Abstract
A first extensive survey in the inland waters of Apulia was carried out with the aim to detect occurrences of large branchiopods and to assess their diversity and distribution in the region. Samples were gathered during each wet season between 2004 and 2016, in several cases with multiple collections per site in different years. A total of 240 water bodies were studied. Based on a careful review of the available literature data and the results of this survey, 10 large branchiopod species (six Anostraca, two Notostraca, two Spinicaudata) are reported to occur in the Apulian inland waters at present. The findings of the spinicaudatans Leptestheria mayeti and Cyzicus tetracerus and of the anostracan Streptocephalus torvicornis are new records for the Italian mainland. Different species assemblages were found to correspond to specific sets of environmental conditions and habitat types. The diversity of large branchiopods in the region indicates the distinctive role of the Apulian inland waters and their ecological importance in the biogeography of the crustacean inland water fauna in the Mediterranean area. The conservation status of the environments where the species were collected is also discussed.
Introduction
In the last few decades, there has been an increasing interest in temporary inland water ecosystems studies. In Europe, this trend led the scientific community to promote the launch of an international forum named the “European Pond Conservation Network” (EPCN Citation2008; www.europeanponds.org) with the aim to promote awareness, understanding and conservation of ponds with their biodiversity in a changing European landscape (Oertli et al. Citation2009). The need for more applied research on pond biology and ecology is one of the main topics of EPCN in order to support best practices in pond conservation, management, creation and monitoring. Among the different types of inland waters, the Mediterranean Temporary Ponds (hereafter MTPs) were indicated as priority habitats (habitat code 3170*) for conservation by Council Directive 92/43/EC (European Commission Citation1992).
Branchiopoda fulfil an important ecological role in the biological community of these characteristic water ecosystems. Indeed, they include species responsible for the energy transfer from producers to consumers; moreover, several species offer a crucial contribution to the recycling of organic matter (Dumont & Negrea Citation2002 and references therein). A subgroup of Branchiopoda is represented by the so-called “large branchiopods”, namely Anostraca (fairy shrimps), Notostraca (tadpole shrimps), Spinicaudata (clam shrimps), Laevicaudata and Cyclestherida (Olesen & Richter Citation2013). In particular, the first three groups are considered to be flagship animals of the temporary waters for their highly specialised life cycle. Despite the periodical drying up of ponds, the large branchiopods are able to persist in the sediments thanks to their resting stages (Dumont & Negrea Citation2002), which also give them a high dispersal ability (but see also Incagnone et al. Citation2015). Large branchiopods are extraordinary animals that have conserved their basic morphological structure since the Devonian (Thiéry Citation1996; Voigt et al. Citation2008; Lagebro et al. Citation2015; Gueriau et al. Citation2016). Their phylogeny has been recently rearranged thanks to modern molecular tools (Stenderup et al. Citation2006; Richter et al. Citation2007), especially in the group of the Notostraca (Mantovani et al. Citation2004, Citation2009; Korn et al. Citation2006, Citation2010, Citation2013; Vanschoenwinkel et al. Citation2012).
About 550 different species of large branchiopods have been described so far at the global scale, most of them (313 species) belonging to Anostraca (Brendonck et al. Citation2008; Ahyong et al. Citation2011). According to the last updated inventory by Mura (Citation2006) and the additional record by Scanabissi et al. (Citation2006), 25 species (16 Anostraca, four Notostraca, five Spinicaudata) are known to occur currently in Italy. Nevertheless, Mura (Citation2006) noted a very scant knowledge for several Italian areas because of the paucity of field studies that affects the temporary waters especially in the southern regions, Apulia being a case in point.
Recent limnological studies focused on Apulian ponds (Alfonso et al. Citation2011) have already achieved an updated inventory and distribution of Calanoida (Crustacea, Copepoda) (Alfonso & Belmonte Citation2011) and new records for the Italian fauna (Alfonso & Belmonte Citation2013). Conversely, data on the occurrence and distribution of large branchiopods are still scattered and dated, and often refer to single records of species, therefore resulting in an uneven set of data.
Due to this gap of knowledge, the present research aims to: (i) update the checklist and distribution of large branchiopods in Apulia by reviewing existing literature and conducting new surveys; (ii) identify patterns of species assemblages typical of the Apulian biogeographical province; (iii) evaluate the influence of the environmental features and the habitat type on the species occurrence; (iv) evaluate the conservation status of the species and their occurrence sites.
Materials and methods
Study area
Apulia is a peninsular region of 19,357 km2, and represents the south-easternmost part of the Italian mainland pointing towards the Balkans with the closest point less than 75 km away across the Adriatic Sea. The region has a north-west–south-east orientation and is located between 42°15ʹN-39°45ʹN parallels and the 15°00ʹE-18°30ʹE meridians. The Bradano Valley represents the natural boundary separating Apulia from the mountain chain of the Apennines. The Apulian landscape is mainly karst and characterised by lowlands, with the exceptions of the central area (Murgia plateau, maximum altitude 686 m asl), the Gargano promontory in the north and the Dauni Mountains in the north-west (these last two areas have maximum altitudes of around 1000 m asl) (ISTAT Citation2004).
Climate and vegetation are typically Mediterranean (Macchia et al. Citation2000). The average annual rainfall ranges between 500 and 750 mm (Zito et al. Citation1991). Most rainfall generally occurs in the period October–January, with monthly average values ranging between 70 and 90 mm (exceptionally, 100 mm) and progressively decreasing to the minimum values of 10–20 mm in July. The average annual temperature is around 16°C with possible maximum values slightly over 40°C in summer in some inner plane areas in the north and in the southernmost part of the region (data from www.agrometeopuglia.it). These environmental and meteorological conditions are ideal for the presence of surface waters with temporary hydroperiods (Alfonso et al. Citation2011), which are also suitable for hosting large branchiopods. Apulia is different from the other Italian biogeographical provinces: (Alpine, Apenninic, Padanian, Sardinian and Sicilian), but all of them concur to represent the Italian biogeographic scenario in the context of Mediterranean area (Minelli et al. Citation2006).
Data collection and species identifications
Qualitative samples of large branchiopods were collected between 2004 and 2016, in the framework of an extensive limnological survey carried out mainly during the wet season and until the late spring (i.e. from October to May) in 240 inland water bodies throughout Apulia. Samples were collected using a hand-net (mesh size of 500 µm, mouth opening of 25 cm) and a towing plankton net (mesh size of 125 µm, mouth opening of 20 cm).
Some environmental features were detected along with the collection of the biological samples. They included the geographical coordinates and altitude, directly recorded by a portable geographic positioning system (GPS) and checked with Google Earth Pro version 7.1.5.1557. The same software was used to measure the maximum surface of ponds on the satellite images. The maximum depth and water transparency were measured in situ by a turbidity tube (Dahlgren et al. Citation2004). A multi-probe provided values of temperature, pH, electrical conductivity and dissolved oxygen. Existing and potential threats to each pond conservation were also noted during samplings. Data on the active protection measures were acquired from the institutional website of the Apulia Region (www.sit.puglia.it). About 14% of the occurrence data of species was obtained through citizen science (mainly from www.argonauti.org), which data were verified and validated by specific surveys on sites. All the examined sites were codified with an alphanumerical code containing the letters “PU” (for Puglia, the Italian name of the region) and three digits indicating a progressive number in the specific dataset.
