Abstract
Soil microbial biomass has been used as an early indicator of change in soil properties resulting from urbanization. We analyzed the effect of urbanization along a rural–urban gradient on soil microbial biomass and physico-chemical properties of the soil. The mean microbial biomass carbon (MBC) value were 107.4, 121.3, and 134.2 μg g−1 of soil, respectively, for urban, sub-urban and rural sections of the gradient. Whereas, the mean microbial biomass nitrogen (MBN) was 10.2, 11.5, and 12.5 μg g−1 of soil for urban, sub-urban, and rural gradient. Similarly, the mean values of microbial biomass phosphorus (MBP) were 5.1, 5.8, and 6.3 μg g−1 of soil, for urban, sub-urban, and rural gradient, respectively. ANOVA and Tukey’s Honest Significant Difference (HSD) analyses showed significant difference (P ≤ 0.05) in microbial biomass with physico-chemical characteristics of soils. Maximal soil microbial biomass was reported for rural soils followed by sub-urban and urban soil. Disturbance in soil texture, increased in BD and decrease in soil moisture content as major factors responsible for depletion in soil microbial biomass in urban soils. . Thus, suggesting that the urbanization adversely effected soil microbial biomass by altering natural soil characteristics.
Introduction
Soil microbial biomass is a labile pool of organic matter acting both as source and sink of plant nutrients (Singh, Raghubanshi, Singh, & Srivastava, Citation1989). It is considered as one of the main determinants of soil fertility (Jenkinson & Ladd, Citation1981). Change in microbial biomass adversely affects the cycling of soil organic matter, ecosystem stability and fertility (Smith & Paul, Citation1990). Studies on soil microbial biomass carbon (MBC), nitrogen (MBN), and phosphorus (MBP) in different natural and disturbed ecosystems showed them to be an important labile pool of carbon (C) and mineral nutrients ( Smith & Paul, Citation1990; Wardle, Citation1992). Consequent upon decomposition nutrients are released into the environment affecting soil nutrient content, hence, primary productivity of the ecosystems (Franzluebber, Hons, & Zuberor, Citation1994; Gregorich, Liang, Drury, Mackenzie, & McGill, Citation2000; Haney, Franzluebbers, Hons, Hossner, & Zuberer, Citation2001). Therefore, any disturbance in the microbial population in response to the variation in soil properties such as moisture, bulk density, organic C, nutrients, EC, pH will have serious implications on overall productivity of the ecosystem.
Advancing urbanization could have serious ecological and agronomic consequences for developing countries like India. In India, majority of the lands used for establishment of housing colonies are fertile agricultural lands. Urban soils get altered by anthropogenic activities such as compaction, construction, mixing, land filling, and degradation. Topsoil usually get filled up with stones, construction rubble, bricks, and other building materials, contributing to poor soil fertility (Jin, Ye, Xu, Shen, & Huang, Citation2011). Soil compaction due to urbanization affects the soil carbon cycle which in turn can alter the soil biological activity (Deurer et al., Citation2012; Nawaz, Bourrie, & Trolard, Citation2012). Beside soil carbon, compaction also affects the amount and distribution of MBC (Beylich, Oberholzer, Schrader, Hoper, & Wilke, Citation2010). A study carried out at a military training site in Fort Benning, Georgia showed that MBC decreased as the level of disturbance resulting from training activities increased (Silveira, Comerford, Reddy, Prenger, & DeBusk, Citation2010). Nevertheless, a little is known about the impact urbanization could have on soil microbial biomass.
Therefore, microbial biomass could be used as a valuable tool for understanding and predicting the long-term effects of land use change (Sharma, Rai, Sharma, & Sharma, Citation2004; Singh & Yadava, Citation2006). Climatic seasonality has been reported to influence the microbial populations (Diaz-Ravina, Acea, & Carballas, Citation1993) and soil microbial biomass (Granatstein, Bezdicek, Cochran, Elliott, & Hammel, Citation1987; Lynch & Panting, Citation1980) either directly or by influencing microbial responses to changes or, indirectly by influencing plant metabolism. Here, we measured the soil microbial biomass along a rural–urban and correlated it with change in soil characteristics such as pH, EC, texture, organic carbon, macro (N, P, K), and micro (Fe, Cu, Zn, Mn) nutrients.
