Abstract
It is known that arsenic has different toxicological properties dependent upon both its oxidation state for inorganic compounds, as well as the different toxicity levels exhibited for organic arsenic compounds. The field of arsenic speciation analysis has grown rapidly in the recent years, especially with the utilization of chromatographic analysis. Extraction method as sequential extraction combined to microwave assisted digestion were applied to water and sediments samples. This paper describes the essential background and toxicity of arsenic in environment, and more importantly, some currently used chromatographic applications and sample handling procedures necessary to accurately detect arsenic in its various forms. Experiments and work using ultra high-performance liquid chromatography and inductively coupled plasma optical emission spectrometry for arsenic speciation of environmental samples are presented in this research.
1. Introduction
Chromatographic techniques to determine arsenic have typically focused on the total level of arsenic, rather than chemical form or the oxidation state. The actual toxicity levels of the different arsenic compounds vary greatly. There is a need for up to date analytical methods. Elevated concentrations of arsenic have previously been reported in soils, streams waters, and sediments. Drinking water tend to be the largest source of arsenic exposure worldwide. As a result, contaminated water lead to detrimental health impact. Inorganic arsenic is considered acutely more toxic. Arsenite (AsIII) is more toxic than arsenate (AsV) (Chen et al., Citation2016). Inorganic salts of arsenic are the most toxic, in particular trivalent arsenic (III) such as arsenic trioxide and pentavalent arsenic (V).
Acute arsenic poisoning also affects the haematological system causing haemolysis and coagulopathy. It causes acute renal and hepatic failure, an acute neurological insult with delirium and seizures (Rosenberg et al., Citation2016). In his study Tang, Kang, Wang, and Zhao (Citation2016) showed that methylated As species, especially DMA (V) were more toxic than As (V) or MMA (V) to a plant species of Arabidopsis thaliana. This shows the difference in phytotoxicity mechanism between different arsenic species.
Carcinogenic role of arsenic has been extensively studied. More recently a capacity of arsenic in unducing generation of cancer stem cells shed light on the important impact of arsenic-induced cellular pathology through its carcinogenic effect (Li & Chen, Citation2016; Sadee, Foulkes, & Hill, Citation2016).
Elemental speciation is defined as the analytical activity in combination with electrochemical principles, which uses the ability of electroanalysis to indicate the changes in chemical equilibrium and redox states of various element. Consequently this allows together with determination of their total content, the identification and quantification of the individuals forms and their actual distribution (Navrátilová & Švancara, Citation2015). Studies of water wells monitoring for arsenic pollution in Asian, European and American countries have shown widespread detection of arsenic in ground and surface waters were they have been classified as potential risk for environmental ecosystems and human’s health (Koch et al., Citation1999; Mahanta et al., Citation2015; Ryker, Citation2003; Smith et al., Citation2016).
Several methods have been developed and applied for sample preparation, Chromatographic separation, and detection of arsenic species in water and sediments samples. Sequential extraction (SE) is an established technique that has been used for the extraction of organic pollutants from marine sediments and soil samples. Although some work in speciation analysis has been done using electrochemical detection of inorganic forms of arsenic, inductively coupled plasma optical emission spectroscopy for the determination of both organic and inorganic compounds remain an up to date detector. Its level of sensitivity coupled with ultra high-performance liquid chromatography (UHPLC), offers the versatility of a wide analysis for both organic and inorganic arsenic compounds. This is amongst the highly favoured detection technique for speciation analysis of arsenic (Table ).
Table 1. Some of the common arsenic compounds found in speciation analysis.
2. Chromatographic analysis
All glassware were washed with liquid soap and rinsed properly with distilled water.