The biological samples were fixed in situ and preserved in 96% ethanol. Species were observed with a stereomicroscope and identified according to the main morphological characters described by Daday de Deés (Citation1914, Citation1923), Longhurst (Citation1955), Cottarelli and Mura (Citation1983), Brtek et al. (Citation1984), Alonso (Citation1996) and Thiéry (Citation1996). Drawings of morphological features were realised with a camera lucida mounted on the stereomicroscope.
Data analysis
The efficiency of the sampling effort was evaluated with the software EstimateS (version 9.1.0) (Colwell Citation2013), the input data being based on presence/absence values; the estimators computed were Chao 2 and Bootstrap. The coordinates of sites were uploaded in a dataset file along with the occurrence data of each species, and distribution maps were outlined using the QGIS software (version 2.8.2). The reference system was WGS 84.
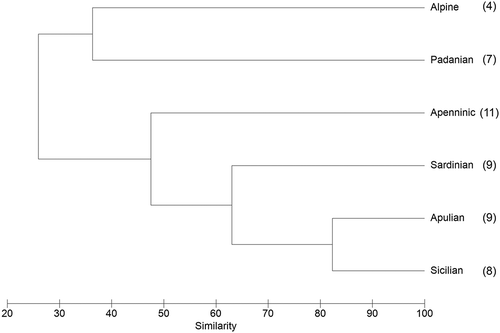
A comparison with the checklists of the other five Italian biogeographical provinces was performed with a cluster analysis with the Primer 6 plus software (Clarke & Gorley Citation2006). The Apulian data derived from a past literature review as well as from this research. Data of the other biogeographical provinces came mainly from Mura (Citation2005) and was updated using the following studies and surveys (Marrone et al. Citation2009; Gandolfi et al. Citation2015).
Since more than one species co-occurred in several sites, the coexistence patterns were analysed for each pair of species by the Fager’s Index of affinity (Fager Citation1957) and reported in a table also containing the number of joint occurrences following Maeda-Martínez et al. (Citation1997). The three species occurring in the two coastal saline ponds were excluded from this analysis because of their habitat type which is different from most of the analysed temporary freshwater ponds and obviously constitutes a separated group. For the same reason, the two coastal saline ponds were left out of the environmental analyses involving the other 59 freshwater sites.
A distance-based redundancy analysis (dbRDA; Legendre & Anderson Citation1999) was applied to the similarity matrix (based on “S8 Sørensen” as resemblance measure) using the software Primer 6 plus (Clarke & Gorley Citation2006) to correlate patterns of species assemblages with environmental features. The non-parametric Spearman’s correlation values were used for superimposing species as vectors on the ordination plot. The dbRDA plots display sites with different symbols according to the factor “MTP”. The identification of MTPs is based on Alfonso et al. (Citation2011, Citation2016). In addition, a matrix of correlations among all the environmental features was computed using the R software version 3.2.5 (R Core Team Citation2016). The correlations between species occurrence probability (y-axis) and environmental variables (x-axis) were also calculated in order to support the dbRDA plots.
Results
Species diversity and distribution
Overall, 10 species of large branchiopods (six Anostraca, two Notostraca and two Spinicaudata; ) have been detected in 61 Apulian temporary waters (online Appendix I) out of 240 water bodies sampled (). Anostraca, with their occurrences in 49 different ponds (80% of ponds with large branchiopods) was the most frequent taxon; Notostraca occurred in 32 ponds (52%), and Spinicaudata in 12 ponds (19%). Streptocephalus torvicornis (Waga, 1842), Cyzicus tetracerus (Krynicki, 1830) and Leptestheria mayeti (Simon, 1885) are new records for the Italian Peninsula. Lepidurus sp. still represents an undefined taxon. First-hand drawings were made to highlight the main morphological features of these last four species.
Table I. Checklist of Apulian large branchiopods and their occurrence sites. Systematics according to Olesen and Richter (Citation2013). (*) = allochthonous species. References: (a) Artom (Citation1921); (b) Ghigi (Citation1921); (c) Belmonte et al. (Citation1993); (d) Cottarelli and Mura (Citation1995); (e) Mura et al. (Citation1999); (f) Mura (Citation2001); (g) Moscatello et al. (Citation2002); (h) Mura et al. (Citation2006b); (i) Mura et al. (Citation2006a); (j) Scanabissi et al. (Citation2006); (k) Alfonso et al. (Citation2011); (l) Alfonso et al. (Citation2016); (m) Korn et al. (Citation2013); (n) this study.
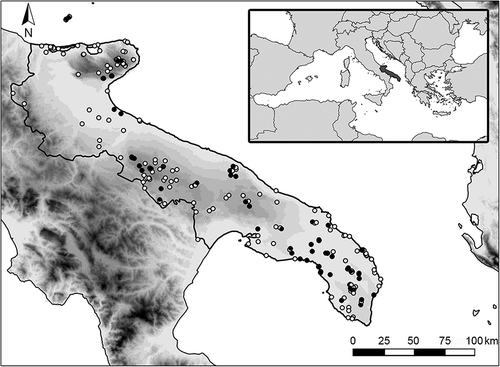
Figure 1. Map of Apulia and distribution of the sampled sites. White dots represent sites without large branchiopods; black dots are sites hosting at least one species.
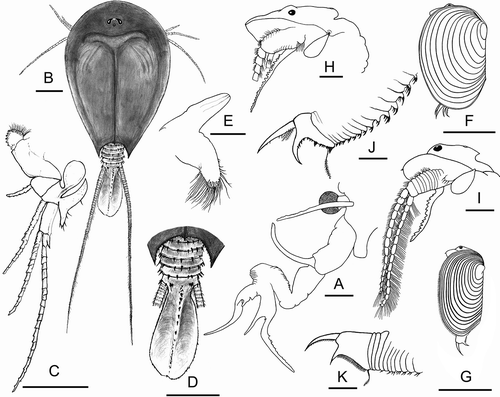
S. torvicornis was easily identified based on the peculiar morphology of the second antennae (). This feature showed a marked variation in the denticulation of the thumb among the four different Apulian populations, even within the same population. The row of denticles generally terminated before the major inflexion and denticles increased in size towards the proximal end. The notostracan Lepidurus sp. () had the exopodite of the first leg with two lobes (). The supra-anal plate was very developed and shaped as in . The maxilla was strong and largely covered by very subtle setae in its distal margin; the annexed deferent glandular duct had a conic shape (). Length of C. tetracerus males () was maximum 12 mm. The head was rounded in its posterior margin () and the telson equipped with about 20 markedly different spines on its dorsal margin (). Males of L. mayeti () were about 9–10 mm long. Head was sharp and armed with a small apical spine (). The telson had about 60 thin spines of similar length ().