Materials and methods
Study area and sampling
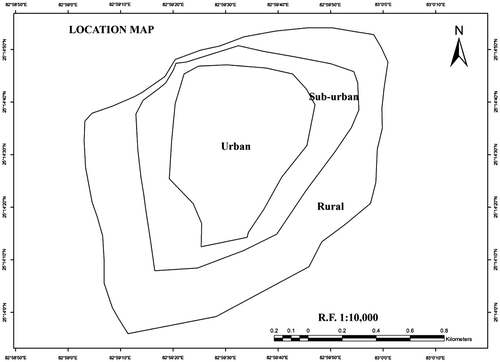
We selected a fertile tract of land in the district Varanasi (Uttar Pradesh, India), located between 25°19′14·86 N latitude and 82°58′12·30 E longitude (Figure ). The tract representing rural, semi-urban, and urban areas, was previously used for agriculture but, in a decade, a part of it, has undergone intense urbanization. The level of urbanization was characterized on the basis of the amount of built-up area (buildings, roads, and asphalt covered paths). The built-up area in rural, sub-urban, and urban was 5, 30, and 60%, respectively. Sampling area was measured 1 km2 and experimental design was random. Soil samples (0–15 cm depth) were randomly collected in triplicate from different sites of each location using a steel corer. Sampling was done from September 11 to 21, 2015. Samples were collected in plastic bags, immediately brought to the laboratory and stored at 4 °C for further processing. Soil samples were air dried and sieved (2 mm) prior to their physico-chemical analysis.
Physico-chemical analyses of soil samples
Soil characteristics such as electrical conductivity (EC) and pH were determined using EC and pH metre, as described by Sparks (Citation1996). Soil texture (clay, silt, and sand) was determined using international pipette method (International Society of Soil Science, [ISSS], Citation1929). Bulk density (BD) and particle density (PD) of soil were determined by core sampler (Veihmeyer & Hendrickson, Citation1948) and pycnometer method, respectively. Soil moisture content was determined by drying the soil to a constant weight at 105 °C. Water holding capacity (WHC) of the soil was determined by Keen Rackzowski box (Black, Citation1965). Soil organic carbon (OC) was determined using K2Cr2O7-H2SO4 oxidation method (Walkley & Black, Citation1934).
Micronutrients such as Fe, Cu, Zn, and Mn were extracted using diethylene triamine penta-acetic acid (DTPA) and determined by the method of Lindsay and Norwell (Citation1978). Available nitrogen (N), phosphorus (P), and potassium (K) were determined as per Subbiah and Asija (Citation1956), Olsen, Cole, Watanable, and Dean (Citation1954), and Hanway and Heidal (Citation1952), respectively. Whereas, soil microbial biomass carbon (SMBC), nitrogen (SMBN), and phosphorus (SMBP) were analyzed according to Vance, Brookes, and Jenkinson (Citation1987), Brookes, Landman, Pruden, and Jenkinson (Citation1985) and Brookes, Powlson, and Jenkinson (Citation1982), respectively.
Statistical analyses
The statistical analyses were performed using software SPSS 20 version. ANOVA and Tukey’s Honest Significant Difference (HSD) were analyzed to determine the statistical significance between samples along the gradient. PC-ORD software package (McCune & Mefford, Citation1999) was used to create ordinations that indicate the correlation between soil parameters and microbial biomass.
Results
Table showed the values of each soil variable suggesting that soil properties varied greatly along the gradient. EC value was high (271.4) for the rural gradient than sub-urban (246.4) and urban gradient (210.5). Soil pH ranged from 6.8 to 7.9 along the rural–urban gradient. Urban soil has higher BD (1.39 Mg m−3) than that of sub-urban (1.36 Mg m−3) and rural soils (1.31 Mg m−3). Soils of rural and urban areas have maximum clay (13.6%), and silt content (29.4%) whereas, soil of sub-urban part has highest (58.3%) sand content.
