ABSTRACT
The problem of the generic placement of some peculiar Mediterranean and Atlantic sabellids, showing a mixture of features found in the genera Euchone and Paradialychone, is addressed. Due to the high similarity to E. pseudolimnicola, this peculiar taxon had previously been informally named Euchone cfr. pseudolimnicola. However, while the presence of a simple pre-pygidial depression without lateral wings or a distinct ridge in the uppermost margin is similar to Dialychone and Paradialychone, the dentition of thoracic and abdominal uncini resembles that found in Paradialychone. In this study, the morphological features of Mediterranean and British material were re-examined and compared to E. pseudolimnicola specimens, and a cladistics analysis was performed. The genus Euchone appears not well defined; E. cfr. pseudolimnicola and E. pseudolimnicola are located close to each other and also located in the area of the phylogenetic tree containing the other examined Euchone species. We suggest to maintain both these taxa within the genus Euchone until a revision based on the examination of type material of all the Euchone species is performed. The new species Euchone anceps sp. nov. has been described, and the large morphological variability existing in some Mediterranean material is also addressed. An updated taxonomic key for the species of the considered genera present in Northeast Atlantic and Mediterranean Sea is also provided.
Introduction
Cochrane (Citation2003) introduced the term “Chonea” for a group including the highly speciose sabellid genera Chone Krøyer, Citation1856, and Euchone Malmgren, Citation1866. The genera within this group have a very similar external morphology and their taxonomy has been problematic since the first phylogenetic analysis was performed by Fitzhugh (Citation1989), and another later by Nogueira et al. (Citation2010). The cladistics analysis by Cochrane (Citation2003) showed Euchone to be paraphyletic due to the disposition of pinnules on radioles in the branchial crown, and the number of chaetigers forming the pre-pygidial depression. Phylogenetic relationships were assessed within Chone by Tovar-Hernández (Citation2008), whose analysis revealed the existence of three monophyletic genera previously assigned to Chone: Dialychone Claparède, Citation1870, Paradialychone Tovar-Hernández, Citation2008 and Chone sensu stricto. This last genus is characterised by squared abdominal uncini with similar morphology in all the abdominal segments, and the absence of a pre-pygidial depression. The other genera have anterior abdominal uncini differing in shape from the posterior ones, and a pre-pygidial depression is present.
Among the diagnostic characters normally utilised in defining all of these genera, the shape of the abdominal uncini is a problematic feature, taking into consideration the great variability within a given region of the abdomen or even within the same fascicle (Banse Citation1972; Fitzhugh Citation1989; Bick & Randel Citation2005). With regard to the pre-pygidial depression, in Dialychone and Paradialychone, it appears only as a terminal flattened portion (simple depression), and only Euchone has a distinctive depression that appears hollowed. In addition, in most of the Euchone species the pre-pygidial depression is characterised by the presence of lateral wings. However, this feature cannot be considered an apomorphy for the genus Euchone since they are lacking in Euchone pseudolimnicola Giangrande & Licciano, Citation2006, and Euchone limnicola Reish, Citation1959, but are present also in the genus Euchoneira Licciano, Giangrande & Gambi, Citation2009 (Licciano et al. Citation2009). As for the internal structure of the crown, the definitions of the “Chonea” genera show that the presence/absence of dorsal radiolar appendages has been considered important. The assessment of these features, however, has produced varied interpretations. Although Cochrane (Citation2003) considered Euchone to lack these structures, according to Fitzhugh (Citation2003) the genus is characterised by dorsal lips with radiolar appendages supported only by blood vessels, even though histological analysis by Bick and Randel (Citation2005) showed the presence of a radiolar skeleton in Euchone analis (Krøyer Citation1856). Tovar-Hernández (Citation2008) considered radiolar appendages to be present in Euchone and absent in Chone, Dialychone and Paradialychone. Finally, Capa et al. (Citation2011) confirmed the presence of radiolar appendages in Euchone, and in Dialychone australiensis (Hartmann-Schröder Citation1979), stating that they are likely present in both Dialychone and Paradialychone.
Understanding the divisions among these taxa is important because the “Chonea” sabellids are widespread in soft-bottom habitats and are useful in monitoring studies. However, many problems and doubts arise when people try to identify specimens. Indeed, some species can confidently be assigned to one of the currently defined genera, while there are other species that share diagnostic features from two or more genera. As an example, some species have been described and easily placed within Dialychone or Paradialychone (Nishi et al. Citation2009; Tovar-Hernández & Dean Citation2010; Selim et al. Citation2012), whereas for others, it was difficult to determine the taxonomic status due to the presence of several diagnostic features typically attributed to Chone, Dialychone and Paradialychone (Capa & Murray Citation2015).