Figure 2. Streptocephalus torvicornis, male: A, part of head, first and second antenna. Lepidurus sp., male: B, Habitus; C, first leg; D, supra-anal plate; E, maxilla. Cyzicus tetracerus, male: F, habitus; H, head; J, telson. Leptestheria mayeti, male: G, habitus; I, head; K, telson. Scale bars: B–D, F, G = 5 mm; A, E, H–K = 1 mm.
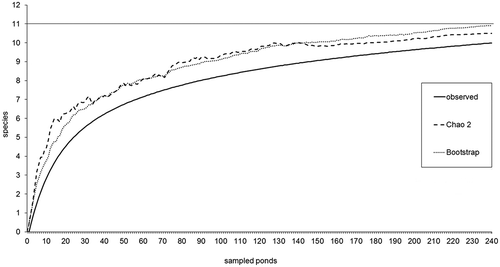
The estimators of species richness, computed along with the species rarefaction curve (), indicated the efficiency of the sampling effort revealing that 92–95% of the large branchiopod fauna in Apulia was covered by this study.
Figure 3. Species rarefaction curve of large branchiopods in Apulia (observed) and performance of estimators (Chao 2, Bootstrap).
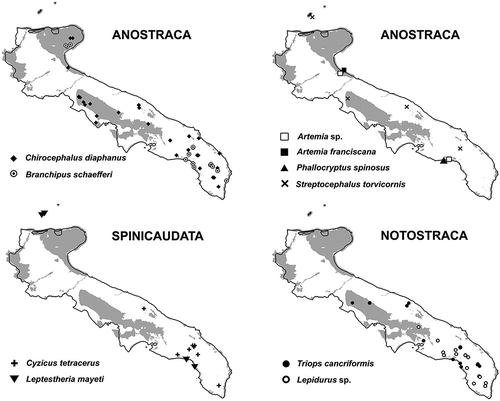
The distribution of species is reported in . The anostracan Chirocephalus diaphanus Prévost, 1803 was the most common species (33 occurrence sites; ) and the one with the widest distribution. The notostracan Lepidurus sp. (19 sites) was found exclusively in the southern part of the region (Salento Peninsula), similarly to the rarer C. tetracerus (eight sites). L. mayeti and S. torvicornis (four sites, one shared by the two species) were the rarest species found although their distribution covered the entire region, including the Tremiti Islands. Artemia sp. was known from both saltworks present in Apulia, “Margherita di Savoia” (PU073) and “Torre Colimena” (PU196). Based on data from the literature (Mura et al. Citation2006b), the allochthonous species Artemia franciscana Kellogg, 1906 co-occurs with the “native” Artemia in PU073. Unfortunately, it was not possible to check the presence of A. franciscana after 2004. Phallocryptus spinosus (Milne-Edwards, 1840) (see Marrone et al. Citation2016b for the updated binomen) was once present in the abandoned southern saltworks of Torre Colimena, currently a regional park. In the framework of this study, this site was repeatedly sampled in different seasons and years, but only specimens of Artemia sp. parthenogenetic population (sensu Mura et al. Citation2005; Baxevanis et al. Citation2006; Rogers Citation2013) were collected.
Figure 4. Distribution map of the large branchiopods in Apulia. Some symbols, related to single ponds, might be overlapped. The protected areas are shaded in grey.
The comparison of the Apulian large branchiopods checklist with those of the other five Italian provinces was evaluated with a cluster analysis that identified two main groups (). One cluster included the Alpine and the Padanian provinces (with a very low percentage of similarity), the second comprising all the other provinces with the subgroup Sardinian–Apulian–Sicilian reaching more than 60% of similarity. The higher similarity value (more than 80%) emerged for the Sicilian and the Apulian provinces.
Co-occurrences of species and environmental features
The species incidence percentages per site are summarised in and reveal that the higher the number of co-occurring species, the lower the number of sites where the co-occurrences took place. Out of the 61 ponds with large branchiopods, there were 34 sites hosting only one species (56%); in most cases (17 sites) the single species was Chirocephalus diaphanus (see also ). There were 16 ponds hosting two species (26%), 10 with three species (16%) and only one site (about 2%) with four co-occurring species synchronically. This site, called Palude Balsamo (PU001) and located in the Sandonaci floodplain, was the largest freshwater temporary pond in the studied area and hosted up to six large branchiopods during the year. In particular, Lepidurus sp., Cyzicus tetracerus, Chirocephalus diaphanus and (more rarely) Branchipus schaefferi Fischer, 1834 were detected in the cold season, while Streptocephalus torvicornis and Triops cancriformis (Bosc, 1801) were usually found in the late spring when the pond exceptionally might remain flooded. The Fager’s affinity index of co-occurrences () showed the highest value for the couple T. cancriformis–B. schaefferi (55) followed by Lepidurus sp.–C. diaphanus (47), Lepidurus sp.–C. tetracerus (44) and C. diaphanus–C. tetracerus (35). Among the cases of three co-occurring species (10 sites), the co-occurrence of Lepidurus sp.–C. diaphanus–C. tetracerus was the most frequent (seven sites), followed by the combination of T. cancriformis–B. schaefferi–L. mayeti (two sites).
Table II. Percentage of occurrences of species number of large branchiopods (LBs) per site.
Table III. Co-occurrence patterns for each pair of species of large branchiopods in Apulian freshwater temporary ponds. The upper side of the matrix shows the number of joint co-occurrences. The lower side shows the values of the Fager’s Index of affinity per 100.
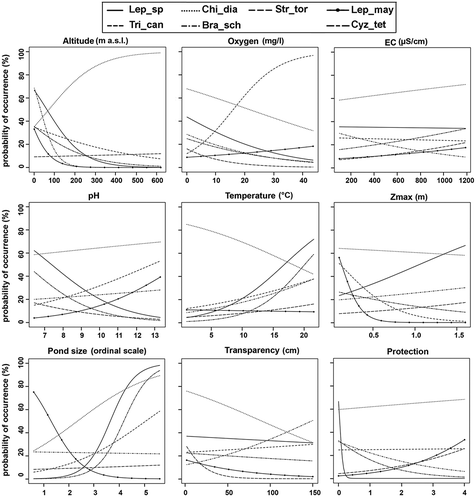
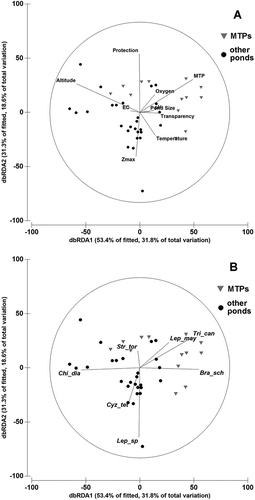
Information on the Apulian ponds with large branchiopods is provided in Appendix I. Although the altitude of sites ranged from sea level up to 785 m, most sites (52 out of 61) were under 500 m asl. The pond size, measured as the maximum the inundation area, varied from 4 to 410,000 m2 for the freshwater sites. The depth values (averages for each pond) generally did not exceed 1.50 m, and most ponds (55) were less than 1 m deep. The dbRDA analysis () shows that the explained constrained variation of the first two axes was 84.7% of total variation (unconstrained variation 50.4%), indicating a highly significant correlation between species distribution and predictor variables. A detectable grouping along the habitat type showed MTPs as separated from the other pond types. The first axis in the dbRDA plot with environmental features as vectors () represented an altitudinal gradient linked to the MTP habitat type. The second axis was highly correlated with pond depth and protection measures. Vectors plotting species () clearly showed that Triops cancriformis, Leptestheria mayeti and Branchipus schaefferi were significantly associated to the MTPs, contrarily to the assemblage composed by Chirocephalus diaphanus, Cyzicus tetracerus and Lepidurus sp. In addition, Lepidurus sp. and C. tetracerus characterised the deeper and “less protected” ponds.