Table 1 Mean value of selected (± SD) soil properties (0–15 cm) along the rural–urban gradient.
Rural soil showed maximal WHC compared to that of the sub-urban and urban area. Soil moisture content was 7.2% for rural, (6.8%) for sub-urban and urban gradient (6.4%) for urban soils, respectively. The soil of rural parts has higher SMBC, SMBN, and SMBP (134.2, 12.5, and 6.3 μg g−1 soil) than that of sub-urban (121.3, 11.5, and 5.8 μg g−1 soil) and urban (107.4, 10.2, and 5.1 μg g−1 soil) parts, respectively. Similarly, rural soil possessed higher OC (0.44%) than sub-urban (0.42%) and urban (0.36%) soils. The soil of rural gradient had higher levels of macronutrients (N, P, K) than sub-urban and urban gradient soil. Among nutrients rural soil contended more Fe and Zn than the sub-urban and urban gradient soils. Contrary to this, levels of Cu and Mn were more in the sub-urban soils than the rural and urban soils (Table ).
Soil properties differed significantly along the gradient (p < 0.05 Table ). Soil pH, EC, texture (except sand), BD, PD, WHC, moisture, microbial biomass, and macronutrients showed significant variations (p < 0.05) along the gradient. Among the micronutrients Fe and Mn showed significant variation (p < 0.05) along the gradient. Soil pH, BD, PD, and silt content increased from rural to urban section of the gradient (Table ). While, EC, clay, WHC, moisture, microbial biomass, organic carbon, macro and micronutrients increased from urban to rural section of the gradient (Table ).
Canonical correspondence analysis (CCA)
Figures represent the relationship between the microbial biomass and physico-chemical variables of soils analyzed using canonical correspondence analysis (CCA) ordination. Summary of the CCA analysis was given in Table . Eigen values of MBC, MBN, MBP on ordination axis 1 and 2 were 0.002 and 0.000, respectively. Results suggest, between MBC and soil variables, the ordination axis one explained 83.3% variability while axis second reported 16.7% of total variation. In case of MBN, the ordination axis one explained 88.8% variability while the axis second explained 11.2% of total variation. Similarly for MBP, the ordination axis one explained 86.3% variability while the axis two explained 13.7% of total variation. In ordination plots, the soil variables, OC, Av. K, Fe, moisture, clay, EC, Av. P, Av. N, WHC, Cu are associated very closely to the rural–urban gradient and microbial biomass, affecting microbial biomass more. While sand, BD, PD, silt, pH, Zn, and Mn have less effect.
Figure 2. Canonical correspondence analysis (CCA) ordination illustrating the relationship between the microbial biomass carbon (MBC) and soil variables (pH, EC, texture, N, P, K, OC, Fe, Cu, Zn, Mn) along the urbanization gradient.
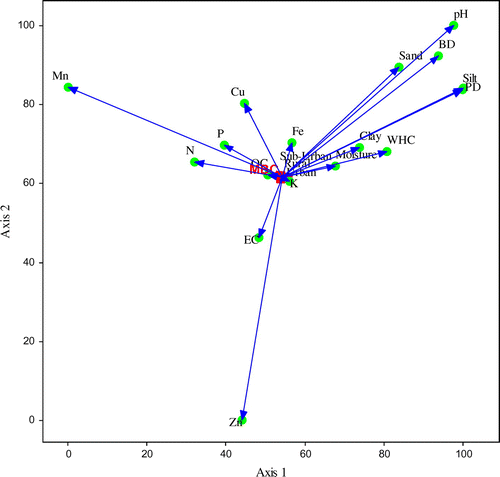
Figure 3. Canonical correspondence analysis (CCA) ordination illustrating the relationship between the microbial biomass nitrogen (MBN) and soil variables (pH, EC, texture, N, P, K, OC, Fe, Cu, Zn, Mn) along the urbanization gradient.