This was also the case for the fairly common and interesting specimens collected from soft-sediment benthic surveys conducted in the Gulf of Salerno (Mediterranean Sea) and along British coasts (Shetland Isles and off the south-western coast of England), where individuals exhibited a mix of features found in Euchone, Dialychone and Paradialychone. Due to a similarity with the Mediterranean taxon E. pseudolimnicola, specimens were recorded as Euchone cfr. pseudolimnicola, whilst the British material was initially identified as Chone cfr. collaris. This was mainly due to the presence of a crenulated collar, but later, this material was also informally named Euchone cfr. pseudolimnicola. A more careful examination, however, showed this taxon to be different from E. pseudolimnicola. To further complicate matters, more recently, similar specimens, also identified as Euchone cfr. pseudolimnicola but showing a high morphological variability, have been collected in the northern Mediterranean area (Gulf of Lion).
The aim of the present work is to understand the generic placement, and to provide a detailed description, of this particular taxon. For this purpose a phylogenetic analysis was performed using related taxa for which detailed descriptions were available. Furthermore, the features of E. pseudolimnicola have been re-examined, using the type material, specimens from the Mediterranean and other localities, and specimens identified as Euchone cfr. pseudolimnicola.
Materials and methods
The examined material of Euchone cfr. pseudolimnicola came from ecological studies conducted along the Italian coast (Lorenti et al. Citation2011), and British (SGS M-Scan, Ltd. Citation2012, Citation2014) and French coasts (material provided by Dr Celine Labrune), while E. pseudolimnicola was collected only along the Italian coast (Giangrande & Licciano Citation2006). Material of E. limnicola collected from shallow subtidal areas of Tees Estuary on the east coast of England (April 2013) was examined during the NE Atlantic Marine Biological Analytical Quality Control Scheme (NMBAQC) course, held in Newcastle, 2014.
Holotypes are housed at the Museum Nacional de Ciencias Naturales de Madrid, Spain (MCNM). Paratypes and additional material are located at the Polychaete Collection of the Laboratory of Zoology of the University of Salento (Lecce), Italy (PCZL).
Drawings were made with the aid of a camera lucida attached to stereo and compound microscopes; photographs were taken using a stereomicroscope equipped with a Nikon Coolpix 990 camera. Staining patterns were obtained using methyl green stain, while the internal structure of the crown and ventral sacs were evidenced utilising a textile fibre identification stain (Shirlastain A).
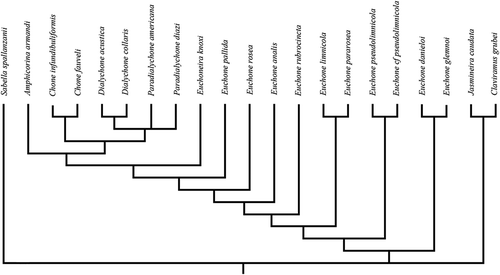
Phylogenetic analysis was carried out on a matrix of 21 taxa for 27 characters (). Representative taxa were selected from among the most plesiomorphic sabellid genera (Fitzhugh Citation1989; Cochrane Citation2003; Nogueira et al. Citation2010), already considered in a previous analysis by Licciano et al. (Citation2009), and using Sabella spallanzanii (Gmelin Citation1791) as outgroup. Descriptions of character states are given in . The analysis included species for which detailed descriptions were available, and specimens that were readily available for examination (e.g. Mediterranean material). When possible, the type species for each genus was included. The character state matrix was computed based on the analysis by Tovar-Hernández (Citation2008), and the data matrix and tree diagrams were created using MacClade version 3.08 (Maddison & Maddison Citation2005). Analysis was carried out using PAUP version 4.0b8 (Swofford Citation2002). A heuristic search for the most parsimonious trees was carried out by using the default settings of PAUP (tree-bisection-reconnection (TBR) branch-swapping, MULTREES and COLLAPSE options in effect). A random stepwise addition sequence of 100 replicates was used, and a strict consensus tree using the accelerated transformation principle (ACCTRAN) was compiled for all minimum length trees retained.
Table I. Character state distribution for 21 taxa and 27 characters used in the present analysis.
Table II. List of characters and character states.
Results
Cladistic analysis
The analysis produced 60 trees, from which the strict consensus tree is reported (96 steps, CI: 0.67, RI: 0.78; ), where CI is the consistency index, and RI the retention index.