2.1. Inductively coupled plasma – optical emission spectrometry
The sediment samples were filtered using nylon syringe filter of 0.45 μm prior to the analysis (Ashraf, Maah, & Yusoff, Citation2012) by inductively Coupled Plasma Optical Emission Spectroscopy at the Hydrogeology Lab, Geology Department, University of Malaya (Perkin Elmer Optima 5300 V). Standard solutions were prepared accordingly from single certified standard for the calibration purposes. Both qualitative and quantitative analyses can be realized using this method. The emission of a spectra characterize the identification of a specific element in case it is for a qualitative study and the measurement of the intensity of the radiation is specific of the quantitative study, which provide a quantitative spectrochemical analyses by determining the concentration of the element of interest within the sample.
2.2. Ultra high-performance liquid chromatography
A combination of analytical techniques is often required to achieve both high sensitivity and selectivity in detection of low concentration of arsenic. The coupling instrumentation of HPLC with ICP provide a multi-element capability, large dynamic range of measurement and extremely high sensitivity. It shows this instrumentation to be a most effective tool in arsenic research. In the determination of total arsenic concentration, sample storage and collection are to prevent contamination and reduce the loss of trace levels of analytes. HPLC has the advantage of performing separations of non-volatiles species. Ion exchange chromatography is the based design for arsenic speciation in this study by HPLC. The mode of extraction was anion exchange chromatography. The ionic strength of the solute, the pH of the mobile phase, the ionic strength and concentration of the buffer, and the temperature influence the separation and the retention of analytes in the ion-exchange HPLC. The buffer was of HPLC grade. Mobile Phases A and B were water-methanol (94:6, v/v). The gradient program was 0–7 min, 100%A to 100%B and hold at 100%B. The baseline resolution of the arsenic species was obtained by a polar column C18.
3. Study case
3.1. Geological characteristics
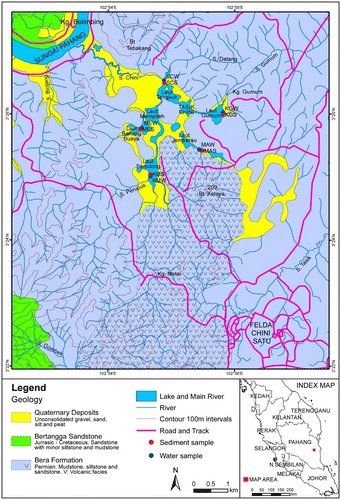
The location of the study is shown in the maps, GPS was used in order to give the sampling coordinates at the study area. Structural and geomorphological study was made through references in books and articles. The Peninsular Malaysia can be divided into three geologically distinct provinces of western, central and eastern belts (Hutchison, Citation1977; Scrivenor, Citation1928). Although the Three belts are apparent, there is no clear agreement on the demarcation and origin of their margins (Hutchison, Citation1977; Khoo & Tan, Citation1983; Tan, Citation1984). Subduction–collision models have been widely used to explain the evolution and juxtaposition of these belts (Hutchison, Citation1977; Metcalfe, Citation2013), though such models have been questioned by others (Tan, Citation1984). Tasik Chini is located in the eastern part of the Central range of Peninsular Malaysia and delimitated between the coordinates 03°26′35.7″N; 03°25′52.8″N and 102°54′29.3″E; 102°54′41.9″E. The oldest rocks known in the area are thought to be of Permocarboniferous age, which are underlain predominantly by the Bera formation. They are in association with volcanic facies, siltstones, mudstones quartzite-conglomerate, quartzite, shale, chert, phyllite, schist from the cretaceous formation and referred, provisionally, to the Triassic. The Bertangga Sandstone occur at the southwestern corner of the area, while the low-lying areas around the lakes and along the main rivers are underlain by Quaternary deposits (Figure ).
