Abstract
In ecological studies, topological indices are defined to test the ecological and chemical characteristics, such as oxidation, melting point, boiling point, toxicity, and other biological activity. As a basic molecular structure, dendrimers widely appeared in chemical, biology, pharmacy, medicine, and material engineering. In this paper, we study the chemical properties of two kinds of dendrimers from the mathematical point of view. Several vertex distance-based indices are determined. Furthermore, the edge distance-based indices of one class of nanocore structure are also considered.
1. Introduction
It has been over 35 years since the studies of topological indices for molecular structures were conducted. Distance-based and degree-based topological indices are numerical parameters of molecular structure, which are of great importance in physics, chemistry, and pharmacology science.
Specifically, let G = (V(G), E(G)) be a molecular graph with vertex set V(G) and edge set E(G), then a topological index can be regarded as a positive real function f: G → . Being numerical descriptors of the molecular structure deduced from the corresponding molecular graph, topological indices can be applied in theoretical chemistry, like QSPR/QSAR study. For example, harmonic index, Wiener index, PI index, Randic index, and sum connectivity index can be used to reflect certain structural features and chemical characteristics of organic molecules. In recent years, several articles made contribution to covering certain distance-based and degree-based indices of special molecular graph (Gao, Farahani, & Shi, Citation2016; Gao & Siddiqui, Citation2017; Gao, Siddiqui, Imran, Jamil, & Farahani, Citation2016; Gao & Wang, Citation2015, 2016, 2017; Gao, Wang, & Farahani, Citation2016; Gao, Yan, & Shi, Citation2017; Gao et al., Citation2017; Sardar, Zafar, & Zahid, Citation2017). The notations and terminologies are used but undefined in this paper (Bondy & Murty, Citation2008).
For any e = uv ∊ E(G), let
Some researchers have introduced a distance-based version of the atom-bond connectivity index, and it was called the second atom-bond connectivity index being denoted as
The edge version of atom-bond connectivity index (the third atom-bond connectivity index) was defined as
raised a distance-based version of the geometric–arithmetic index, and was called the second geometric–arithmetic index which can be stated as
The third geometric–arithmetic index was introduced as
As a traditional topological index, the vertex PI index and PI index of molecular graph G were defined as
The vertex PI polynomial, as the extension of vertex PI index, was introduced being stated as
The corresponding PI polynomial was stated as
Other distance-based index, the Szeged index, and edge Szeged index are defined as
The corresponding distance-based polynomial, the Szeged polynomial, and edge Szeged polynomial are defined as
For any edge e = uv, let
Then, the modified version of Szeged index for a molecular graph G was defined as
In past decades, the computation of distance-based indices for certain special chemical molecular structure raised much attention among chemists. Although there have been many results in distance-based indices of molecular graphs, the research of indices for special chemical structures are still largely limited. In addition, as widespread and critical chemical structures, tetrathiafulvalene (TTF) and 4,4′-bipyridinium dendrimers are widely applied in medical science and pharmaceutical engineering. For all these reasons, we give a deep discussion on the computation of the two dendrimers mentioned above (Ashrafi, Manoochehrian, & Yousefi-Azari, Citation2006; Graovac & Ghorbani, Citation2010; Gutman & Ashrafi, Citation2008; Tabar, Purtula, & Gutman, Citation2010).
The main contribution of our work is twofold. On the one hand, we will manifest certain vertex distance-based indices of TTF. On the other hand, some important vertex distance-based indices of 4,4′-bipyridinium dendrimers will be discussed. At last, as supplement contribution, the edge distance-based indices of CNC7[n] are presented.
2. Main results and proofs
In this part, we present our main results on vertex distance-based indices of two finite families of dendrimer: TTF and 4,4′-bipyridinimu dendrimers. Then, as supplemental results, the edge distance-based indices of one-heptagonal carbon nanocones are discussed.
2.1. Vertex distance-based indices of TTF
The dendrimers built from a starting atom, such as nitrogen, to which carbon are part of a new group of macromolecules. Other elements are added by a repeating series of chemical reactions that produce a spherical branching structure. In a divergent synthesis of a dendrimer, one starts from the core (a multi-connected atom or group of atoms) and grows out to the periphery. In each repeated step, a number of monomers will be added to the actual structure. In a radial manner, resulting quasi concentric shells are called generations. The periphery in a convergent synthesis is first set up and then the branches which are called Dendron’s are connected to the core.