The analysis did not solve the relationships among genera. Only Jasmineira Langerhans, Citation1880 and Claviramus Fitzhugh, Citation2002 appeared well separated, branching off at the base of the tree. Among the “Chonea” taxa, the region of the tree containing Chone, Dialychone and Paradialychone appears better defined than that containing Euchone and Euchoneira, which represents the most unresolved area of the tree. Chone, which is the only genus without a pre-pygidial depression, also lacks dorsal radiolar appendages. Chone does have pinnular appendages, squared abdominal uncini with similar morphology in all the abdominal segments, and paleate chaetae in the thorax. Paleate chaetae are also characteristic of Paradialychone and Dialychone. According to the analysis of Tovar-Hernández (Citation2008), pinnular appendages are present in Chone, Euchone and Paradialychone, but are lost in Dialychone. Furthermore, while thoracic uncini have teeth of similar size over the main fang in Chone, they are decreasing in size in Dialychone, and have a secondary tooth over the main fang in Paradialychone.
Among all the considered Euchone species, which are scattered along a branch of the tree, E. limnicola, which, according to the absence of lateral wings appeared to be very similar to E. pseudolimnicola, clustered instead with Euchone pararosea Giangrande & Licciano, Citation2006. The recently described species Euchone danieloi Capa & Murray, Citation2015 and Euchone glemnoi Capa & Murray, Citation2015 are close to each other, and E. pseudolimnicola and E. cfr. pseudolimnicola are closely related.
The genus Euchone should be defined by the presence of dorsal lips with radiolar appendages, and the absence of paleate chaetae in the thorax. Most of the Euchone species have thoracic uncini with teeth of similar size over the main fang, well-defined ventral shields, variation of abdominal uncinal shape between segments, a well-defined and hollowed pre-pygidial depression with lateral wings, and a ventral incision in the anterior collar margin, a feature also present in Jasmineira and Euchoneira. However, several exceptions to this pattern can be highlighted. Euchone limnicola has an entire ventral collar margin and a pre-pygidial depression without wings, a feature also present in E. pseudolimnicola and E. cfr. pseudolimnicola, which, however, have the ventral collar deeply incised ventrally. These latter two species also differ from the other Euchone in having no defined ventral shields and thoracic and abdominal uncini with a secondary tooth over the main fang, a state that according to the present analysis appears homoplastic, being present also in the genus Paradialychone. The pre-pygidial depression without wings of E. pseudolimnicola and E. cfr. pseudolimnicola, however, is hollowed and thus more defined in comparison to the flattened type present in Dialychone and Paradialychone species, from which they also differ by the presence of radiolar appendages and in the shape of the inferior thoracic notochaetae.
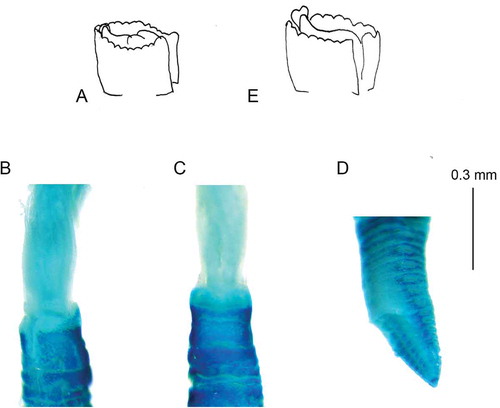
Lastly, E. pseudolimnicola and E. cfr. pseudolimnicola are characterised not only by the presence of a very long incision on the anterior collar margin but also by the presence of parallel lamellae. These structures were never described before for any representative of the “Chonea” group. Recently, however, they were reported for E. danieloi and E. glemnoi (Capa & Murray Citation2015). Parallel lamellae are connected to the ventral lips, and in E. pseudolimnicola and E. cfr. pseudolimnicola, they form a fold under the collar, right behind the peristomial tip. This fold, especially in E. cfr. pseudolimnicola, appears as a bilobed structure (, ) which are here interpreted as ventral sacs. The different development of these structures is the main feature separating E. pseudolimnicola from E. cfr. pseudolimnicola.
Figure 2. Euchone pseudolimnicola. (A) Scheme of the peristomial lobe; (B) staining pattern of the collar dorsal view; (C) staining pattern of the collar ventral view; (D) staining pattern of the pre-pygidial depression; (E) scheme of the peristomial lobe from Euchone anceps sp. nov.
Notwithstanding the above underlined problems within the genus Euchone, the present analysis does not suggest the erection of a new genus for E. pseudolimnicola and E. cfr. pseudolimnicola.