The lake Chini is edged by Sungai Pahang and its major tributaries Sungai chini, Sungai Kencau, Sungai Bintang, Sungai Mentiga which operates to drain the lake. Structurally, the area is dominated by two major tectonic axes, that of the Main range and that of the Gunong Benom Range, situated beyond the western and eastern of map. The Central Belt is a part of the Sukhotai Arc constructed in the late Carboniferous–early Permian on the margin of the Indochina Block. This Arc was then separated in the Permian by a back arc spreading from Indochina. The Sukhotai Arc basin opened in the early Permian and finally collapsed to be closed in the middle-late Triassic. The Tethys ocean basin destroyed by subduction beneath the Indochina Block and Sukhotai Arc produced the Permian–Triassic andesitic volcanism and I-type granitoids observed in the central. Thus, causing the mineralizations in presence at that area (Metcalfe, Citation2013).
3.2. Land use and topography
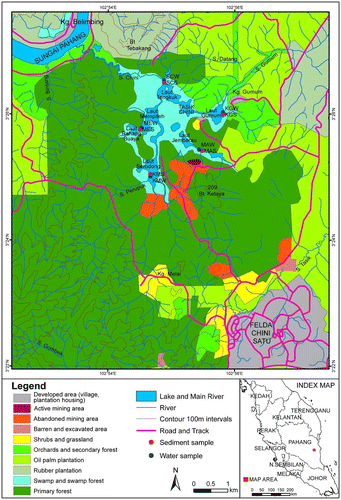
In gauging the different land use changes of the catchment (Figure ), this study reveals that the upstream conditions and the surrounding area can effect both water quality and the natural flora and fauna of the lake. Studies have found that the type and the intensity of land Use have a strong influence on the receiving water resource quality. Tremendous point and non-point sources can be the source of pollution.
Figure shows the map of the Tasik Chini land use. The lake is surrounded by oil plantation at the east at Kampung Gumum. At the southern part is the mining area that has been opened as a mining station which is the one of the sampling point. At the west side of the catchment, the primary forest is surrounding the location site of Mepatih and Kampong Melai.
The map shows different type of land use that the catchment has been switched to. All around the Tasik Chini lake, at the northwestern side, the primary forest is covering the land. That portin of the catchment was investigated at the sampling location Mepatih (ME) and Kampong Melai (KM). At the north-east of Tasik Chini area, at Sungai Chini, the field was once covered by primary forest. The forest is now cleared, instead there is orchads and secondary forest. These are the remaining of the previous primary forest and that side of the catchment is now transformed to an oil plantation.
This oil plantation is extended until the southeastern side of the Tasik Chini, From Laut Gumum to Laut Jerembau. Those location were, respectively, investigated at the sampling site Kampung Gumum (KG) and the mining area (MA). As it is shown in the map, oil plantation is covering almost 70% of Tasik Chini catchment.
At Laut Jerembau, the mining area sampling, there is an abandoned mining area. Whereas an activated Manganese mining area is excavated for exploitation. The area is also surrounded by primary forest and oil plantation.
4. Sampling and preservation
Due to large study area, Global Positioning System was use in order to determinate the actual position of the sampling site and to reconfirm the location of the sampling position during the subsequent sampling period.
4.1. Water investigation
Five sampling points from the downstream to upstream of the catchment were investigated for physico-chemicals parameters. This was made by Hydrolab HACH MS5 on the front of a fibre glass engine for quantitative estimation of heavy metals. Water samples were collected 10 cm below the surface water using HDPE bottle 500 mL. Samples were preserved by few drops of nitric acid (70%) and stored in an icebox and transported to laboratory. Sample were analysed by inductively coupled plasma optical emission spectrometry (ICP-OES).
4.2. Sediment investigation
For sediments multiple subsamples were taken from each location. Samples were homogenized into composites with stainless spoon and then subsampled by spoon into each sample container to get accurate results. Sediments were extracted from the lake by Ekman dredge. The samples were stored at 4 °C until the analysis, then dried till 100 °C in a forced air oven, then crushed in a mortar pestle and sieved up to 0.038 mm. The fraction under 63 μm was used for the purpose of analysis by Perkin Elmer A Analyst 800 atomic absorption spectrometer and UHPLC.
A two-step modified sequential extraction were experimented on sediments from all the sampling points of the catchment before through the ICP-OES and UHPLC.
