatic species program – Biodiesel from Algae, NERL/TP-580-24190. Golden, CO: National Renewable Energy Laboratory, 80401.
- Singh, L., Pavankumar, A.R., & Lakshmanan, R. (2012). Effective removal of Cu2+ ions from aqueous medium using alginate as biosorbent. Ecological Engineering, 38, 119–124.10.1016/j.ecoleng.2011.10.007
- Sialve, B., Bernet, N., & Bernard, O. (2009). Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnology Advances, 27, 409–416.10.1016/j.biotechadv.2009.03.001
- Soeder, C.J., Payer, H.D., Runkel, K.H., Beine, J., & Briele, E. (1978). Sorption and concentration of toxic minerals by mass cultures of Chlorococcales. Mitt. Internat. Verein. Limnol., 21, 575–584.
- Tastan, B.E., Duygu, E., & Dönmez, G. (2012). Boron bioremoval by a newly isolated Chlorella sp. and its stimulation by growth stimulators. Water Research, 46, 167–175.10.1016/j.watres.2011.10.045
- Travieso, L., Benitez, F., & Dupeiron, R. (1992). Sewage treatment using immobilized microalgae. Bioresource Technology, 40, 183–187.10.1016/0960-8524(92)90207-E
- Torzillo, G., Pushparaj, B., Bocci, F., Balloni, W., Materassi, R.R., & Florenzano, G. (1986). Production of Spirulina biomass in closed photobioreactors. Biomass, 11, 61–74.10.1016/0144-4565(86)90021-1
- Tuzen, M., & Sarı, A. (2010). Biosorption of selenium from aqeous solution by green algae (Cladophora hutchinsiae) biomass: Equilibrium, thermodynamic and kinetic studies. Chemical Engineering Journal, 158, 200–206.10.1016/j.cej.2009.12.041
- US, DOE. (2009). National algal biofuels technology roadmap. Office of energy efficiency and, renewable energy (p. 214). Retrieved from http://biomass.energy.gov
- Werblan, D., Smith, R.J., Van der Valk, A.G., & Davis, C.B. (1978). Treatment of waste from a confined hog feeding unit by using artificial marshes. In H.L. Mickim (Ed.), Proceedings of international symposium on land treatment of wastewater (pp. 1–13). New Hapshire: Hannover.
- Wright, P.J., & Weber, J.H. (1991). Biosorption of inorganic Tin and Methyltin compounds by estuarine macrolagae. Environmental Science & Technology, 25, 287–294.10.1021/es00014a011
- Wolverton, B.C. (1982). Hybrid wastewater treatment system using anaerobic microorganisms and red (Phragmites communis). Economic Botany, 36, 373–380.
- Woertz, I.C., Lundquist, T.J., Feffer, A.S., & Nelson, Y.M. (2009). Lipid productivity of algae grown during treatment of dairy and municipal wastewaters. Journal of Environmental Engineering, 135, 1115–1122.10.1061/(ASCE)EE.1943-7870.0000129
- Wu, X.F., & Kosaric, N. (1991). Removal of organochlorine compounds in an upflow flocculated algae photo-bioreactor. Water Science Technology, 24, 221–232.
- Yee, N., Benning, L.G., Phoenix, V.R., & Ferris, F.F. (2004). Characterization of metal-cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environmental Science & Technology, 38, 775–782.10.1021/es0346680
- Yen, H.-W. (2004). Anaerobic bioassay of methane potential of microalgal biomass (Ph.D. dissertation). Biosystems Engineering, Clemson University, Clemson.
- Yen, H.-W., & Brune, D.E. (2007). Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresource Technology, 98, 130–134.
- Yüce, M., Nazır, H., & Dönmez, G. (2010). An advanced investigation on a new algal sensor determining Pb(II) ions from aqueous media. Biosensors and Bioelectronics, 26, 321–326.10.1016/j.bios.2010.08.022