Taxonomic accounts
Genus Euchone
Euchone pseudolimnicola Giangrande & Licciano, Citation2006: pp. 1307–1308
()
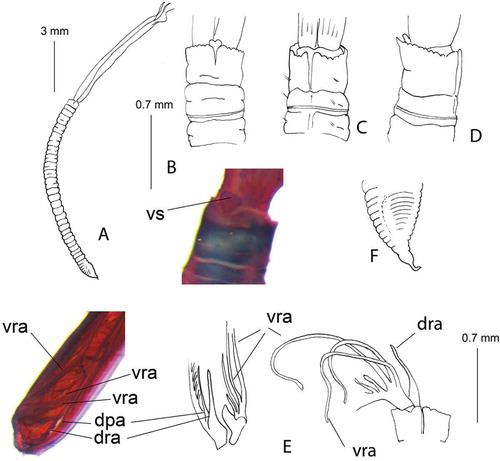
Figure 3. Euchone anceps sp. nov. holotype. (A) Entire worm; (B) collar ventral view (drawn and photographed); (C) collar dorsal view; (D) collar lateral view; (E) internal structures of the crown (stained with SHIRLASTAIN); (F) pre-pygidial depression.
dpa: dorsal pinnular appendage; dra: dorsal radiolar appendage; vs: ventral sacs; vra: ventral radiolar appendage.
Material examined
PCZL: Mediterranean material from the original description, seven specimens from South Coast of Ustica Island, 50 m depth, 38°42ʹ00“N, 13°11ʹ00”E. Sediment mainly composed of medium sand, biogenic and volcanic particles together with a significant amount of red calcareous algae.
Description
The description of the species is available in Giangrande and Licciano (Citation2006).
Addition to the original description
Ventral peristomial lobe triangular not exposed. Parallel lamellae forming small rounded structure interpreted as ventral sacs that appear covered by ventral lappets of the collar (). After methyl green staining the collar showed the apical part less coloured than the basal part (, c and ). Pre-pygidial depression highly developed and formed of nine chaetigers without wings but generally with a distinct ridge in the uppermost part (). Uncini of the pre-pygidial depression not highly modified, but with more teeth of similar size over the main fang compared to teeth of the pre-pygidial depression.
Figure 4. Euchone anceps sp. nov. British material. (A) collar staining pattern (Methyl green stain) lateral view; (B) collar staining pattern ventral view; (C) entire worm; (D) collar staining pattern dorsal view; (E) pre-pygidial depression.
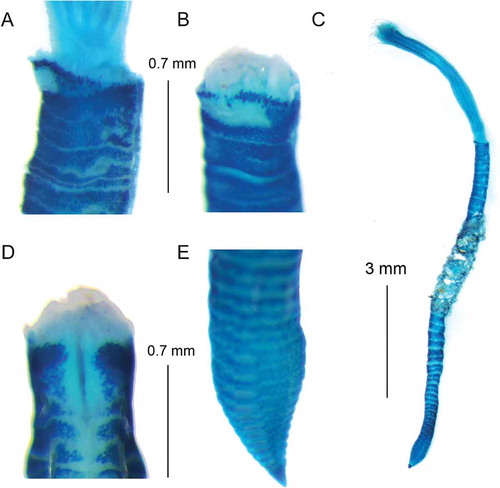
Figure 5. Euchone anceps sp. nov. holotype. (A) Superior thoracic chaeta; (B) inferior thoracic chaeta; (C) thoracic uncini; (D) abdominal chaeta; (E) abdominal uncinus from first abdominal chaetiger; (F) abdominal uncinus from pre-pygidial depression.
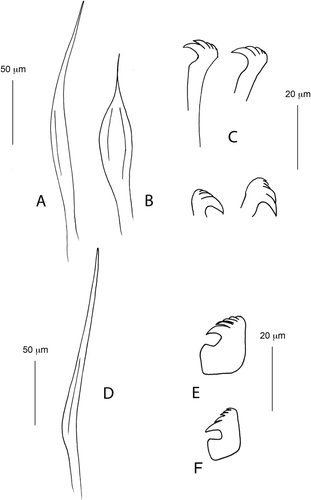
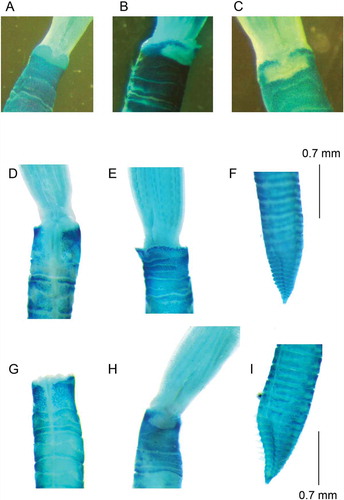
Figure 6. Comparison of collar staining pattern from E. pseudolimnicola (A) and E. anceps sp. nov. Holotype (B–C); variability of collar staining pattern (Methyl green) and development of pre-pygidial depression in two different specimens of E. anceps sp. nov. from the Gulf of Lion: (D–F) (Beauduc) and (G–I) (Levant).