Figure 6. Plots of the distance-based redundancy analysis (dbRDA) using the first two axes. Explained constrained variation: 84.7%; unconstrained: 50.4% of the total variation. Percentage values for each axis are in parentheses. A, Vectors refer the environmental variables. B, vectors refer to the species using Spearman as correlation type. Sites represented by triangles include the habitat type “Mediterranean Temporary Ponds” (MTP). Species abbreviations are as in .
The independence of the environmental features is reported in the correlogram of Appendix II. The correlation between the gradients of each environmental feature and the probabilities of the species occurrences () was also evaluated. Most species occurred at lower altitudes (less than 200 m asl), with the exception of C. diaphanus. Triops cancriformis was the species better correlated with higher oxygen values. The exclusion of the two coastal saline ponds from the analyses reduced the electrical conductivity range considered to 100–1190 µS/cm, which did not have relevant influence on the species occurrences. Lepidurus sp. and C. tetracerus preferred waters with lower pH values (6.5) contrarily to T. cancriformis and L. mayeti (found more frequently in alkaline waters). Most species were collected within the temperature range 10–20°C, but C. diaphanus was mainly detected at lower temperatures. Deeper waters seemed to be preferred by Lepidurus sp. The larger the pond, the higher the probability of occurrence for Lepidurus sp. and C. tetracerus, but not for L. mayeti. Water transparency appeared suitable for B. schaefferi, contrarily to C. diaphanus and Lepidurus sp. The factor “protection” had a faint influence only on the occurrences of S. torvicornis and L. mayeti more than on the other species. Nevertheless, about half of the Apulian ponds hosting large branchiopods had no kind of protection (see online Appendix I and ).
Discussion
This study provides a first comprehensive overview on the current occurrence and distribution of the large branchiopods in Apulia. Concerning Notostraca, Ghigi (Citation1921) described the species “Thriops apulius” on the basis of specimens collected in the Salento Peninsula. This taxon was later synonymised by Longhurst (Citation1955) with Triops cancriformis (Bosc, 1801), a species confirmed to be present in central and southern Apulia. The Apulian populations of Lepidurus were identified by Scanabissi et al. (Citation2006) as Lepidurus couesii Packard, 1875, a new record for the Italian fauna. The morphological analysis provided in the present study highlighted that the exopodite of the first leg has two opposite lobes (). Indeed, this feature would characterise L. couesii according Brtek et al. (Citation1984) and Thiéry (Citation1996) (based on Mongolian and Syrian populations respectively), and it differs in Lepidurus apus (Linnaeus, 1758) which bears only one lobe. The shape and length of the male anal plate () is also in good accordance with the descriptions by Alonso (Citation1996) and Thiéry (Citation1996). However, Alonso (Citation1996) identified the Iberian Lepidurus as L. apus despite the fact that the exopodites of their first legs had two lobes. Notwithstanding, the shape of the maxilla and its glandular duct () also resembled the description of Alonso (Citation1996). Although the Apulian Lepidurus are molecularly close to L. couesii (Scanabissi et al. Citation2006; Mantovani et al. Citation2009), Mathers et al. (Citation2013) indicated that this taxon might belong to a new putative species, presumably separated from other Lepidurus spp. about 15 Mya. Accordingly, Korn et al. (Citation2013) hypothesised a possible case of cryptic endemism and suggested designating the Apulian Lepidurus as Lepidurus sp., waiting for an accurate revision of the genus.
The main morphological features of the Apulian specimens of Leptestheria mayeti fit with the identification of L. cf cortieri provided by Cottarelli and Mura (Citation1983) based on specimens collected in Sicily, and with the descriptions of Jaume (Citation1989) and Alonso (Citation1996) for the Balearic population. Alonso (Citation1996) proposed the binomen Isaura mayeti (Simon, 1885), stating that the Sicilian population corresponded with that taxon. However, the current accepted name for this spinicaudatan is Leptestheria mayeti (Simon, 1885) (www.faunaeur.org). The morphological features of the other spinicaudatan species () fit with the descriptions of Cyzicus tetracerus provided by Cottarelli and Mura (Citation1983) and Alonso (Citation1996). The Apulian specimens of Streptocephalus torvicornis () showed a wide variability in the denticulation of the thumb of the male second antenna. This kind of variability was already discussed by other authors for comparisons among populations belonging to a wider geographic scale (Dumont et al. Citation1991). The lack of digitiform protuberances on the peduncle of the distal outgrowth, together with other features of the male second antenna, led Dumont et al. (Citation1995) to distinguish the presumed subspecies S. torvicornis bucheti from S. t. torvicornis. As also noted by Mura and Cottarelli (Citation1998), something like small digitiform processes were present in the population of the Tremiti Islands (PU131), but not in the other Apulian population here reported for the first time. As the morphological variability of this taxon is large, further studies could be useful to clarify the validity of the presumed subspecies.
In Italy, S. torvicornis was known only from the Tremiti Islands (Cottarelli & Mura Citation1995); C. tetracerus from seven occurrence sites in north-western Sicily (Cottarelli & Mura Citation1979; Marrone & Mura Citation2006), and one in southern Sardinia (Cottarelli & Mura Citation1995); and L. mayeti only from a single temporary pond of north-eastern Sicily (Cottarelli & Mura Citation1979). In this last case the deterioration of the pond was documented (Marrone & Mura Citation2006), and consequently the extinction of L. mayeti in Italy. For this reason, the findings of L. mayeti in Apulia can be considered a re-discovery for the Italian fauna. Moreover, the records of L. mayeti in Apulia provide new data on its distribution (earlier considered chiefly North African–Arabian) in continental Europe in addition to the recent discovery of a population in eastern Spain (Miracle et al. Citation2008). At present, the Apulian ponds with L. mayeti (those at the Tremiti Islands in particular) represent the northernmost occurrence sites of its known distribution, which hence could be better defined as Mediterranean–Arabian. Brtek and Thiéry (Citation1995) supposed that L. mayeti have colonised Europe from North Africa. A similar North African origin is shared with other crustacean species typical of temporary ponds, the anostracan S. torvicornis (see Dumont et al. Citation1995) and the calanoid copepod Neolovenula alluaudi (Guerne & Richard, 1890) (see Alfonso & Belmonte Citation2013), both detected in Apulia as well and currently in no other pond in Italy. Considering the known distribution of these species, it is more likely that the Apulian populations of S. torvicornis (like those of the calanoid N. alluaudi) descend from colonisers coming from the Balkans, rather than entailing a direct arrival from the south (as probably happened for L. mayeti). The similarity analysis showed the “Mediterranean” structure of the large branchiopod faunas of Sardinia, Sicily and Apulia, in good accordance with previous studies focused on other crustacean groups (Alfonso & Belmonte Citation2011).