Fraction1: This fraction has demonstrated the adsorption of trace elements, changes in water ionic composition are likely to affect sorption–desorption processes. The sediment was extracted at room temperature for 1 h with the 16 ml of 1 M of sodium acetate solution at pH 7 with continuous agitation.
Fraction 2: Here residue from fraction 1 was leached at room temperature and adjusted to pH 5 with acetic acid (HOAc) for 5 h where continuous agitation was maintained.
Concerning the UHPLC, the mode of extraction was anion exchange chromatography. The ionic strength of the solute, the pH of the mobile phase, the ionic strength and concentration of the buffer, and the temperature influence the separation and the retention of analytes in the ion-exchange HPLC. The buffer was of HPLC grade. Mobile Phases A and B were water-methanol (94:6, v/v). The gradient program was 0–7 min, 100%A to 100%B and hold at 100%B. The baseline resolution of the arsenic species was obtained by a polar column C18.
5. Results and discussions
5.1. Physico-chemicals parameters
The concentration of aqueous species in forms of cations and anions are determined to provide the hydrochemical characteristics at Sg Chini, Kampung Gumum, Mining area, Kampong Melai and Mepatih. The availability of the heavy metals is controlled by the geochemical processes such as adsorption, solubility. However, man-made sources also contribute to the elevated concentration of minor or trace elements. In Table are summarized the statistical values calculated for the cation and the anion species measured at respective sampling locations.
Table 2. Statistics for cation and anion species concentration measured at Tasik Chini river.
The highest mean concentration is Fe with 8.61 mg/L followed by Al with 2.66 mg/L and As 0.03 mg/L. The lowest average values are Cu and Zn with, respectively, values of 0.01 and 0.03 mg/L. In comparison with water standard INWQS (Interim National Water Quality Standard), it can be observed that Fe and Al exceed both the standards. The concentration of other species exceed slightly but are still relatively lower compared to the standard. The anion concentration Sg Chini ranges from 0.13 to 5.03 mg/L, which are both concentration of NO3− . The highest concentration of anion species is NO3−, the second highest concentration is measured for Cl− with 0.28 mg/L.
The highest cation concentration measured is Na (2.83 mg/L), followed by Mg (1.52 mg/L) and finally Ca (0.54 mg/L). The highest standard deviation value is Ca (0.54) these values range from 0.54 to 0.20 for Na specie.
For the metal ions species, the highest mean concentration is Fe (1.33 mg/L). The lowest species are As (0.04 mg/L) and Cu (0.01 mg/L). When compared to the INWQS, these concentration slightly exceed the concentration standard. The lowest average anion species concentration is measured for Cl− specie (1.90 mg/L). The concentration value of the anions species vary from 13.10 mg/L for to 0.39 mg/L for Cl− the lowest second concentration of anion value is NO3− (10.80 mg/L) and the highest is
(13.10 mg/L). The specie
has the highest standard deviation value 1.18 followed by Cl− (0.45) and NO3− (0.39).
The highest concentration at the mining area is given for Na (5.80 mg/L) followed by Ca (1.34 mg/L) and Mg (1.11 mg/L) where all the concentrations vary from 5.80 mg/L to 0.66 mg/L. the standard values of 0.54, 0.26 and 0.20 are measured for Ca, Mg, Na species, respectively. Fe specie has the highest concentration value (6.07 mg/L), followed by Al (1.43 mg/L), Zn (0.35 mg/L) and As (0.08 mg/L). The lowest concentration are recorded for Pb and Cu with concentration of 0.04 and 0.01 mg/L, respectively. Standard deviation values vary from 0.01 to 0.02. The highest standard deviation is calculated for Fe (0.02) while 0.01 is calculated for Cu, Al, Pb and As. The measured concentration values of As and Al exceed the INWQS standard. The highest anion species measured at the mining area is NO3− (15.69 mg/L). All the values range from 1.90 (mg/L) to 15.69 (mg/L). The second average value is (13.32 mg/L) followed by Cl− (1.90 mg/L). The highest standard deviation value for anion species is
(1.18), the second is Cl− (0.45) and the last is NO3− (0.39).