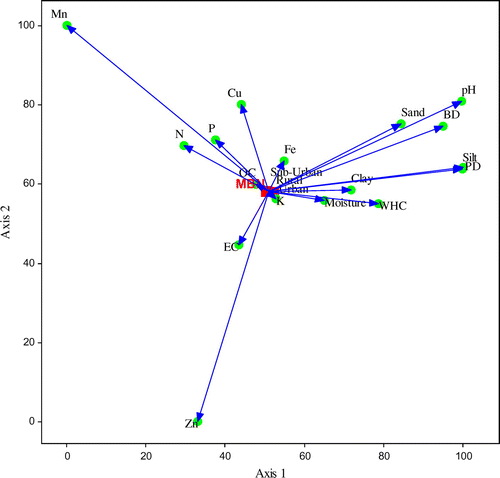
Figure 4. Canonical correspondence analysis (CCA) ordination illustrating the relationship between the microbial biomass phosphorus (MBP) and soil variables (pH, EC, texture, N, P, K, OC, Fe, Cu, Zn, Mn) along the urbanization gradient.
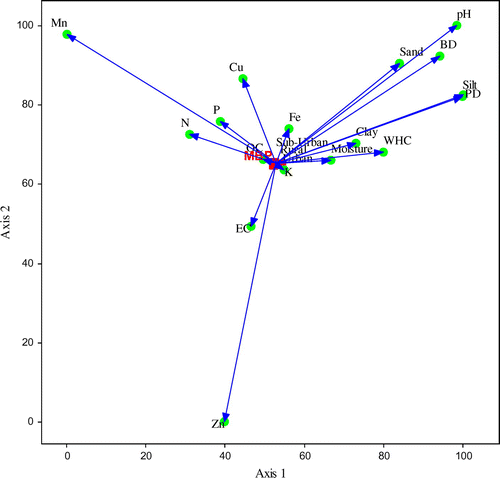
Table 2. Axis summary statistics of CCA analysis between the soil variables and microbial biomass (MBC, MBN, MBP) along the rural–urban gradient.
Discussion
The results suggest that increasing urbanization has adversely affected the microbial biomass, along the gradient, as previously reported by Scharenbroch, Lloyd, and Johnson-Maynard (Citation2005) and McDonnell et al. (Citation1997). Nevertheless, in the study, we analyzed the role of physico-chemical characteristics of soil in bringing this change. Soil pH differed significantly along the rural–urban gradient. Rural soil was slightly acidic, turning out to be alkaline with increasing urbanization. Thereby supporting maximal microbial biomass. Maximal microbial biomass was reported at pH of 6.5 by Tabatabai (Citation1994), Acosta-Martinez and Tabatabai (Citation2000). Jim (Citation1998a) reported that urban roadside soil in Hong Kong was alkaline than natural soil not affected by urbanization. He implicated the release of carbonate from the calcareous construction waste for increase in pH of the soil. We suspect that the use of calcium-enriched water, atmospheric pollution, and liming of soil to correct suspected deficiencies may be responsible for alkalinity of urban and sub-urban soils of Varanasi.
Further, high BD of the urban soil compared to that of sub-urban and rural soils may be due to relatively high soil compaction in urban areas (Nowak, Hoehn, Crane, Stevens, & Walton, Citation2007; Pouyat, Szlavecz, Yesilonis, Groffman, & Schwarz, Citation2010). Increased BD results in depletion of soil moisture and air space, leading to reduction in WHC of the soil (Jim, Citation1998a). Depletion in moisture and increase in BD may lead to reduction in soil OC and PD of urban soil (Scharenbroch et al., Citation2005).