The decreasing number of sites in relation to higher numbers of co-occurring species found comparable patterns with few other geographic areas (e.g. Maeda-Martínez et al. Citation1997). The higher Fager’s index values for combinations involving one notostracan and one anostracan species were comparable with values detected in Moroccan ponds (Van Den Broeck et al. Citation2015). The long hydroperiod and the large surface area of the Sandonaci floodplain (pond codes PU001-4) probably promoted the rare conditions that allowed the alternation of different large branchiopods assemblages in the same site (up to six syntopic species). This extraordinary species richness is unparalleled in Italy and, in the Mediterranean area, finds some counterparts only in the Iberian Peninsula (Sahuquillo & Miracle Citation2010) and Maghreb (Van Den Broeck et al. Citation2015, for Morocco; Stoch et al. Citation2016, for Tunisia), appearing typical of floodplains located close to the border of different climatic belts (Stoch et al. Citation2016). Likewise, the Sandonaci ponds (PU001-4) lie in a floodplain (at the centre of the Salento Peninsula) with micro-climatic conditions different from those of the neighbouring areas (Macchia et al. Citation2000). These ponds, remarkable for their large branchiopods diversity, also host calanoid copepods belonging to the genus Hemidiaptomus (cf. Alfonso & Belmonte Citation2011); therefore, they may be categorised as “Hemidiaptomus ponds” (Sahuquillo & Miracle Citation2013; Alfonso et al. Citation2016; Marrone et al. Citation2016a). Similarly, in the west Mediterranean, few areas remarkable for large branchiopod diversity also have the rare “Hemidiaptomus ponds”: the Chaouia plain in Morocco (cf. Ramdani Citation1988), the Medjerda floodplain in Tunisia (cf. Turki & Turki Citation2010), and Sinarcas in Spain (cf. Sahuquillo & Miracle Citation2010).
The combination Lepidurus sp.–Chirocephalus diaphanus–Cyzicus tetracerus was the most frequent among the cases of three-species assemblages in the Apulian ponds. The assemblage Branchipus schaefferi–Triops cancriformis–Leptestheria mayeti, very rare in Apulia, found resemblance with the association Branchipus-Triops-Leptestheria already reported for Tunisia and Algeria (Gauthier Citation1928), Western Morocco (Thiéry Citation1991), Balearic Islands (Jaume Citation1989; Alonso Citation1996) and Eastern Spain (Miracle et al. Citation2008). The steno-Mediterranean climate and the characteristic geographic position in the Mediterranean probably promote in Apulia the occurrence of species with a Palaearctic distribution (C. diaphanus, B. schaefferi, T. cancriformis and C. tetracerus) and species with a circum-Mediterranean gravitation (L. mayeti, S. torvicornis, P. spinosus). In addition, if the preliminary results of Mathers et al. (Citation2013) and Korn et al. (Citation2013) are confirmed with further evidence, the Apulian Lepidurus could belong to a new species endemic to southern Apulia. The dbRDA analysis (in good accordance with the species occurrence probability analysis) allowed us to identify the ecological preference of species and even a niche separation among species belonging to the same group. The detected preference of C. diaphanus for sites located at high altitude could be explained by the wide ecological valence of this species, able to live also in sites not shared by other large branchiopods. Conversely, B. schaefferi showed a marked preference for ponds located at lower altitudes. Triops cancriformis preferred well-oxygenated, alkaline and shallow ponds, contrarily to Lepidurus sp., which was found more frequently in deeper ponds, often together with the spinicaudatan C. tetracerus. These last two species were also detected more frequently in slightly acidic waters (pH values 6–7). The most frequent three-species assemblage (Lepidurus sp.–C. diaphanus–C. tetracerus) occurred in the deeper temporary ponds of the Salento Peninsula with turbid and low oxygenated waters. In contrast, the assemblage T. cancriformis–L. mayeti–B. schaefferi was associated with the MTP habitat type. Current protection measures appear to be mainly unrelated with most of the species occurrences, which even showed that the rare species C. tetracerus and the putative endemic Lepidurus sp. are present in ponds with no protection measures. These ponds, more than others, suffer periodic ploughing for farming, and waste accumulation, which are potential threats to the conservation of both habitats and species. Furthermore, the protected sites suffered a wider spectrum of threats including pollution, introduction of allochthonous species, vehicle transit, habitat modification and even inappropriate management.
Conclusions
The remarkable diversity of large branchiopods in Apulia highlights the important biogeographic role of this area for the Mediterranean temporary ponds and their typical crustacean fauna. The results of this research suggest that the large number of temporary water bodies located in Apulia promotes a high incidence and diversity of large branchiopods in a relatively small geographic area. Considering that the current checklist of large branchiopods in the Italian fauna includes 23 species (see Ruffo & Stoch Citation2006; but also Belk & Brtek Citation1995; Zarattini et al. Citation2001), it is evident that Apulia (with 10 species) alone hosts an important part of the Italian large branchiopod diversity. Apulia also appears to be one of the rarest Mediterranean areas noteworthy for large branchiopod diversity and co-occurrences. Threats to conservation of large branchiopods in Apulia are represented by factors of different nature. The invasive species Artemia franciscana could represent a problem for the native parthenogenetic population of Artemia sp. in the Margherita di Savoia saltworks (PU073), as recently is happening in other regions of the Mediterranean area (Muñoz et al. Citation2014). In other cases, even though ponds lie in protected areas, the management was revealed to be inadequate to large branchiopod conservation. Recently, the saltworks of Salina dei Monaci (PU196) were connected with the sea by a permanently opened channel, compromising the specific features of the saline system. As a paradox, the less-protected subarea in Apulia (the Salento Peninsula) is that with a higher number of species and co-occurrences. The low number of sites hosting the rare species L. mayeti and S. torvicornis (exclusive in Italy) and the small size of ponds, although located in protected areas, make them vulnerable sites for species conservation. In addition, the periodic ploughing of lands surrounding ponds (and even of the pond bed when dried) contributes to a progressive reduction of these habitats year by year.
Therefore, it is suggested that local administrations and management agencies should pay more attention to occurrences and ecological requirements of large branchiopods in order to adopt specific protection measures for ponds as special biodiversity hot-spots.