The highest cation concentration for alkaline and alkali earth element at kampong Melai is Na (3.10 mg/L) followed by Mg (1.49 mg/L), and Ca (0.52 mg/L). The standard deviation value varies from 0.54 to 0.20 with the highest value calculated for Ca (0.54) and the lowest value is for Na (0.20). For metal ion species, Fe species show the highest concentration (0.59 mg/L) followed by Al (0.09 mg/L), Pb (0.06 mg/L) and As (0.034 mg/L). The lowest concentration value is 0.01 mg/L for the ions species Cu and Zn. The highest standard deviation value for metals ions species is expressed for Fe species (0.02) followed by Pb, Al, Cu, As for (0.01). At kampong Melai the value of As, Pb and Fe have exceed WHO 2011 and INWQS where the other metals exhibit lower mean concentration value. The average anion species concentration varies from (11.2 mg/L) to (0.13 mg/L) where the highest concentration measured is species (11.2 mg/L) and the lowest is Cl− species (0.13 mg/L). The highest standard deviation value for anion species is calculated for Cl− species (0.45) followed by
(1.18) and NO3− (0.39).
The highest average cation concentration measured at Mepatih is Na (4.03 mg/L) followed by Mg (1.49 mg/L) and Ca (0.95 mg/L). Ca species has the highest standard deviation value (0.54) compared to the other values which are 0.26 for Mg and 0.20 for Na. For metal ion species, Fe species has the highest concentration value (0.62 mg/L) followed by Al (0.12 mg/L) and Pb (0.08 mg/L). The lowest concentration are expressed for Cu and Zn with, respectively, values of (0.001 mg/L) and (0.01 mg/L). the concentration level of As and P exceed the WHO (2011) drinking water standard and INWQS standard while other metal have lower concentration value compared to both standard. The concentration of anions species at Mepatih ranges from 11.21 to 1.78 mg/L with the highest value expressed for (11.21 mg/L) and the second highest value for NO3− (4.81) and Cl− (1.78 mg/L). The highest standard deviation value for anion species is calculated for
species (1.18) followed by Cl−(0.45) and finally NO3− species (0.39).
5.2. Metal spatial trend
The concentration in sediments of metals was explored in the catchment. Table displays from Sungai Chini to Mepatih fluctuating trends of heavy metals concentration.
Table 3. Metal concentration in sediment samples at the catchment (mg/kg).
Metal concentration at Sg Chini show a distinct increase of As and Pb in comparison with the others which are gradually decreasing from 1200 to <200 mg/kg.
From Mepatih to Kampong Gumum, a clear decrease for the metals can be observed. Pb show the most increase in metals concentration whereas the lowest was displayed for Al. The richness of all heavy metals in sediment at the mining catchment indicate that metals are highly derived from the mining activities which are associated with ore mineralization.
At the other sampling locations known as Kampong Gumum, Kampong Melai and Mepatih, heavy metals exhibit a somewhat decrease. The lowest decrease is of Fe at kampong Melai while the highest decrease is Zn with less than 10 mg/kg in concentration. Almost all metals show fluctuating trend in concentration values
5.3. Chromatographic analysis
UHPLC has the advantage of performing separations of non-volatiles species. After the determination of total arsenic concentration by ICP-OES, the coupled instrumentation with UHPLC provides a crucial chemical speciation of arsenic. This a most effective tool since it has a sensitive limit detection.
Extraction was determined by anion exchange chromatography.
The chromatographs of sediments at location 3 show to give more insight of the arsenic species present at the mining area. The others location in the catchment show also to have concentration of arsenic species. This arsenic speciation provide more information about the species in presence in the sediments of the mining area.