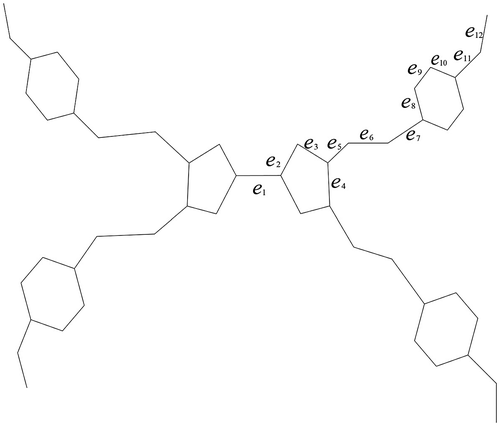
We first discuss a kind of dendrimers named TTF, and see Figure as an example of this molecular structure where TTF units were used as branching centers. Several contributions on TTF and other dendrimers. In this subsection, we aim to determine the distance-based indices of TTF by means of distance computation.
Figure 1. Molecular graph of dendrimers using TTF units as branching centers. D (Gao, Wang, Jamil, & Farahani, Citation2016).
We use D[k] to denote the molecular graph TTF with k generation. By simply calculating, we have . In order to determine the vertex distance-based index of D[k], we divide E(D[k]) into two parts A and B (see Figures and , respectively) where A includes the edges of the core and B is the subset containing all remaining edges of D[k]. In view of the symmetry of core, we yield the value of nu(e),nv(e), and the number of such kind of edges (as we indicate in Figures and , there are 12 kinds of edges in part A and 19 kinds of edges in part B) which are manifested in Tables and for part A and part B, respectively.
Table 1. Distance computation for part A.
Table 2. Distance computation for part B (1 ≤ i ≤ k).
Using the definition of distance-based indices, we get
2.2. Vertex distance-based indices of 4,4′-bipyridinium dendrimers
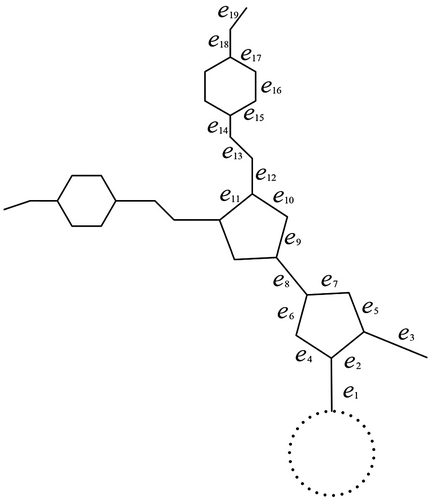
In this subsection, we present the above-mentioned distance-based indices of 4,4′-bipyridinium dendrimers. The structure of 4,4′-bipyridinium dendrimers with n stages (which is denoted by Gn) can be referred to Figure . Some contributions on these structures in the field of chemical science can be referred to (Balzani et al., Citation2006; Bongard, Moller, Rao, Corr, & Walder, Citation2005; Ewais, Nagdy, & Hameed, Citation2006; Giansante et al., Citation2009; Kathiresan & Walder, Citation2011; Kim et al., Citation2007; Oh et al., Citation2004; Ong et al., Citation2005; Marchioni et al., Citation2004; Rajakumar & Srinivasan, Citation2014). By computation, we yield
Let hi be a hexagon in the i-stage and be the edge connected hi and hi–1. Suppose that e ∊ E(hn) for all six edges, we have nu(e) = 3 and there are 2n such hexagons. If e ∊ E(hn–1), we get nu(e) = 11 for four of edges, nu(e) = 19 for other two edges, and there are 2n–1 such hexagons. Continue such computation until we reach the first stage. If e ∊ E(h1), we obtain nu(e) = 4 · 2n − 5 for four of edges, nu(e) = 8 · 2n − 13 for other two edges, and there are two such hexagons. Suppose that e ∊ E(h0) for all six edges, we have nu(e) = 8 · 2n − 6 and there are two such hexagons.
Next, we determine the value of nu(e) for (the presentation of edges can be referred to Figure ). Obviously, we infer
and there are 2n such edges. Moreover, we deduce
,
,
and there are 2n–1 such edges. Continue such computation until we reach the first stage. We check that
,
and there are two such edges. Assume that e is the edge between two central hexagons, we verify nu(e) = 8 · 2n − 3.