Supplemental Material
Download MS Word (30.1 KB)Acknowledgements
This paper is gratefully dedicated to the memory of Professor Graziella Mura (7 December 1940–23 March 2016) who considerately encouraged the author to realise this work. She spent her life in the study of large branchiopods and passionately transmitted her knowledge to several limnologists.
The banking foundation Fondazione Cassa di Risparmio di Puglia and the Italian National Park Parco Nazionale dell’Alta Murgia partially contributed to support this research. Francesco Chetta, Michel di Bari, Ugo Ferrero, Marco Gargiulo, Cristiano Liuzzi, Giuseppe Mascia, Fabio Mastropasqua, Salvatore Moscatello, Giuseppe Nuovo, Arianna Pisconti, Ventura Talamo and the members of the naturalist network www.argonauti.org are acknowledged to have provided information on the occurrence of some species. I thank Isabel Darrer for the English review. Francesco Cozzoli is acknowledged for supervision of the data analysis. I am grateful to Graziella Mura, Vezio Cottarelli, Fabio Stoch and Federico Marrone for useful comments on a first draft of the manuscript.
Supplemental data
Supplemental data for this article can be accessed here.
References
- Ahyong ST, Lowry JK, Alonso M, Bamber RN, Boxshall GA, Castro P, Gerken S, Karaman GS, Goy JW, Jones DS, Meland K, Rogers DC, Svavarsson J. 2011. Subphylum Crustacea Brünnich, 1772. In: Zhang ZQ, editor. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa. Vol. 3148. Auckland: Magnolia Press. pp. 1–237.
- Alfonso G, Beccarisi L, Pieri V, Frassanito A, Belmonte G. 2016. Using crustaceans to identify different pond types. A case study from the Alta Murgia National Park, Apulia (South-eastern Italy). Hydrobiologia 782:53–69. DOI: 10.1007/s10750-016-2669-y.
- Alfonso G, Belmonte G. 2011. Calanoida (Crustacea Copepoda) from the inland waters of Apulia (south-eastern Italy). Journal of Limnology 70:57–68. DOI: 10.4081/jlimnol.2011.57.
- Alfonso G, Belmonte G. 2013. Neolovenula alluaudi (Guerne and Richard, 1890) (Calanoida: Diaptomidae: Paradiaptominae): first record in Italy and review of geographical distribution. Journal of Limnology 72:251–261. DOI: 10.4081/jlimnol.2013.e20.
- Alfonso G, Belmonte G, Ernandes P, Zuccarello G. 2011. Stagni temporanei mediterranei in Puglia. Biodiversità e aspetti di un habitat poco conosciuto. Lecce: Ed. Grifo. ISBN: 9788896801680.
- Alonso M. 1996. Crustacea, Branchiopoda. Fauna Iberica. Vol. 7. Madrid: Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Cientificas.
- Artom C. 1921. Dati citologici sul tetraploidismo dell’Artemia salina di Margherita di Savoia. Rendiconti Accademia Dei Lincei 30:66–69.
- Baxevanis AD, Kappas I, Abatzopoulos T. 2006. Molecular phylogenetics and asexuality in the brine shrimp Artemia. Molecular Phylogenetics and Evolution 40:724–738. DOI: 10.1016/j.ympev.2006.04.010.
- Belk D, Brtek J. 1995. Checklist of the Anostraca. Hydrobiologia 298:315–353. DOI: 10.1007/BF00033826.
- Belmonte G, Bernardini M, Brizio S. 1993. Ambienti e itinerari naturalistici della Provincia di Lecce. Lecce: Conte Editore. ISBN: 88-85979-01-7.
- Brendonck L, Rogers DC, Olesen J, Weeks S, Hoeh WR. 2008. Global diversity of large branchiopods (Crustacea: Branchiopoda) in freshwater. Hydrobiologia 595:167–176. DOI: 10.1007/s10750-007-9119-9.
- Brtek J, Forró L, Ponyi JE. 1984. Contributions to the knowledge of the Branchiopoda (Crustacea) fauna of Mongolia. Annales Historico-Naturales Musei Nationalis Hungarici 76:91–99.
- Brtek J, Thiéry A. 1995. The geographic distribution of the European Branchiopods (Anostraca, Notostraca, Spinicaudata, Laevicaudata). Hydrobiologia 298:263–280. DOI: 10.1007/BF00033821.
- Clarke KR, Gorley RN. 2006. PRIMER v6: user manual/tutorial. Plymouth: PRIMER-E.
- Colwell RK 2013. EstimateS: statistical estimation of species richness and shared species from samples. Version 9. Available: http://purl.oclc.org/estimates. Accessed Oct 2016 31.
- Cottarelli V, Mura G. 1979. Reperti inediti di crostacei Anostraci, Notostraci e Concostraci di Calabria e Sicilia. Bollettino del Museo Civico di Storia Naturale di Verona 6:353–361.
- Cottarelli V, Mura G. 1983. Guide per il riconoscimento delle specie animali delle acque interne italiane. Anostraci, Notostraci, Concostraci. Vol. 18. Verona: Consiglio Nazionale delle Ricerche.
- Cottarelli V, Mura G. 1995. Ricerche zoologiche della nave oceanografica “Minerva” (C.N.R.) sulle isole circumsarde. (24) Ulteriori reperti di Anostraci, Notostraci e Spinicaudati (Crostacei Branchiopodi) della fauna italiana. Annali Del Museo Civico Di Storia Naturale “G. Doria”, Genova 90:599–607.
- Daday de Deés E. 1914. Monographie systématique des Phyllopodes conchostracés. 1.re partie, Caenestheridae. Annales Des Sciences Naturelles, Zoologie 20:39–330.
- Daday de Deés E. 1923. Monographie systématique des Phyllopodes conchostracés. 2.re partie, Leptestheridae. Annales Des Sciences Naturelles, Zoologie 6:331–390.
- Dahlgren R, Van Nieuwenhuyse E, Litton G. 2004. Transparency tube provides reliable water-quality measurements. California Agriculture 58:149–153. DOI: 10.3733/ca.v058n03p149.
- Dumont HJ, De Walsche C, Mertens J. 1991. Distribution and morphological variation of Streptocephalus torvicornis (Waga, 1842) in Northern Africa. Hydrobiologia 212:203–208. DOI: 10.1007/BF00026002.
- Dumont HJ, Mertens J, Maeda-Martinez A. 1995. Historical and morphological differentiation of Streptocephalus torvicornis (Waga) since the Würm II-glaciation. Hydrobiologia 298:281–286. DOI: 10.1007/BF00033822.
- Dumont HJ, Negrea S. 2002. Introduction to the Class Branchiopoda. In: HJ Dumont, editor. Guides to the Microinvertebrates of the Continental Waters of the World. Leiden: Backhuys.
- EPCN (European Pond Conservation Network). 2008. The Pond Manifesto. http://freshwaterhabitats.org.uk/wp-content/uploads/2016/06/EPCN-MANIFESTO.pdf
- European Commission. 1992. Council Directive of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora, 92/43/EC. Official Journal L 206, 22 Jul 1992:44.
