Abstract
An attempt is made to examine prevalence of insect pests and their seasonality in Senna alata L. from Puducherry region, India covering a period of one year. Totally, five insects found to make use of S. alata for ovideposition viz. butterfly (Catopsilia pyranthe), Jewel bug (potamid Chrysocoris stollii), flower bud worm (Hendecasis duplifascialis) Aphides (Aphis sp.), and Mealy bugs. All egg masses collected from leaves and flower buds are reared and developmental stages are documented. Secondly, two virulent borers viz. flower bud borer H. duplifascialis and pod borer Etiella zinckenella and their developmental stages are also documented for the first time in S. alata; thirdly, the extent of caterpillars that destroy medicinally important leaves of S. alata mostly young leaves, has also been documented. Adult insects viz. aphides (Aphis sp.) and green stink bug Acrosternum hilare, are also found feeding on leaves of S. alata. The last but unique observation made during field study is sighting of a worm inside anther for the first time. The seasonal study revealed that ovideposition is influenced by temperature and rainfall and showed higher level of ovideposition during late post-monsoon and early summer. The overall picture on association of insect pest with S. alata indicated a low level of insect pest faunal diversity. Present report would be considered as the important input into the annals of medicinally important member of the genus Senna.
Introduction
Senna alata L. belonging to family Fabaceae, is an invasive species in India from South America. S. alata is reported to possess many medicinally important phytoconstituents which form as principal ingredient in a variety of antifungal balm and cream formulations (Abubacker, Ramanathan, & Kumar, Citation2008) and hence it has got export value also (ZAUBA, Citation2015). Moreover, potted plants are raised in nurseries as ornamental plant and sold (https://nurserylive.com/buy-avenue-trees-plants-online-in-india/cassia-alata-plants-in-india.) and seeds are also collected, screened for quality and traded (https://www.amazon.com/Candle-Senna-Alata-Cassia-Shrub/dp/B00WJCTK0I). In this context, insect pests are the major negative biological factor in view of health and yield of crops and it is more concerned in the case of medicinally important plants like S. alata. Leaves of plants and flowers are preferred by few insects for ovideposition and various attributes have been put forward to explain host selection behaviour in female insects (Brody, Citation1992; Brody & Waser, Citation1995; Jiménez-Pérez & Wang, Citation2003; Pettersson, Citation1992; Zimmerman, Citation1980). Ovideposition by emigrant butterfly Catopsilia pyranthe in species of Cassia have been reported by Atluri, Ramana, and Reddi (Citation2004). Studies in bud borer Hendecasis duplifascialis Hampson is taken up by David (Citation1958), Amutha (Citation1994); Suganthi, Chandrasekaran, and Regupathy (Citation2006) and Meenatchi, Giraddi, Patil, Vastrad, and Biradar (Citation2011). Recently, Kamala, Chinniah, Kennedy, Kalyanasundaram, and Suganthy (Citation2017) have made a survey in major jasmine growing districts of Tamil Nadu and assessed distribution, infestation level and the relative importance of jasmine budworm, Hendecasis duplifascialis. Regarding pod borer, Etiella zinckenella, Sarma, Senthilkumar, and Das (Citation2008) have made extensive survey on the pest in selected medicinal plants and reported E. zinckenella, oviposition egg Permana, Johari, Putra, Sastrodihardjo, and Ahmad (Citation2012) have reported a variety of insect pests including pod-feeding Lepidoptera larvae of E. zinckenella. The larvae of polyphagous pest, gram pod borer, E. zinckenella, found to cause damage to W. somnifera leaves and flower buds (Sharma, Kumar, Mehta, & Singh, Citation2014). Mealy bugs are also recorded in different medicinal plant including species of genus Cassia other than S. alata (Mahalingam et al., Citation2010; Muniappan et al., Citation2008; Selvaraju & Sakthivel, Citation2012; Tanwar, Jeyakumar, & Vennila, Citation2010).
Present scenario on the use of members of genus Senna is reported to be most important in terms of their medicinal and export potential. However, being important medicinal plant scientific information on prevalence of insect pest in S. alata L. and its seasonality is not available from tropical regions. Hence, present study has been undertaken to prevalence and seasonality of insect pests in medicinally important plant S. alata L. profusely growing under tropical climate in the Pondicherry region.