In terms of the definition of distance-based indices, we get
2.3. Edge distance-based indices of one-heptagonal carbon nanocones
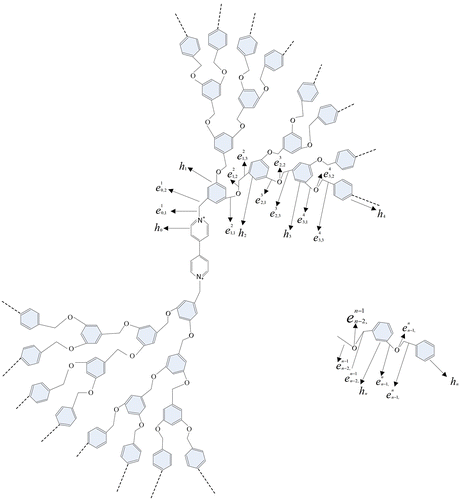
As a supplement, we present the edge distance-based indices of one-heptagonal carbon nanocones CNC7[n] which is widely used in chemical engineering. The detail molecular structure and some important results can be found (Adil, Assal, Khan, Al-Warthan, & Siddiqui, Citation2013; Alipour & Ashrafi, Citation2009; Ashrafi & Gholami-Nezhaad, Citation2009; Doynov, Bozadjiev, Dimitrov, & Bozadjiev, Citation2011; Siddiqui, Warad, Adil, Mahfouz, & Al-Arifi, Citation2012). In view of molecular structure analysis, we get . The whole molecular graph CNC7[n] can be divided into seven parts since its symmetry structure and the center is C7. Hence, by means of its symmetry, the edge distance-based indices can be calculated and stated as follows.
3. Conclusion
In our paper, according to the analysis of molecular graph, structural, distance calculating, and mathematical derivation, we mainly determine the distance-based indices of two classes of dendrimers: TTF and 4,4′-bipyridinium dendrimer. As supplement conclusion, we report the edge distance-based indices of CNC7[n]. The theoretical formulations obtained in our work illustrate the promising prospects of their application for the pharmacy and chemical engineering.
Disclosure statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding
This work was supported in part by the National Natural Science Foundation of China [11401519].
Acknowledgements
We thank the reviewers for their constructive comments in improving the quality of this paper.
References
- Adil, S.F., Assal, M.E., Khan, M., Al-Warthan, A., & Siddiqui, M.R.H. (2013). Nano silver-doped manganese oxide as catalyst for oxidation of benzyl alcohol and its derivatives: Synthesis, characterization, thermal study and evaluation of catalytic properties. Oxidation Communications, 36, 778–791.
- Alipour, M.A., & Ashrafi, A.R. (2009). A numerical method for computing the wiener index of one-heptagonal carbon nanocone. Journal of Computational and Theoretical Nanoscience, 6, 1204–1207.10.1166/jctn.2009.1168
- Ashrafi, A.R., & Gholami-Nezhaad, F. (2009). The PI and edge Szeged indices of one-heptagonal carbon Nano-cones. Current Nanoscience, 5, 51–53.10.2174/157341309787314629
- Ashrafi, A.R., Manoochehrian, B., & Yousefi-Azari, H. (2006). On the PI polynomial of a graph. Util. Math., 71, 97–102.
- Balzani, V., Bandmann, H., Ceroni, P., Giansante, C., Hahn, U., Klamer, F.G., … Vogtle, F. (2006). Host-guest complexes between an aromatic molecular tweezer and symmetric and unsymmetric dendrimers with a 4,4′-bipyridinium core. Journal of the American Chemical Society, 128, 637–648.10.1021/ja056615j
- Bondy, J.A., & Murty, U.S.R. (2008). Graph Theory. Springer, Berlin, 244, XII–655.
- Bongard, D., Moller, M., Rao, S.N., Corr, D., & Walder, L. (2005). Synthesis of non-symmetrically N, N′-diaryi-substituted 4,4′-bipyridinium salts with redox-tunable and titanium dioxide (TiO2)-anchoring properties. Helvetica Chimica Acta, 88, 3200–3209.10.1002/(ISSN)1522-2675
- Doynov, M.I., Bozadjiev, R.L., Dimitrov, T.I., & Bozadjiev, L.S. (2011). Nano spinel powders and pigments on their basis. Oxidation Communications, 34, 726–730.
- Ewais, H.A., Nagdy, M.A., & Hameed, S.A. (2006). Periodate oxidation of Aqua-N-(2-Acetamido) Iminodiacetato-Cobalt (II) complex involving maleic acid co-ligands. Oxidation Communications, 39, 41–52.
- Gao, W., Farahani, M.R., & Shi, L. (2016). Forgotten topological index of some drug structures. Acta Medica Mediterranea, 32, 579–585.