- Fager EW. 1957. Determination and analysis of recurrent groups. Ecology 38(4):586–595. DOI: 10.2307/1943124.
- Gandolfi A, Zarattini P, Rossi V. 2015. Re-evaluation of three related species of the genus Branchipus Schaeffer, 1766 (Branchiopoda: Anostraca) by morphological and genetic analyses. Journal of Crustacean Biology 35(6):804–813. DOI: 10.1163/1937240X-00002382.
- Gauthier H. 1928. Recherches sur la faune des eaux continentales de l’Algérie et de la Tunisie. Algiers: Minerva ed.
- Ghigi A. 1921. Ricerche sui Notostraci di Cirenaica e di altri paesi del Mediterraneo. Atti Della Società Italiana Di Scienze Naturali E Del Museo Civico Di Storia Naturale Di Milano 60:161–188.
- Gueriau P, Rabet N, Clément G, Lagebro L, Vannier J, Briggs DEG, Charbonnier S, Olive S, Béthoux O. 2016. A 365-million-year-old freshwater community reveals morphological and ecological stasis in branchiopod crustaceans. Current Biology 26:383–390. DOI: 10.1016/j.cub.2015.12.039.
- Incagnone G, Marrone F, Barone R, Robba L, Naselli-Flores L. 2015. How do freshwater organisms cross the “dry ocean”? A review on passive dispersal and colonization processes with a special focus on temporary ponds. Hydrobiologia 750:103–123. DOI: 10.1007/s10750-014-2110-3.
- ISTAT. 2004. Annali di Statistica - Regione Puglia: territorio. Rome: Istituto Nazionale di Statistica.
- Jaume D. 1989. Metadiaptomus chevreuxi (Copepoda: Calanoida: Diaptomidae) and Leptestheria mayeti (Branchiopoda: Conchostraca: Leptestheriidae), two African freshwater crustaceans recorded in Majorca. Limnética 5:101–109.
- Korn M, Green AJ, Machado M, Garcia-de-Lomas J, Cristo M, Cancela da Fonseca L, Frisch D, Pérez-Bote JL, Hundsdoerfer AK. 2010. Phylogeny, molecular ecology and taxonomy of southern Iberian lineages of Triops mauritanicus (Crustacea: Notostraca). Organisms Diversity & Evolution 10:409–440. DOI: 10.1007/s13127-010-0026-y.
- Korn M, Marrone F, Perez-Bote J, Machado M, Cristo M, Da Fonseca LC, Hundsdoerfer AK. 2006. Sister species within the Triops cancriformis lineage (Crustacea, Notostraca). Zoologica Scripta 35:301–322. DOI: 10.1111/zsc.2006.35.issue-4.
- Korn M, Rabet N, Ghate HV, Marrone F, Hundsdoerfer AK. 2013. Molecular phylogeny of the Notostraca. Molecular Phylogenetics and Evolution 69:1159–1171. DOI: 10.1016/j.ympev.2013.08.006.
- Lagebro L, Geriau P, Hegna TA, Rabet N, Butler AD, Budd GE. 2015. The oldest notostracan (Upper Devonian Strud locality, Belgium). Palaeontology 58(3):497–509. DOI: 10.1111/pala.12155.
- Legendre P, Anderson MJ. 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69:1–24. DOI: 10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2.
- Longhurst AR. 1955. A review of the Notostraca. Bulletin of the British Museum (Natural History): Zoology 3(1):1–57. DOI: 10.5962/bhl.part.4119.
- Macchia F, Cavallaro V, Forte L, Terzi M. 2000. Vegetation and climate of Apulia. In: Marchiori S, De Castro F, Myrta A, editors. La cooperazione italo-albanese per la valorizzazione della biodiversità. Bari: CIHEAM. Vol. 53. pp. 33–49.
- Maeda-Martínez AM, Belk D, Obregón-Barboza H, Dumont HJ. 1997. Large branchiopod assemblages common to Mexico and the United States. Hydrobiologia 359:45–62. DOI: 10.1023/A:1003102601542.
- Mantovani B, Cesari M, Scanabissi F. 2004. Molecular taxonomy and phylogeny of the ‘living fossil’ lineages Triops and Lepidurus (Branchiopoda: Notostraca). Zoologica Scripta 33:367–374. DOI: 10.1111/zsc.2004.33.issue-4.
- Mantovani B, Cesari M, Scanabissi F. 2009. Molecular taxonomy and phylogeny of Italian Lepidurus taxa (Branchiopoda: Notostraca). Italian Journal of Zoology 76(4):358–365. DOI: 10.1080/11250000802687461.
- Marrone F, Castelli G, Naselli-Flores L 2009. Sicilian temporary ponds: an overview of the composituioin and affinities of their Crustacean biota. International Conference on Mediterranean Temporary Ponds. In: Fraga I, Arguimbau P, editors. International Conference on Mediterranean Temporary Ponds. Proceedings & Abstracts. Consell Insular de Menorca. Recerca, 14. Mao´, Menorca. pp. 189–202.
- Marrone F, Havenstein K, Tiedeman R, Ketmaier V. 2016a. Identification and characterization of five polymorphic microsatellite loci in the freshwater copepod Hemidiaptomus gurneyi (Copepoda: Calanoida: Diaptomidae). Italian Journal of Zoology 83:146–150. DOI: 10.1080/11250003.2015.1126363.
- Marrone F, Korn M, Stoch F, Naselli-Flores L, Turki S. 2016b. Updated checklist and distribution of large branchiopods (Branchiopoda: Anostraca, Notostraca, Spinicaudata) in Tunisia. Biogeographia 31:27–53.
- Marrone F, Mura G. 2006. Updated status of Anostraca, Notostraca and Spinicaudata (Crustacea Branchiopoda) in Sicily (Italy): review and new records. Naturalista Siciliano 30:3–19.
- Mathers TC, Hammond RL, Jenner RA, Hänfling B, Gómez A. 2013. Multiple global radiations in tadpole shrimps challenge the concept of ‘living fossils’. PeerJ 1:e62. DOI: 10.7717/peerj.62.
- Minelli A, Ruffo S, Vigna Taglianti A. 2006. The Italian faunal provinces. In: Ruffo S, Stoch F, editors. Checklist and distribution of the Italian fauna. Memorie del Museo Civico di Storia Naturale di Verona - 2. serie – Sezione Scienze della Vita 17:37–39.
- Miracle MR, Sahuquillo M, Vicente E. 2008. Large branchiopods from freshwater temporary ponds of Eastern Spain. Verhandlungen Des Internationalen Verein Limnologie 30:501–505.
- Moscatello S, Belmonte G, Mura G. 2002. The co-occurrence of Artemia parthenogenetica and Branchinella spinosa (Branchiopoda: Anostraca) in a saline pond of south eastern Italy. Hydrobiologia 486:201–206. DOI: 10.1023/A:1021307019891.