We reported that the clay content was high in the rural soils than that of sub-urban and urban soils. Clay particles interact with soil organic matter to form aggregates that protect the organic matter from decomposition (Hassink & Whitmore, Citation1997). Soils with higher clay contents tend to have greater organic matter (Hassink, Bouwman, Zwart, Bloem, & Brussard, Citation1993; Jenkinson, Citation1988), which is crucial in determining the microbial biomass, microbial activity, and composition of microbial community (McCulley & Burke, Citation2004). Soils with high clay content lead to more stabilization of soil OC and higher microbial biomass (Schimel et al., Citation1994).
We observed a significant variation in OC along the gradient. OC was high in rural soil than that of the sub-urban and urban soils. Similar results were reported by Jim (Citation1998a, Citation1998b) and Chen, Liu, and Tao (Citation2013). The availability and amount of OC is the key factor affecting activity and structure of the microbial community and microbial biomass content in the soils (Degens, Schipper, Sparling, & Vojvodic-Vukovic, Citation2000). Change in soil moisture, texture, temperature, altered soil community, soil hydrophobicity etc., due to physical disturbance, land management practices, and local climate fluctuation are major factors directly affecting the soil carbon pool from natural to urban system (Bandaranayake, Quian, Parton, Ojima, & Follet, Citation2003; Pouyat, Yesilonis, & Nowak, Citation2006).
We reported high concentrations of macronutrients (N, P, K) rural soils. High soil pH could have affected the nitrogen mineralization and nitrification processes in urban soil (Baxter, Pickett, Dighton, & Carreiro, Citation2002), resulting in depletion of nitrogen content in urban soil to that of the sub-urban and rural soils (Jim, Citation1998a; Zhang, Xu, & Wang, Citation2010). White and Mcdonnell (Citation1988) observed that trampling and high concentration of heavy metals in the urban areas reduced the numbers and diversity of soil microbes and invertebrates. This resulted in decrease in the nitrogen mineralization and nitrification, ultimately reducing the microbial biomass nitrogen in urban soil. Similar trend was reported for available P and K. Jim (Citation1998a) and Baxter et al. (Citation2002) suggested that the lower concentration of available P in urban soil is likely a result of the reduced organic inputs. Bennett (Citation2003) reported low concentration of available P in urban land surrounding agricultural land. Carbonates that are abundantly available in the urban region bind with soil P further limit its availability (Hong, Zehou, & Junsheng, Citation2001).
Soil microbial biomass was high in rural area than the sub-urban and urban areas. A similar result was obtained by Carreiro, Howe, Parkhurst, and Pouyat (Citation1999). High soil OC and its fast mineralization in the rural soil could result in increased MBC in the rural soils. Groffman, Pouyat, McDonnell, Pickett, and Zipperer (Citation1995) compared the C pools along the urban–rural gradient and found that urban areas contained more passive pools of C. Low MBC could be expected due to the high turnover rate for C mineralization. Silveira et al. (Citation2010) suggested that MBC decreases with increasing level of disturbance due to decreased level of labile C pool. In the present study, the high value of available N in rural soils resulted in greater potential of N mineralization.
We could not find any relationship between available micronutrients and urbanization. The concentrations of Cu and Mn were maximal in sub-urban soil while, the concentration of Fe and Zn was maximal in rural soil. Further, amongst these, only Fe and Mn showed significant difference along the gradient.
Our study suggests that the disturbance in soil texture, increased BD and decrease in soil moisture content are the major factors responsible for depletion in soil microbial biomass in the urban area. The resulting reduction in the rate of mineralization of organic matter in the urban soils further resulted in decrease of soil microbial biomass (MBC, MBN, and MBP) from rural to urban gradient.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the University Grants Commission [Pradeep Kumar Rai].
Acknowledgements
We thank the Head, Department of Botany, Banaras Hindu University for providing necessary facilities. PKR gratefully acknowledges the financial support received from the UGC, New Delhi, in form UGC Research Fellowship.
References
- Acosta-Martinez, V., & Tabatabai, M.A. (2000). Enzyme activities in a limed agricultural soil. Biology and Fertility of Soils, 31, 85–91.10.1007/s003740050628
- Bandaranayake, W., Quian, Y. L., Parton, W. J., Ojima, D. S., & Follet, R. F. (2003). Estimation of soil organic carbon changes in turf grass systems using the CENTURY model. Journal of Agronomy, 95, 558–563.