Material and methodology
Study area
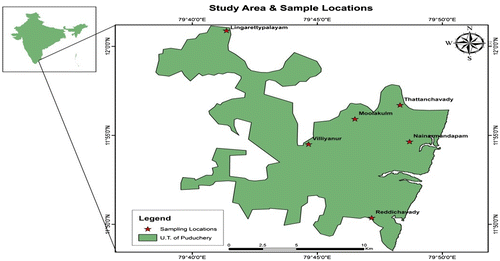
S. alata L. it is an erect herb, 2–3 m tall with compound leaves. Terminal inflorescence is a spike with a beautiful cluster of golden yellow flower resembles yellow candlesticks. The study region comes under tropical climatic experiencing two rainy seasons; one north-east monsoon shower (October–December) which is the principal period of precipitation received by Pondicherry region on the south-east coast of India and another short spell during south-west monsoon (April–June). The region receives an annual rainfall of about 2400 mm of rainfall. The maximum and minimum temperatures are 34.4 °C and 25.2 °C during summer and monsoon, respectively. The relative humidity of the study region ranged from 86% and a minimum of 64%. Totally, six sites where S. alata is profusely growing in and around Pondicherry region, are selected for the study (Figure (a) and (b)). As insect pests are mostly influenced by local environmental factors like temperature and rainfall, presently, data relating to study region have been obtained from Department of statistics and commerce, Government of Pondicherry for the study period. The investigation is done for one complete year during 2015 covering four seasons.
Sample collection
The field study on insect pests in S. alata is made more effective using a hand lens (10 cm dia) and butterfly/insect nets. It is learnt from trial field survey that pest could be categorized into two viz. swiftly flying insects and slow moving. Therefore, an indigenous technique has been adopted to maximize pest collection. A single plant S. alata is totally covered by a large transparent plastic bag down to the base of the plant to trap the entire flying and slow moving pest as fast as possible. The mouth of bag is tied at the base of the plants and its plant cut at the base off and whole plant with trapped insects and worms seen inside the bag. A small amount of cotton chocked in 5% formalin is dropped into the bag. After 15 min, plant is shaken well and all the insects and worms are collected and brought to the laboratory for further identification studies.
Secondly, the egg masses noticed on the leaves and flower buds are collected gently along with leaves and buds and brought to the laboratory and reared to find out ovidepositor. Besides, worms from flower buds and infected pods are also collected, photographed and reared in the laboratory to find out the adult. Images of adult insects, leaves with ovideposition and developmental stages of larvae are done using digital microscope, USB, under 500× (Duratool-gaosuo). Data collected on number of egg masses on leaves and on flower buds of S. alata and insect pest prevalent in the S. alata during 12 months period covering four seasons viz. post-monsoon, summer, pre-monsoon, and monsoon are presented as graphs. Adult insect pests are identified with Entomology Dept of Agriculture, Govt of Pondicherry, Horticulture department of the Pondicherry University and Tamil Nadu Agriculture University, Tamil Nadu.
Results
Seasonal field survey on insect pests associated with S. alata has brought out very interesting and unique findings. The outcome of seasonal field study is dealt as six categories. (1) leaves used as a site for ovideposition: five types of eggs masses, four laid on leaves and other one on flower bud viz. butterfly C. pyranthe, Jewel bug, Chrysocoris stollii, mealy bugs, and aphids and Hendecasis duplifascialis, respectively; Among five types, three without any cover- or cocoon-like structure and two with a spongy cover made of white fibrous material; (2) One borer found inside bud and matured flowers (Hendecasis duplifascialis); (3) Borers found inside pod (Etiella zinckenella); (4) Caterpillars of a butterfly and borer; (5) A tiny worm inside anther; (6) Adult insects feeding on leaves of S. alata (other than ovidepositors) viz.