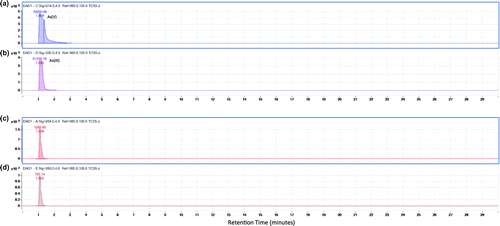
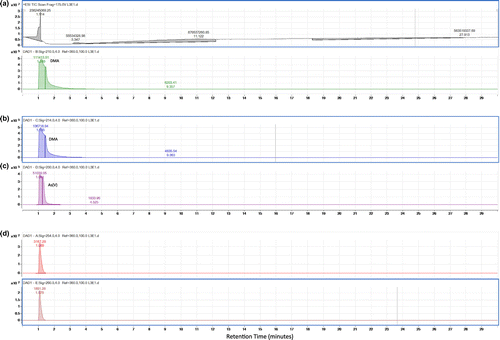
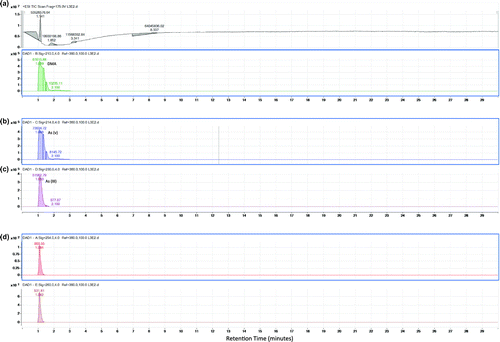
In the upper layer of sediment at the mining area, the chromatogram of the Q-TOF LC/MS (Figure ) displays the presence of As (V) and As (III) and also other unknown arsenic species are, respectively, in Figure (a)–(d). The following Figures display the results after first extraction of the sediment (Figure ). This is the arsenic species bound to the exchangeable fraction. It can be seen that DMA appears both in Figure (a) and (b). As (V) and other unknown arsenic species appear, respectively in Figure (c), (d) and (e). Lastly Figure, (a)–(c), (d) and (e), respectively, illustrates the presence of DMA, As (V), As (III) and unknown arsenic species which are bound to the Fe/Mn oxides fraction of sediment.
5.4. Arsenic distribution in water
Water is the greatest medium to exacerbate metals in natural ecosystems. This is due to the importance of water layer as a medium for materials being transported from one station to another station. The results show good interpretation in arsenic occurrence at the water catchment.
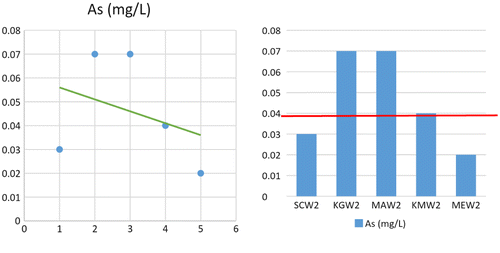
Comparison between arsenic concentration and its observed flow for distribution purposes shows a slow regression coefficient, R2 (0.12) with a negative slope value (−0.005) (Table , Figure ). In term of arsenic dissemination by the water medium, the correlation results in general, show that there is no significant relationship. However, arsenic concentration show to be in higher concentration at the mining site and Kampung Gumum where it is slightly higher than the allowed limit of World Health Organisation [WHO] (Citation1999) in drinking water.
Table 4. Statistical parameters and value for arsenic distribution at the different sampling points.
The higher concentration of arsenic at sampling location 3 in fact is due to the mining activities. However the concentration of arsenic at Kampong Gumum at the same rate with the concentration at the mining area show that Kampong Gumum receive directly mining effluent from mining activities through the water flow.