- Baxter, J.W., Pickett ,S.T.A., Dighton ,J., & Carreiro ,M.M. (2002). Nitrogen and phosphorus availability in oak forest stands exposed to contrasting anthropogenic impacts. Soil Biology and Biochemistry, 34, 623–633.
- Bennett, E.M. (2003). Soil phosphorus concentrations in Dane County, Wisconsin, USA: An evaluation of the urban-rural gradient paradigm. Environmental Management, 32, 476–487.10.1007/s00267-003-0035-0
- Beylich, A., Oberholzer, H.R., Schrader, S., Hoper, H., & Wilke, B.M. (2010). Evaluation of soil compaction effects on soil biota and soil biological processes in soils. Soil and Tillage Research, 109, 133–143.10.1016/j.still.2010.05.010
- Black, C.A. (1965). Methods of soil analysis, vol. I and II. Madison, WI: American Society of Agronomy.
- Brookes, P.C., Landman, A., Pruden, G., & Jenkinson, D.S. (1985). Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology and Biochemistry, 17, 837–842.10.1016/0038-0717(85)90144-0
- Brookes, P.C., Powlson, D.S., & Jenkinson, D.S. (1982). Measurement of microbial biomass phosphorus in soil. Soil Biology and Biochemistry, 14, 319–329.10.1016/0038-0717(82)90001-3
- Carreiro, M.M., Howe, K., Parkhurst, D.F., & Pouyat, R.V. (1999). Variation in quality and decomposability of red oak leaf litter along an urban-rural gradient. Biology and Fertility of Soils, 30, 258–268.10.1007/s003740050617
- Chen, M.X., Liu, W.D., & Tao, X.L. (2013). Evolution and assessment on China’s urbanization 1960–2010: Under-urbanization or over-urbanization? Habitat International, 38, 25–33.10.1016/j.habitatint.2012.09.007
- Degens, B.P., Schipper, L.A., Sparling, G.P., & Vojvodic-Vukovic, M. (2000). Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biology and Biochemistry, 32, 189–196.10.1016/S0038-0717(99)00141-8
- Deurer, M., Muller, K., Kim, I., Huh, K., Young, I., Jun, G., & Clothier, B. (2012). Can minor compaction increase soil carbon sequestration? A case study in a soil under a wheel-track in an orchard. Geoderma, 183–184, 74–79.10.1016/j.geoderma.2012.02.013
- Diaz-Ravina, M., Acea, M.J., & Carballas, T. (1993). Seasonal fluctuations in microbial populations and available nutrients in forest soils. Biology and Fertility of Soils, 16, 205–210.10.1007/BF00361409
- Franzluebber, A.J., Hons, F.M., & Zuberor, D.A. (1994). Seasonal changes in soil microbial biomass and mineralizable C and N in wheat management systems. Soil Biology and Biochemistry, 26, 1469–1475.10.1016/0038-0717(94)90086-8
- Granatstein, D.M., Bezdicek, D.F., Cochran, V.L., Elliott, L.F., & Hammel, J. (1987). Long-term tillage and rotation effects on soil microbial biomass carbon and nitrogen. Biology and Fertility of Soils, 5, 265–270.
- Gregorich, E.G., Liang, B.C., Drury, C.F., Mackenzie, A.F., & McGill, W.B. (2000). Elucidation of the source and turnover of water soluble and microbial biomass carbon in agricultural soils. Soil Biology and Biochemistry, 32, 581–587.10.1016/S0038-0717(99)00146-7
- Groffman, P.M., Pouyat, R.V., McDonnell, M.J., Pickett, S.T.A., & Zipperer, W.C. (1995). Carbon pools and trace gas fluxed in urban forest soils. In R. Lat, J. Kimble, E. Levine, & B.A. Steward (Eds.), Advances in soil science, soil management and greenhouse effect (pp. 147–158). Boca Raton, FL: CRC Press.