- Gao, W., & Siddiqui, M.K. (2017). Molecular descriptors of nanotube, oxide, silicate, and triangulate networks. Journal of Chemistry, 2017, Article ID 6540754, 10 pages. doi:10.1155/2017/6540754
- Gao, W., Siddiqui, M.K., Imran, M., Jamil, M.K., & Farahani, M.R. (2016). Forgotten topological index of chemical structure in drugs. Saudi Pharmaceutical Journal, 24, 258–264.10.1016/j.jsps.2016.04.012
- Gao, W., & Wang, W.F. (2015). The vertex version of weighted wiener number for bicyclic molecular structures. Computational and Mathematical Methods in Medicine, 2015, Article ID 418106, 10 pages. doi:10.1155/2015/418106
- Gao, W., & Wang, W.F. (2016). The eccentric connectivity polynomial of two classes of nanotubes. Chaos, Solitons & Fractals, 89, 290–294.10.1016/j.chaos.2015.11.035
- Gao, W., & Wang, W.F. (2017). The fifth geometric arithmetic index of bridge graph and carbon nanocones. Journal of Difference Equations and Applications, 1–10. doi:10.1080/10236198.2016.1197214
- Gao, W., Wang, W.F., & Farahani, M.R. (2016). Topological indices study of molecular structure in anticancer drugs. Journal of Chemistry, ID 3216327, 8 pages. doi:10.1155/2016/3216327
- Gao, W., Wang, W.F., Jamil, M.K., & Farahani, M.R., (2016). Electron energy studying of molecular structures via forgotten topological index computation. Journal of Chemistry, ID 1053183, 7 pages, doi:10.1155/2016/1053183
- Gao, W., Yan, L., & Shi, L. (2017). Generalized Zagreb index of polyomino chains and nanotubes. Optoelectronics and Advanced Materials – Rapid – Communications, 11, 119–124.
- Giansante, C., Mazzanti, A., Baroncini, M., Ceroni, P., Venturi, M., Klarner, F.G., & Vogtle, F. (2009). Tweezering the core of dendrimers: Medium effect on the kinetic and thermodynamic properties. The Journal of Organic Chemistry, 74, 7335–7343.10.1021/jo9014134
- Graovac, A., & Ghorbani, M. (2010). A new version of atom-bond connectivity index. Acta Chimica Slovenica, 57, 609–612.
- Gutman, I., & Ashrafi, A.R. (2008). The edge version of the Szeged index. Croatica Chemica Acta, 81, 263–266.
- Kathiresan, M., & Walder, L. (2011). Shell-by-shell inside-out complexation of organic anions in flexible and rigid pyridinium dendrimers. Macromolecules, 44, 8563–8574.10.1021/ma201615w
- Kim, S.Y., Ko, Y.H., Lee, J.W., Sakamoto, S., Yamaguchi, K., & Kim, K. (2007). Toward high-generation rotaxane dendrimers that incorporate a ring component on every branch: Noncovalent synthesis of a dendritic [10] pseudorotaxane with 13 molecular components. Chemistry – An Asian Journal, 2, 747–754.10.1002/(ISSN)1861-471X
- Marchioni, F., Venturi, M., Ceroni, P., Balzani, V., Belohradsky, M., Elizarov, A.M., … Stoddart, L.F. (2004). Complete charge pooling is prevented in viologen-based dendrimers by self-protection. Chemistry-A European Journal, 10(24), 6361–6368.10.1002/(ISSN)1521-3765
- Oh, M.K., Bae, S.E., Yoon, J.H., Roberts, M.F., Cha, E., & Lee, C.W.J. (2004). Synthesis, characterization, and electrochemical behavior of viologen-functionalized poly(amidoamine) dendrimers. Bulletin of the Korean Chemical Society, 25, 715–720.
- Ong, W., Grindstaff, J., Sobransingh, D., Toba, R., Quintela, J.M., Peinador, C., & Kaifer, A.E. (2005). Electrochemical and guest binding properties of Frechet- and Newkome-type dendrimers with a single viologen unit located at their apical positions. Journal of the American Chemical Society, 127, 3353–3361.10.1021/ja049530b
- Rajakumar, P., & Srinivasan, K. (2014). Synthesis and electrochemical properties of dendrimers containing meta-terphenyl peripheral groups and a 4,4′-bipyridinium core. Tetrahedron, 60, 10285–10291.
- Sardar, M.S., Zafar, S., & Zahid, Z. (2017). Computing topological indices of the line graphs of Banana tree graph and Firecracker graph. Applied Mathematics and Nonlinear Sciences, 2, 83–92.10.21042/AMNS.2017.1.00007
- Siddiqui, M.R.H., Warad, I., Adil, S.F., Mahfouz, R.M., & Al-Arifi, A. (2012). Nano-gold supported nickel manganese oxide: Synthesis, characterization and evaluation as oxidation catalyst. Oxidation Communications, 35, 476–481.
- Tabar, G.F., Purtula, B., & Gutman, I. (2010). A new geometric–arithmetic index. Journal of Mathematical Chemistry, 47, 477–486.10.1007/s10910-009-9584-7