5.5. Arsenic distribution in the sediments
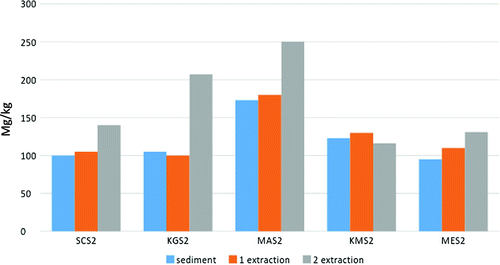
Sediment of natural ecosystems have a great adsorption ability unto heavy metals. High spatial variability of As concentrations appears to be related to regional and local variation in the nature of clay minerals associated in sediments. And thus for feasible mitigation measures, the concentration of arsenic bond to sediment can serve as an indicator for remediation process. Figure illustrates the arsenic pattern in the sediment column.
The two-step sequential extraction procedure performed in the two fraction of sediment at each sampling location suggest that arsenic was detected at high concentrations (Figure ) as compared to others metals. Arsenic concentration distribution was the highest at the second extraction followed by the first fraction, and then the overlaying sediment fraction. This means that arsenic concentrations occur in decreasing trend, respectively, from the fraction bound to Fe and Mn oxides (second extraction), then the exchangeable fraction bound to carbonates and finally the overlaying fraction.
The fraction bound to iron oxides serve as cement between particles. Thus they are excellent scavengers for trace metals and actually for arsenic. However this fraction is unstable under anoxic condition. The exchangeable fraction is more susceptible to changes of pH. Tasik Chini receive water from its different feeder’s rivers, so there is actually movement of exchange water which is not favourable for anoxic conditions. This make the conditions favourable for arsenic adsorption by the fraction bound to Fe oxides.
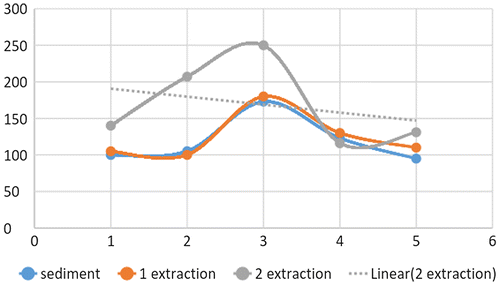
Figure shows the relation between the different fractions and their concentration based on sampling site. The resulting relationship reflects that there is a positive relation between the first fraction and the second fraction. However that relation is not highly significant. So the processes involved in the adsorption of arsenic in the first fraction are totally different from those responsible of arsenic adsorption in the second fraction.
Due to the attraction between the outer surface of the solid particle and arsenic, a risk assessment with respect to human health and environment is of concern. These arsenic concentrations found in both exchangeable and Fe/Mn oxides fractions are considered to be mobile and associated with anthropogenic pollution. The water and sediment analysis of the studied area shows that the greatest probability of metals being leached because of high concentrations were likely to result in soluble metals that can leach in case of favourable changes in the natural conditions.
6. Conclusion
Arsenic distribution appear to vary in concentration at the different sampling sites. Nevertheless the greatest concentration is at the mining area, as the mining activities exacerbate these concentrations through water channels. The research focuses on the level of arsenic concentration and its speciation in Tasik Chini catchment and thus its distribution in the natural ecosystem. The importance of the study is very essential as it helps to understand the arsenic pattern through the different areas involved in geochemical speciation.
This research show that arsenic were present in almost all locations in relative low concentration in water samples. However in sediment samples, it showed to be in high concentration in both exchangeable and Fe/Mn oxides fraction. This revealed the important natural role that clay played as adsorbent in the catchment. There were a positive significant relation between arsenic distribution and its concentration at these different fraction.
Further research can be carried out on the speciation and bioavailability of arsenic in plants and aquatic species living in the vicinity of Lake Chini. This is to apprehend a possible risk of pollution which can be a concern for human lives.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by University Malaya [grant number PG133-2014 B].
References
- Ashraf, M.A., Maah, M.J., & Yusoff, I. (2012). Speciation of heavy metals in the sediments of former tin mining catchment. Iranian Journal of Science and Technology, 36, 163.