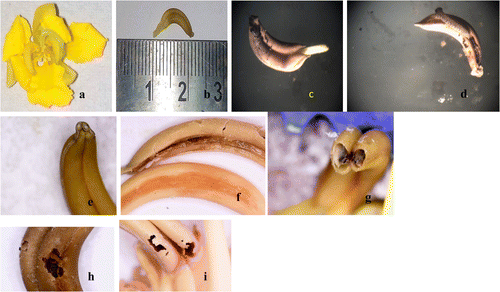
All egg masses and larvae collected from infected flower bud and pods are reared under normal laboratory conditions to a maximum of 30 days. By 21st day, the adult emerged as Butterfly, C. pyranthe. The second creamy white developed into Jewel bug C. stolli and third one collected from surface of the flower bud emerged as Hendecasis duplifascialis Hampson (Pyrauptidae: Lepidoptera). All developmental stages are photographed and presented (Figures (a)–(f) and (a)–(h)). The striking observation made with regard to pupa of a butterfly, is the surface appearance of the pupa. The pupa actually mimics the surface of a leaf having venation-like ornamentation with green colour and mid rib (Figure (d)) and the length of pupa ranged from 2.5 to 3.0 cm. After the emergent of adult from the pupa, the greenish pupa turned into creamy white and dried off (Figure (e)). Similarly, pure creamy white eggs of Jewel bug collected along with the leaf are reared in the lab and after 10 days nymph of jewel bug is formed. Nymphs looked like dark red dots (equal to the pin head-1–2 mm). The nymph feed on debris of leaves which were previously bearing eggs. After one week, the nymph transformed into an adult bug and it is identified as C. stolli, a potamid bug. From third type of egg collected, after 14 days, there emerged larvae of Hendecasis duplifascialis and at the end of 23rd day, the adult insect emerged. Fourth and fifth types are not successful. However, based on morphological features of eggs and previous reports, it is reasonably presumed that it might be the eggs of aphides and another presumed as Mealy bugs. Adult aphides and mealy bugs are also noticed in the host plant feeding on the leaves and soft stems. Two borer worms are recorded in the present study one on flower buds and another one young pods. The well-grown worm is found to bore into flower buds and eats up all the floral parts leaving plenty of excreta (Figure (a)–(e)). On rearing the worm for 7 days, an adult insect came out and it is identified as H. duplifascialis, emerged out after passing thro pupation. Regarding pod borer, the small holes are seen in young pods and on rearing for 30 days i.e., after passing through two types of larval stages (Figure (a)–(f)) the adult insect, E. zinckenella, emerged out. The third category pest is the Caterpillars feeding mostly on young leaves. Caterpillars of the mottled emigrant butterfly are prevalent during post-monsoon to late summer in S. alata. Caterpillars inflict damage by eating foliage and stems voraciously and defoliate a plant in a short period of time (Figure (a)–(e)). These caterpillars also reared and emerged adult is identified as a migrant butterfly, Catopsilia pyranthe.
Figure 2. Life stages of eggs of butterfly collected from leaves of S. alata reared in laboratory. (a) Eggs on the leaves, (b) larva feeding on leaves, (c) pupa, (d) close-up view of pupa, (e) white pupa after emergent of adult, (f) adult mottled emigrant butterfly.
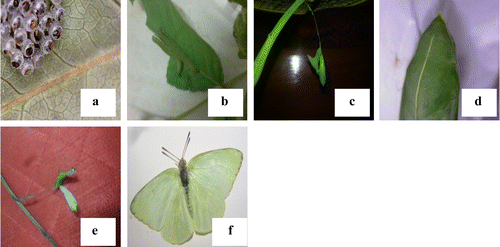
Figure 3. Life stages of eggs of Jewel bug (C. stolli) collected from the leaves of S. alata reared in laboratory. (a) adult bugs in the polythene bag, (b) eggs collected from wild, (c) embryo development inside eggs, (d) size of egg (pin head for comparison), (e) fully developed embryo, (f) nymph just come out from embryo sac, (g) nymph of jewel bug, (h) adult bug C. stolli.
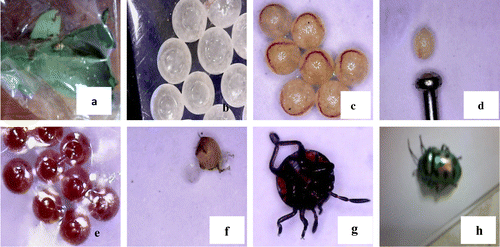
Figure 4. Flower bud boring by flower bud borer: (a) flower bud borer collected from infected bud; (b) bore made by worm; (c) worm enters into flower bud; (d) worm smashing internal floral parts; (e) end of flower bud.
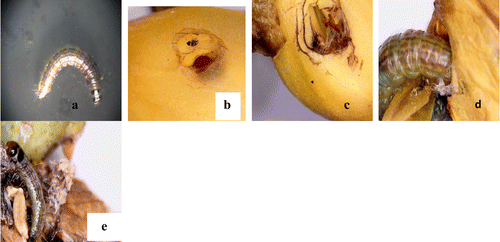
Figure 5. Devastation of pods of S. alata by pod borer: (a–b) pod borer, (c) worm coming out of the pod, (d–e) reared cocoon, (F) adult.
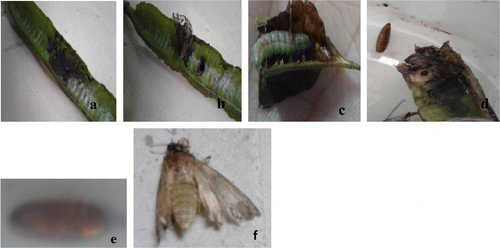
Figure 6. Devastation of leaves of S. alata by caterpillars: (a–c) perforated leaves by caterpillar; (d–e) caterpillars feeding on both from dorsal and ventral sides.