- Muñoz J, Gómez A, Figuerola J, Amat F, Rico C, Green AJ. 2014. Colonization and dispersal patterns of the invasive American brine shrimp Artemia franciscana (Branchiopoda: Anostraca) in the Mediterranean region. Hydrobiologia 726:25–41. DOI: 10.1007/s10750-013-1748-6.
- Mura G. 2001. Updating Anostraca (Crustacea, Branchiopoda) distribution in Italy. Journal of Limnology 60:45–49. DOI: 10.4081/jlimnol.2001.45.
- Mura G. 2005. Crustacea Branchiopoda Anostraca, Notostraca, Conchostraca. In: Ruffo S, Stoch F, editors. Checklist and distribution of the Italian fauna. Memorie del Museo Civico di Storia Naturale di Verona, 2. Serie, Sezione Scienze della Vita 17. 16. pp. 85–86.
- Mura G, Alfonso G, Fancello G. 2006a. Contributo alla conoscenza della fauna ad Anostraci (Crustacea Branchiopoda) delle Puglie. Thalassia Salentina 29:21–27.
- Mura G, Baxevanis AD, Lopez GM, Hontoria F, Kappas I, Moscatello S, Fancello G, Amat F, Abatzopoulos TJ. 2005. The use of a multidisciplinary approach for the characterization of a diploid parthenogenetic Artemia population from Torre Colimena (Apulia, Italy). Journal of Plankton Research 27:895–907. DOI: 10.1093/plankt/fbi063.
- Mura G, Cottarelli V. 1998. On the occurrence of Streptocephalus torvicornis Waga 1842 (Crustacea Anostraca) on a coastal island off Italy. Hydrobiologia 367:15–19. DOI: 10.1023/A:1003253201147.
- Mura G, Ferreri D, Belmonte G. 1999. Prima segnalazione di Branchinella spinosa Milne Edwards 1840 (Crustacea, Branchiopoda, Anostraca) per l’Italia peninsulare. Thalassia Salentina 23:59–65.
- Mura G, Kappas I, Baxevanis AD, Moscatello S, D’Amico Q, Lopez G, Hontoria F, Amat F, Abatzopoulos T. 2006b. Morphological and molecular data reveal the presence of the invasive Artemia franciscana in Margherita di Savoia salterns (Italy). International Review of Hydrobiology 91:539–554. DOI: 10.1002/(ISSN)1522-2632.
- Oertli B, Céréghino R, Hull A, Miracle R. 2009. Pond conservation: from science to practice. Hydrobiologia 634:1–9. DOI: 10.1007/s10750-009-9891-9.
- Olesen J, Richter S. 2013. Onychocaudata (Branchiopoda: Diplostraca), a new high-level taxon in branchiopod systematics. Journal of Crustacean Biology 33:62–65. DOI: 10.1163/1937240X-00002121.
- R Core Team. 2016. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available: https://www.R-project.org/. Accessed Oct 2016 31.
- Ramdani M. 1988. Les eaux stagnantes au Maroc: études biotypologique et biogéographique du zooplankton. Travaux De L’institut Scientifique, Série Zoologie 43:1–40.
- Richter S, Olesen J, Wheeler WC. 2007. Phylogeny of Branchiopoda (Crustacea) based on a combined analysis of morphological data and six molecular loci. Cladistics 23:301–336. DOI: 10.1111/cla.2007.23.issue-4.
- Rogers DC. 2013. Anostraca catalogus (Crustacea: Branchiopoda). The Raffles Bulletin of Zoology 61:525–546.
- Ruffo S, Stoch F. 2006. Checklist and distribution of the Italian fauna. Memorie del Museo Civico di Storia Naturale di Verona - 2. serie - Sezione Scienze della Vita 17. 307 pp.
- Sahuquillo M, Miracle MR. 2010. Crustacean and rotifer seasonality in a Mediterranean temporary pond with high biodiversity (Lavajo de Abajo de Sinarcas, Eastern Spain). Limnetica 29:75–92.
- Sahuquillo M, Miracle MR. 2013. The role of historic and climatic factors in the distribution of crustacean communities in Iberian Mediterranean ponds. Freshwater Biology 58:1251–1266. DOI: 10.1111/fwb.2013.58.issue-6.
- Scanabissi F, Alfonso G, Bergamaschi S, Mantovani B. 2006. Primo ritrovamento di Lepidurus couesii Packard, 1875 in Italia. Thalassia Salentina 29:113–124.
- Stenderup JT, Olesen J, Glenner H. 2006. Molecular phylogeny of the Branchiopoda (Crustacea)–multiple approaches suggest a ‘diplostracan’ ancestry of the Notostraca. Molecular Phylogenetics and Evolution 41:182–194. DOI: 10.1016/j.ympev.2006.06.006.
- Stoch F, Korn M, Turki S, Naselli-Flores L, Marrone F. 2016. The role of spatial environmental factors as determinants of large branchiopod distribution in Tunisian temporary ponds. Hydrobiologia 782:37–51. DOI: 10.1007/s10750-015-2637-y.
- Thiéry A. 1991. Multispecies coexistence of branchiopods (Anostraca, Notostraca & Spinicaudata) in temporary ponds of Chaouia plain (western Morocco): sympatry or syntopy between usually allopatric species. Hydrobiologia 212:117–136. DOI: 10.1007/BF00025994.
- Thiéry A. 1996. Large branchiopods (Crustacea: Anostraca, Notostraca, Spinicaudata, Laevicaudata) from temporary inland waters of the Arabian Peninsula. Fauna of Saudi Arabia 15:37–98.
- Turki S, Turki B. 2010. Copepoda and Branchiopoda from Tunisian temporary waters. International Journal of Biodiversity and Conservation 2:86–97.
- Van Den Broeck M, Waterkeyn A, Rhazi L, Brendonck L. 2015. Distribution, coexistence, and decline of Moroccan large branchiopods. Journal of Crustacean Biology 35:355–365. DOI: 10.1163/1937240X-00002316.
- Vanschoenwinkel B, Pinceel T, Vanhove MPM, Denis C, Jocque M, Timms BV, Brendonck L. 2012. Toward a global phylogeny of the “living fossil” crustacean order of the Notostraca. Plos ONE 7:e34998. DOI: 10.1371/journal.pone.0034998.
- Voigt S, Hauschke N, Schneider JW. 2008. On the occurences of fossil notostracans in Germany - an overview. Abhandlungen Und Berichte Für Naturkunde 31:7–24.
- Zarattini P, Magnaschi G, Rossi V, Mura G. 2001. Further evidence of the synonymy between Branchipus schaefferi and B. visnyai (Crustacea, Anostraca). Italian Journal of Zoology 68:79–82. DOI: 10.1080/11250000109356387.
- Zito G, Ruggiero L, Zuanni F 1991. Aspetti meteorologici e climatici della Puglia. Progetto Strategico C.N.R. Clima Ambiente e Territorio nel Mezzogiorno. 1° Workshop, Atti, 11 Dicembre 1989, Taormina. pp. 43–73.