- Haney, R.L., Franzluebbers, A.J., Hons, F.M., Hossner, L.R., & Zuberer, D.A. (2001). Molar concentration of K2SO4 and soil pH effect estimation of extractable C with chloroform fumigation extraction. Soil Biology and Biochemistry, 33, 1501–1507.10.1016/S0038-0717(01)00065-7
- Hanway, J., & Heidal, H. (1952). Soil analysis methods used in Iowa State College soil testing laboratory. Iowa State College Agricultural Bulletin, 57, 1–13.
- Hassink, J., Bouwman, L.A., Zwart, K.B., Bloem, J., & Brussard, L. (1993). Relationship between soil texture, physical protection of organic matter, soil biota, C and N mineralization in grassland soils. Geoderma, 57, 105–128.10.1016/0016-7061(93)90150-J
- Hassink, J., & Whitmore, A.P. (1997). A model of the physical protection of organic matter in soils. Soil Science Society of America Journal, 61, 131–139.10.2136/sssaj1997.03615995006100010020x
- Hong, S., Zehou, Z., & Junsheng, C. (2001). Environmental geochemical characteristics of some microelements in the yellow brown soil of Hubei province. Acta Pedologica Sinica, 38, 89–96.
- International Society of Soil Science. (1929). Minutes of the first commission meetings. International congress of soil science, Washington. Proceedings of International Society of Soil Science, 4, 215–220.
- Jenkinson, D.S. (1988). Soil organic matter and its dynamics. In A.R. Wild (Ed.), Soil conditions and plant growth (pp. 564–607). New York, NY: Longman.
- Jenkinson, D.S., & Ladd, J.N. (1981). Microbial biomass in soil: Measurement and turnover. In E.A. Paul & J.N. Ladd (Eds.), Soil biochemistry (pp. 415–417). New York, NY: Mercel Dekker.
- Jim, C.Y. (1998a). Physical and chemical properties of a Hong Kong roadside soil in relation to urban tree growth. Urban Ecosystems, 2, 171–181.10.1023/A:1009585700191
- Jim, C.Y. (1998b). Urban soil characteristics and limitations for landscape planting in Hong Kong. Landscape and Urban Planning, 40, 235–249.10.1016/S0169-2046(97)00117-5
- Jin, J.W., Ye, H.C., Xu, Y.F., Shen, C.Y., & Huang, Y.F. (2011). Spatial and temporal patterns of soil fertility quality and analysis of related factors in urban-rural transition zone of Beijing. African Journal of Biotechnology, 10, 10948–10956.
- Lindsay, W. L. & Norwell, W. A. (1978). Development of DTPA soil test for Zn, Iron, Manganese and Copper. Soil Science Society of America Journal, 42, 421–428.
- Lynch, J.M., & Panting, L.M. (1980). Cultivation and the soil biomass. Soil Biology and Biochemistry, 12, 29–33.10.1016/0038-0717(80)90099-1
- McCulley, R.L., & Burke, I.C. (2004). Microbial community composition across the great plains. Soil Science Society of America Journal, 68, 106–115.10.2136/sssaj2004.1060
- McCune, B., & Mefford, M.J. (1999). PC-ORD. Multivariate analysis of ecological data [ Version 4]. Gleneden Beach: MjM Software Design.
- McDonnell, M.J., Pickett, S.T.A., Groffman, P., Bohlen, P., Pouyat, R.V., Zipperer, W.C., … Medley, K. (1997). Ecosystem processes along an urban-to-rural gradient. Urban Ecosystems, 1, 21–36.10.1023/A:1014359024275
- Nawaz, M.F., Bourrie, G., & Trolard, F. (2012). Soil compaction impact and modelling. A review. Agronomy for Sustainable Development, 1–19.
- Nowak, D.J., Hoehn, R., Crane, D.E., Stevens, J.C., & Walton, J.T. (2007). Assessing urban forest effects and values: Philadelphia’s urban forest. Resources Bulletin, NRS-7. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northeastern Research Station p. 22.