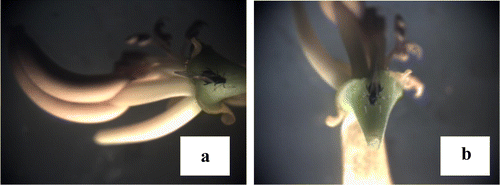
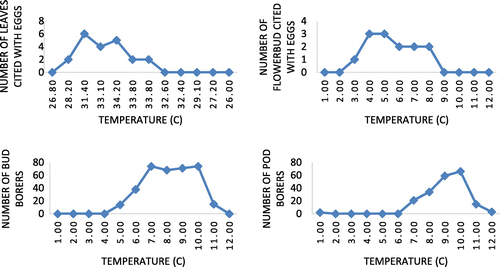
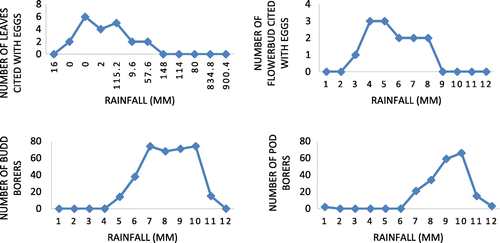
One of the striking observations made in the present study is sighting of a worm inside anther. Anthers measured about 10 mm in length. Since it is interesting and strange to see a worm coming out of an anther, it is then video graphed in addition to photographs (Figure (c)). When anther with worm is split open longitudinally to isolate worm for rearing, we could see full of excreta inside rather than pollen grains. Moreover, as the length is of about 10 mm and worm inside is (6–7 mm) less than anther length, (Figure (b)–(d)), it is very fragile to collect the worm alive. The overall understanding on seasonality of pest infestation in S. alata in relation to climatic factors indicates ovideposition and insect pest prevalence in S. alata are influenced by factors viz. temperature and rainfall and high prevalence of ovideposition during late post-monsoon and summer and the bores during late summer and pre-monsoon periods.
Figure 7. Worm inside anther: (a) flower of S. alata (arrows indicate fertile stamens); (b) individual anther measuring 1 cm; (c) arrow indicates head of worm inside anther; (d) worm taken out from anther; (e) close-up view of distal end of anther showing two tiny openings; (f) single arrow indicates internal content of healthy anther; double arrows indicate internal nature of anther after worm attack; (g) destroyed terminal ends of anther after worm exit of thro distal end.
Discussion
A field-based study on insect pests association with S. alata has brought out many interesting and unique observations. The mottled emigrant butterfly C. pyranthe a member of the family Pieridae, (Lepidoptera: Rhopalocera) is a tropical species, selects S. alata as host plant for ovidepostion. It has been reported that two species of Catopsilia viz., C. crocale and C. pyranthe, feeding freely upon Cassia species (Atluri et al., Citation2004). During its larval stage, it feeds on leaves voraciously and destroys the foliar health and productivity (Figure (b)–(d)). Most adult Lepidoptera moth and butterfly feed on nectar, whereas caterpillars consume mainly structural tissue such as leaves, stems, flowers, and fruits (Liu, Scheirs, & Heckel, Citation2010). In the present study, it is found that butterfly C. pyranthe make use of the plant S. alata for oviposition and to complete its life cycle and leaves serve as food for the caterpillars. This caterpillar nuisance is quite obvious in S. alata during late post-monsoon and summer. Present findings are in conformity with previous reports of various workers in different species of genus Cassia. C. pyranthe is seen laying eggs on the leaves of Cassia javanica, C. alata, C. auriculata, C. fistula, Albizia lebbck (Arju, Bashar, & Moula, Citation2010). Atluri et al. (Citation2004) has listed oviposition host plants as Cassia siamea, C. fistula, C. occidentalis, C. auriculata, C. tora, S. alata, C. siamea (Sharma et al., Citation2014). Thus, among various plants preferred by the butterfly, present study confirms that S. alata is preferred not only for ovideposition but also food for their larvae.
The second type of eggs collected and reared in the laboratory is the eggs of Jewel bug. Adult jewel bug C. stolli. (Heteroptera–Pentatomidae–Scutellerinae). It is polyphagus bug with beautiful coloration (metallic and green blue with black spots), reported to cause considerable loss to many plants of economic value and presently in S. alata also. Present observation on occurrence of jewel bug is in conformity with reports of Dhiman and Kumar (Citation2005, Citation2006) who have done a detailed study on ovideposition behaviour of C. stolli. Kumar and Dhiman (Citation2013) have reported that selection of host for oviposition by Jewel bug, C. stolli in Cassia occidentalis and Croton sparciflorus feeding on the leaves during winter and sheltering in the plant during the summer season. In the present study, similar seasonality has been observed.