- Hutchison, C.S. (1977). Granite emplacement and tectonic subdivision of Peninsular Malaysia. Bulletin of the Geological Society of Malaysia, 9, 187–208.
- Chen, L., Liang, R., Tan, T., Ai, X., Hao, T., Chen, X., & Wang, Y. (2016). Recent development in arsenic speciation and toxicity reduction of inorganic arsenic in food. European Journal of BioMedical Research, 2, 27–31.10.18088/ejbmr.2.1.2016.pp27-31
- Khoo, T.T., & Tan, B.K. (1983). Geological evolution of Peninsular Malaysia. In Proceedings workshop on stratigraphic correlation of Thailand and Malaysia (Vol. 1 253–290). Kuala Lumpur: Geological Society of Thailand and Malaysia.
- Koch, I., Feldmann, J., Wang, L., Andrewes, P., Reimer, K.J., & Cullen, W.R. (1999). Arsenic in the Meager Creek hot springs environment, British Columbia, Canada. Science of the Total Environment, 236, 101–117.10.1016/S0048-9697(99)00273-9
- Li, L., & Chen, F. (2016). Oxidative stress, epigenetics, and cancer stem cells in arsenic carcinogenesis and prevention. Current Pharmacology Reports, 2(2), 57–63.10.1007/s40495-016-0049-y
- Mahanta, C., Choudhury, R., Basu, S., Hemani, R., Dutta, A., Barua, P.P., … Saikia, L. (2015). Preliminary assessment of arsenic distribution in Brahmaputra river basin of India based on examination of 56,180 public groundwater wells. Safe and sustainable use of arsenic-contaminated aquifers in the Gangetic Plain (pp. 57–64). New York, NY: Springer International Publishing.10.1007/978-3-319-16124-2
- Metcalfe, I. (2013). Tectonic evolution of the Malay Peninsula. Journal of Asian Earth Sciences, 76, 195–213.10.1016/j.jseaes.2012.12.011
- Navrátilová, Z., & Švancara, I. (2015). Electroanalysis and chemical speciation. In Environmental analysis by electrochemical sensors and biosensors (pp. 841–854). New York, NY: Springer New York.
- Rosenberg, A., Smith, M., Colebatch, A., Leaf, D., Steel, L., & Fogg, T. (2016). Acute fatal arsenic intoxication: A case report and review of the literature. Journal of Clinical Case Reports, 6(1).
- Ryker, S.J. (2003). Arsenic in ground water used for drinking water in the United States. In Arsenic in Ground Water (pp. 165–178). New York, NY: Springer US.10.1007/b101867
- Sadee, B.A., Foulkes, M.E., & Hill, S.J. (2016). A study of arsenic speciation in soil, irrigation water and plant tissue: A case study of the broad bean plant, Vicia faba. Food Chemistry, 210, 362–370.10.1016/j.foodchem.2016.04.066
- Scrivenor, J.B. (1928). A sketch of Malayan mining. Washington, DC: Mining Publications.
- Smith, A.E., Lincoln, R.A., Paulu, C., Simones, T.L., Caldwell, K.L., Jones, R.L., & Backer, L.C. (2016). Assessing arsenic exposure in households using bottled water or point-of-use treatment systems to mitigate well water contamination. Science of The Total Environment, 544, 701–710.10.1016/j.scitotenv.2015.11.136
- Tan, B.K. (1984). Tectonic framework and evolution of the central belt and its margins, Peninsular Malaysia. Bulletin of the Geological Society of Malaysia, 17, 307–322.
- Tang, Z., Kang, Y., Wang, P., & Zhao, F.J. (2016). Phytotoxicity and detoxification mechanism differ among inorganic and methylated arsenic species in Arabidopsis thaliana. Plant and Soil, 401, 243–257.10.1007/s11104-015-2739-3
- World Health Organisation. (1999). Arsenic in drinking water. Fact sheet No. 210. Geneva: WHO.