- Olsen, S.R., Cole, C.V., Watanable, F.S., & Dean, L.A. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate USDA Circular 939.
- Pouyat, R.V., Szlavecz, K., Yesilonis, I.D., Groffman P.M., & Schwarz, K. (2010 ). Chemical, physical, and biological characteristics of urban soils . In J. Aitkenhead-Peterson & A. Volder (Eds.), Urban ecosystem ecology, Agronomy Monograph 55 (pp. 119–152 ). American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison, WI 53711.
- Pouyat, R.V., Yesilonis, I.D., & Nowak, D.J. (2006). Carbon storage by urban soils in the United States. Journal of Environment Quality, 35, 1566–1575.10.2134/jeq2005.0215
- Scharenbroch, B.C., Lloyd, J.E., & Johnson-Maynard, J.L. (2005). Distinguishing urban soils with physical, chemical and biological properties. Pedobiologia, 49, 283–296.10.1016/j.pedobi.2004.12.002
- Schimel, D.S., Braswell, B.H., Holland, E.A., McKeown, R., Ojima, D.S., Painter, T.T., … Townsend, A.R. (1994). Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biogeochemical Cycles, 8, 279–293.10.1029/94GB00993
- Sharma, P., Rai, S.C., Sharma, R., & Sharma, E. (2004). Effects of land use change on soil microbial C, N and P in a Himalayan watershed. Pedobiologia, 48, 83–92.10.1016/j.pedobi.2003.09.002
- Silveira, M., Comerford, N., Reddy, K., Prenger, J., & DeBusk, W. (2010). Influence of military land uses on soil carbon dynamics in forest ecosystems of Georgia, USA. Ecological Indicators, 10, 905–909.10.1016/j.ecolind.2010.01.009
- Singh, J.S., Raghubanshi, A.S., Singh, R.S., & Srivastava, S.C. (1989). Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature, 338, 499–500.10.1038/338499a0
- Singh, L.I., & Yadava, P.S. (2006). Spatial distribution of microbial biomass in relation to land-use in subtropical systems of north-east India. Tropical Ecology, 47, 63–70.
- Smith, J.L., & Paul, E.A. (1990). Significance of soil microbial biomass estimates in soil. Biochemistry, 6, 357–396.
- Sparks, D.L. (1996). Methods of soil analysis. Part 3 – Chemical methods. Soil Sci Soc Am, Am Soc Agro. Madison, WI.
- Subbiah, B. & Asija, G. L. (1956). Alkaline permanganate method of available nitrogen determination. Current Science, 25, 259–260.
- Tabatabai, M.A. (1994). Soil enzymes. In R.W. Weaver, G.S. Angle, P.S. Bottomley, D. Bezdicek, S. Smith, M.A. Tabatabai, & A. Wollum (Eds.) Methods of soil analysis: Part 2. Microbiological and biochemical properties of soils. Soil science society of America Journal (pp. 775–833). Madison, WI.
- Vance, E.D., Brookes, P.C., & Jenkinson, D.S. (1987). An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry, 19, 703–707.10.1016/0038-0717(87)90052-6
- Veihmeyer, F.J., & Hendrickson, A.H. (1948). Soil density and root penetration. Soil Science, 65, 487–494.10.1097/00010694-194806000-00006
- Walkley, A., & Black, C.A. (1934). Estimation of organic carbon by chromic acid and titration method. Soil Science, 37, 28–29.
- Wardle, D.A. (1992). A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biological Reviews, 67, 321–358.10.1111/brv.1992.67.issue-3
- White, C.S., & Mcdonnell, M.J. (1988). Nitrogen cycling processes and soil characteristics in an urban versus rural forest. Biogeochemistry, 5, 243–262.10.1007/BF02180230
- Zhang, K., Xu, X.N., & Wang, Q. (2010). Characteristics of N mineralization in urban soils of Hefei, East China. Pedosphere, 20, 236–244.10.1016/S1002-0160(10)60011-2