Rearing of fourth type of egg with bright orange colour (Figure (e)) is not successful. However, it has been tried to identify the insect based on morphological features of eggs and mode of deposition in comparison with reports of Tanwar et al.(Citation2010); Mahalingam et al. (Citation2010) and Selvaraju and Sakthivel (Citation2012) on mealy bugs egg types, it is reasonably presumed that the ovidepositor might be the mealy bugs. Muniappan et al. (Citation2008) have reported mealy bugs in varieties of cultivated agricultural and horticultural flower crops, vegetables, and fruits. As fifth type of egg masses covered with cotton fibre-like structure has not shown any positive result on rearing, an attempt is made to locate same type of eggs in the S. alata in the field. Interestingly, when the fibres from the same type of egg masses found on leaves, are removed gently, tiny insects similar to young aphids fly away; moreover, adult aphides are noticed feeding on floral parts and presented (Figure (a) and (b)). Aphids are a small soft bodied sap-sucking insects with the long slender mouth parts for piercing leaf and soft stem and considered as one among the most destructive insect pests on cultivated plants. From an ecological point of view, they are a highly successful due to their asexual reproductive capabilities. Thus, it is clear from field survey followed by the laboratory studies that S. alata is preferred by insects for ovideposition. Host selection for oviposition is complex process involving factors such as shape, colour, texture, size, and chemical composition of the host plant (Courtney, Citation1990). As reported by Zalucki, Daglish, Firempong, and Twine (Citation1986) and (Fitt, Citation1989) ability of ovipositioning to locate and utilize a wide range of hosts, is one of the major factors contributing to the pest status of the plants. In the case of S. alata, factor influences host selection might be chemical response (chemo-receptors/sensores) working between host and insect, that influences host selection for ovideposition in addition to leaf morphology and availability of better host. Availability of suitable host plants for ovideposition could also be a factor because S. alata being an invasive, maintains monospecific population in the study site and no other native plant is available. Therefore, while analyzing factors influencing host selection, availability both in type and quantity of the plant, could not be ignored. Last but not the least, it could be attributed that leaves of S. alata are not grazed by larger herbivores-like cattles.
Figure 8. Eggs collected from leaves and flower bud of S. alata. (a) Catopsilia pyranthe (Butterfly), (b) Chrysochoris stollii (Jewel bug), (c) Hendecasiss duplifascialiss(bud borer), (d) mealy bug, (e) unidentified egg.
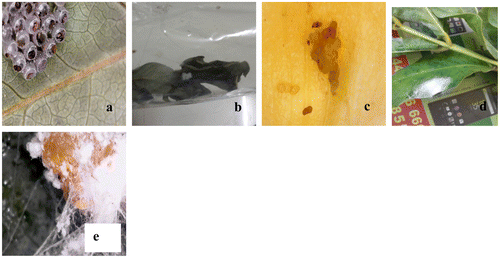
In addition to ovideposition in S. alata, two worms viz. flower bud and pod borer worm are found to feed on young buds and seeds (pod) of S. alata. It is noticed in the field that the well-grown worm is found to bore into the flower buds (Figure (d)) and eats up all the floral parts viz. corolla, style, and stigma – leaving much dark brown excreta. Similarly in the pods, the seeds are eaten by the worm and remaining seeds are succumb to fungal attack. Secondary infection in pods and buds are found to the by pathogenic fungal species viz. Aspergillus niger and A. flavus (Figure (a) and (b)). Similar problem due to bud borer H. duplifascialis has been reported earlier by (Amutha, Citation1994; Meenatchi et al., Citation2011; Suganthi et al., Citation2006) in flowers of various economically important flowers. S. alata is an important plant in terms of the source of medicine aesthetic values, the present finding would help to manage such problems where S. alata is raised in nurseries for commercial purpose.
It is learnt from rearing of worm collected from pod that pods of S. alata are infected by a common polyphagous pest E. zinckenella (Figure (c) and (d)). These borers enter into the pod and start eating seeds. These pod borers are not only recorded in Withania somnifera farmer’s field at Bilaspur (Sharma et al., Citation2014) but also in more crops in the entire South Asian region (Biswas, Citation2014; Fitt,Citation 1989; Yang, Johnson, & Zalucki, Citation2008) have recorded pod borer E. zinckenella in pigeon pea damaging both flower and pod. Thus, reports on attack of flower bud borer and pod borer in S. alata are the first report and considered as important contribution in management of nurseries where S. alata is grown for ornamental–commercial purpose and inflict in leaves which are one of the export-oriented herbal bases in India.
The strange but ecologically significant observation is sighting of worm inside the anthers. The worm is found inside anther and trying to come out by breaking the anther (Figure (g)–(i)). As anther is 10 mm in length and the soft-bodied worm inside anther is less than 10 mm (Figure (b) and (d)) practically it is very difficult to take out the worms alive. This observation eventually raises the question that under which circumstance s the worm is happened to be inside the anther. To address this, we could reasonably presume based on reports already available in this subject matter and observation made in the field that the unknown insect might have laid eggs on the micro pile seen at the distal end of the anther (Figure (e)). The larvae that come out from the eggs start eating the pollen inside. Once they grow bigger, they try to come out by breaking the wall of anther in all possible direction (Figure (g)–(i)). While they are inside, their excreta gets accumulated and spoil anther (Figure (f)). On coming out of the anther, these well-grown worms, start feeding on flower buds. Now, it is assumed that this is the stage at which we have collected and recorded the worm while boring the bud Hendecasis dulplifascialis (Figure ). In view of above observations made, it is presumed that the adult which deposited the eggs might be Hendecasis duplifascialis; however, further intensive study on this specific aspect would definitely bring out the complete life history of the inside anthers of S. alata. The output would open up new concept/scientific thought in the field of ovideposition and host selection process. Besides, worms, adult insects viz. Aphides (Aphis sp.) and green stink bug Acrosternum hilare are the two adult insects feeding on leaves and flowers of S. alata, making tiny holes on the leaves and thereby reducing the photosynthetic surface are of the leaves.
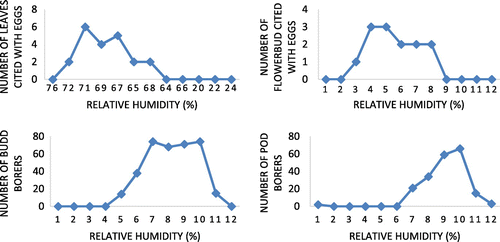
To ascertain the influence of major climatic factors viz. atmospheric temperature and rainfall relating to study region on the prevalence, abundance, and seasonality, Figures and clearly illustrate that ovideposition is activated during post-monsoon till summer and the worms prevalence and abundance are noticed during late summer and early pre-monsoon period. In conclusion, it is stated that as far as S. alata is concerned, infestation in terms of use of leaves for ovideposition, bud and pod destruction by worms and adult leaf feeding insects, seemed to be less serious. Such a low level of infestation may be due to the intrinsic characteristics of an invasive species possessed by S. alata and/or the type of phytochemical constituents present in host plant parts. It is seemed to a potential anti-herbivory not only to invertebrates-like insects but also larger vertebrates-like cattles. These attributes are seem to be relevant from the reports of (Awmack & Leather, Citation2002) that the nutritional quality of plant tissues, concentrations of plant metabolites and morphology of the plant, significantly influence negatively on feeding behaviour and development of native herbivores in invasive plant population. Moreover, reports of (Bezemer, Harvey, & Cronin, Citation2014) have indicated that invasive plants may contain novel secondary compounds that are toxic to native herbivores and their natural enemies, or may produce odours that are attractive to native insects and consequently interfere with interactions of these native insects with native plants. Through a comparative study on insects on related native and invasive plants in invaded habitats, (Carpenter & Cappuccino, Citation2005) reported that abundance of insect herbivores is often lower on invasive plants. Thus, it is clear that the possibilities for heavy pest infestation are very limited in the case of S. alata by the virtue of its phytochemical constituents and /or morphological characteristics. But still, it is felt that these findings on insect pest association and nature of infestation in S. alata and their seasonality would be a significant contribution to S. alata leaf collectors and traders to collect pest-free quality leaves for commercial purpose and quality seeds for those maintain commercial nurseries to raise S. alata plant for ornamental/commercial purpose (Figures and ).
Figure 11. Influence of rainfall on ovideposition in number of leaves, flower bud and bud borer, pod borer in Senna alata.
Conclusion
S. alata, one of the invasive of India, is preferred by minimum number of insects for ovideposition. Ovideposition is influenced by temperature, humidity, and rainfall during late post-monsoon and summer and the borers are influenced during late summer and pre-monsoon period. Reporting on the use of S. alata by the jewel bug C. stolli for feeding as well as ovideposition might be the pioneer one. Infestation in flower buds by borer is higher than pod borers. Sighting and recording of a worm inside the anther is probably be the first time in S. alata (based on the literature survey) One of the worms found developing inside the anther which has to be addressed intensively so as to find out how the worm is happen to be inside the anther. Low level of pest in S. alata in terms of quality and quantity might be due to its phytochemical constituents and/ or intrinsic invasive trait. Therefore, possibilities for heavy insect pest infestation in S. alata are very limited. As scientific information pertaining to insect pest in S. alata and their seasonality are almost not available and S. alata is commercially important plant species, present findings would be more useful to Senna leaf collectors from the wild in understanding pest infestation status during any given period of time.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
We thank Dr. A. Yogamoorthi, and Dr. S M. Sundarapandian, Department of Ecology and Environmental Sciences, Pondicherry University for their suggestion during the study period. We also thank the Pondicherry University for providing facilities required for the study. We also record our sincere thanks to Horticulture Division, Government of Pondicherry and Horticulture wing of Pondicherry University and Tamil Nadu Agriculture University, Coimbatore for providing as field information on crop pests and identification of adult insects collected.
References
- Abubacker, M. N., Ramanathan, R., & Kumar, T. S. (2008). In vitro antifungal activity of Cassia alata Linn. flower extract. Natural Product Radiance, 7, 6–9.
- Amutha, S. (1994). Bioecology and control of three pyralids Hendecasis duplifascialis Hampson. Nausinoe geometralis (Guenee) and Margaronia unionalis Hub. on Jasminum spp ( Ph.D. Thesis). University of Madras, Tamil Nadu, India.
- Arju, M. H., Bashar, M. A., & Moula, G. (2010). Developmental stages of a mottled emigrant butterfly, Catopsilia pyranthe. Dhaka University Journal of Biological Sciences, 19(2), 171–179.
- Atluri, J. B., Ramana, S., & Reddi, C. S. (2004). Ecobiology of the tropical pierid butterfly Catopsilia pyranthe. Current Science, 86(3), 457–461.
- Awmack, C. S., & Leather, S. R. (2002). Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology, 47(1), 817–844.10.1146/annurev.ento.47.091201.145300
- Bezemer, T. M., Harvey, J. A., & Cronin, J. T. (2014). Response of native insect communities to invasive plants. Annual Review of Entomology, 59, 119–141.10.1146/annurev-ento-011613-162104
- Biswas, G. C. (2014). Insect pests of groundnut (Arachis hypogaea L.), nature of damage and succession with the crop stages. Bangladesh Journal of Agricultural Research, 39(2), 273–282.
- Brody, A. K. (1992). Oviposition choices by a pre-dispersal seed predator (Hylemya sp.). II. A positive association between female choice and fruit set. Oecologia, 91, 63–67.10.1007/BF00317242
- Brody, A. K., & Waser, N. M. (1995). Oviposition patterns and larval success of a pre-dispersal seed predator attacking two confamilial host plants. Oikos, 74, 447–452.10.2307/3545989
- Carpenter, D., & Cappuccino, N. (2005). Herbivory, time since introduction and the invasiveness of exotic plants. Journal of Ecology, 93(2), 315–321.10.1111/jec.2005.93.issue-2
- Courtney, S. P. (1990). Mother doesn’t know best: Selection of hosts by ovipositing insects. Insect-plant Interactions, 2, 161–188.
- David, S. K. (1958). Insects and mites affecting jasmine in the Madras state. The Madras Agricultural Journal, 45(4), 146–150.
- Dhiman, S. C., & Kumar, P. (2005). "Food plants and seasonal occurrence of Chrysocoris stolli Wolf. (Heteroptera – Pentatomidae - Scutellerinae)". In VI National symposium on Indian Entomology, Productivity and Health (A silver jubilee celebration), October 2–4, 2005, Abstract 70, page 60. Muzaffarnagar, India: Uttar Pradesh Zoological Society Muzaffarnagar.
- Dhiman, S.C., & Kumar, P.. (2006). "Oviposition, fecundity and egg structure of Chrysocoris stolli Wolf. (Heteroptera – Pentatomidae - Scutellerinae)". In National symposium on Role of Applied Zoology in food Production and Human Health. (A Golden jubilee celebration) 23rd and 24th December 2006, Abstract IP 10, page 43. Saharanpur, India: Department of Zoology, M.S. (P.G.) College.
- Fitt, G. P. (1989). The ecology of Heliothis species in relation to agroecosystems. Annual Review of Entomology, 34(1), 17–53.10.1146/annurev.en.34.010189.000313
- Jiménez-Pérez, A., & Wang, Q. (2003). Oviposition behaviour of ‘Cnephasia’ jactatana Walker (Lepidoptera: Tortricidae) on kiwifruit. New Zealand Entomologist, 26(1), 109–111.10.1080/00779962.2003.9722116
- Liu, Z., Scheirs, J., & Heckel, D. G. (2010). Host plant flowering increases both adult oviposition preference and larval performance of a generalist herbivore. Environmental Entomology, 39(2), 552–560.10.1603/EN09129
- Kamala, M., Chinniah, C., Kennedy, J., Kalyanasundaram, M., & Suganthy, M. (2017). Pesticidal effect of indigenous plant extracts against jasmine bud worm, Hendecasis duplifascialis Hampson. in Jasmine (Jasminum sambac L.). International Journal Of Tropical Agriculture 35(2), 315–323.
- Kumar, P., & Dhiman, S. C. (2013). Chrysocoris stolli Wolf, A Sap Feeder Pentatomidae Bug on the Weed in North Western Districts of Uttar Pradesh. IJASR, 3(2), 89–92.
- Mahalingam, C. A., Suresh, S., Subramanian, S., Murugesh, K. A., Mohanraj, P., & Shanmugam, R. (2010). Papaya mealybug, Paracoccus marginatus – A new pest on mulberry, Morus spp. Karnataka Journal of Agricultural Sciences, 23(1), 182–183.
- Meenatchi, R., Giraddi, R. S., Patil, V. S., Vastrad, A. S., & Biradar, D. P. (2011). Effect of vermitechnologies on jasmine insect pests. Karnataka Journal of Agricultural Sciences, 24 (3).
- Muniappan, R., Shepard, B. M., Watson, G. W., Carner, G. R., Sartiami, D., Rauf, A., & Hammig, M. D. (2008). First report of the Papaya Mealybug, Paracoccus marginatus (Hemiptera: Pseudococcidae), in Indonesia and India. Journal of Agricultural and Urban Entomology, 25(1), 37–40.10.3954/1523-5475-25.1.37
- Permana, A. D., Johari, A., Putra, R. E., Sastrodihardjo, S., & Ahmad, I. (2012). The influence of trichome characters of soybean (Glycine max Merrill) on oviposition preference of soybean pod borer Etiella zinckenella Treitschke (Lepidoptera: Pyralidae) in Indonesia. Journal of Entomology and Nematology, 4(3), 15–21.
- Pettersson, M. W. (1992). Taking a chance on moths: Oviposition by Delia flavifrons (Diptera: Anthomyiidae) on the flowers of bladder campion, Silene vulgaris (Caryophyllaceae). Ecological Entomology, 17(1), 57–62.10.1111/j.1365-2311.1992.tb01039.x
- Sarma, S., Senthilkumar, N., & Das, S. K. R. (2008). Insect pests of medicinal and aromatic plants and their management: An overview. Indian Forester, 134(1), 105–119.
- Selvaraju, N. G., & Sakthivel, N. (2012). Host plants of papaya mealybug (Paracoccus marginatus Williams and Granara de Willink.) in Tamil Nadu. Karnataka Journal of Agricultural Sciences, 24(4).
- Sharma, P. C., Kumar, A., Mehta, P. K., & Singh, R. (2014). Survey studies on insect-pests associated with important medicinal plants in Himachal Pradesh. Evaluation, 2(7), 3.
- Suganthi, A., Chandrasekaran, S., & Regupathy, A. (2006). Pheromone release behaviour of jasmine bud worm, Hendecasis duplifascialis Hampson (Pyraustidae: Lepidoptera). J. Entomol., 3, 236–240.
- Tanwar, R. K., Jeyakumar, P., & Vennila, S. (2010). Papaya mealybug and its management strategies (p. 26). New Delhi: National Centre for Integrated Pest Management.
- Yang, Y., Johnson, M. L., & Zalucki, M. P. (2008). Possible effect of genetically modified cotton on foraging habits of early instar Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) larvae. Australian Journal of Entomology, 47(2), 137–141.10.1111/j.1440-6055.2008.00640.x
- Zalucki, M. P., Daglish, G., Firempong, S., & Twine, P. (1986). The biology and ecology of Heliothis-armigera (Hubner) and Heliothis-punctigera Wallengren (Lepidoptera, Noctuidae) in Australia – What do we know. Australian Journal of Zoology, 34(6), 779–814.10.1071/ZO9860779
- ZAUBA. (2015). Retrieved from https://www.Zauba.com/export-candle%20bush+Cassia
- Zimmerman, M. (1980). Reproduction in Polemonium: Pre-dispersal seed predation. Ecology, 61(3), 502–506.10.2307/1937415