Euchone anceps sp. nov.
(, –, and –)
Euchone cfr. pseudolimnicola. – Giangrande et al. Citation2015, comments, p. 36.
Chone cfr. collaris. – SGS M-Scan Ltd. Citation2012; Citation2014, appendix 8b, p. 365 (non Langerhans, Citation1881).
Type material
Holotype16.01/17716 MNCN: Gulf of Salerno, 15 m depth, February 2007, 40°39ʹN, 14°46ʹE
Paratypes PCZLS.EU. 9.1: Gulf of Salerno, 15 m depth, February 2007, 40°39ʹN, 14°46ʹE, seven specimens.
Additional material
PCZL.S.EU.: Shetlands Little Roe, 51 m depth, June 2012, 60°30ʹN, 1°17ʹ W; Shetlands, Orka Voe, 20 m depth, June 2012, 60°28ʹN, 1°15ʹW (three specimens); Shetlands, Jetty Grid, 26 m depth, June 2012 60°27ʹN, 1°18ʹW (one specimen); Western English Channel 88 m depth, July 2011, 49°33ʹN, 4°36ʹW (one specimen); Western English Channel, 88 m depth, July 2011, 49°33ʹN, 4°36ʹW (one specimen); Shetlands, Calbeck Ness, 53 m depth, June 2014, 60°29ʹN, 1°17ʹW (one specimen); Shetlands, Orka Voe, 17 m depth, June 2014, 60°28ʹN, 1°15ʹW (one specimen); Shetlands, Jetty Grid, 28 m depth, June 2014, 60°27ʹN, 1°18ʹW (two paratypes); Shetlands, Jetty Grid, 19 m, June 2014, 60°27ʹN, 1°16ʹW (one specimen); Shetlands, Jetty Grid, 28 m depth, June 2014, 60°27ʹN 1°18ʹW (one specimen); Leucate: 20 m depth, December 2012, 42°51ʹN, 3°03ʹE (one specimen). Grau du Roi: 9.5 m depth, December 2012, 43°31ʹN, 4°05ʹE (one specimen). Porquerolles: 48 m depth, December 2012, 43°01ʹN, 6°16ʹE (one specimen). Beauduc: 15 m depth, December 2012, 43°23ʹN, 4°34ʹE (three specimens). Faraman: 11 m depth, December 2012, 43°20ʹN, 4°47ʹE (two specimens). Levant: 35 m depth, December 2012, 43°00ʹN, 6°43ʹE (two specimens). Ile Maire: 44 m depth, December 2012, 43°20ʹN, 5°34ʹE (two specimens).
Description
Holotype complete with eight thoracic and 30 abdominal chaetigers (). Branchial crown length 7 mm; total thorax-abdomen length 10 mm; maximum width 0.3 mm. Branchial lobes each with seven fully developed radioles with palmate membrane for about half of their length; radiolar flanges present distal to palmate membrane; radioles terminating as extra-long filaments ( and ). Dorsal pinnular appendages present but poorly developed, dorsal lips pointed with dorsal radiolar appendages (). Ventral lips triangular, parallel lamellae present, three pairs of ventral radiolar appendages of variable length, some of them about three-quarters the length of the radioles (). Collar high, slightly higher ventrally, regularly crenulated except dorsally, where it shows a cleft continuing in a lobe united with each side of the faecal groove (). Ventral margin of collar with a deep incision. Ventral peristomial lobe triangular; not exposed because it is covered by a bilobed structure interpreted as ventral sacs. The ventral sacs are centrally located, not covered by the collar, and appear as folds under the collar ( and ). Lobe of anterior peristomial ring dorsally not covered by collar margin (). After staining, ventral collar markedly stained close to the edge (, and ). Ventral shields visible only after methyl green staining. Apart from the peculiar methyl pattern in the ventral collar, colouration remains quite uniform along the body. Notopodia in chaetiger 1 with six narrowly hooded chaetae. Glandular ridge present on chaetiger 2, narrow all around. Notopodial fascicle from chaetigers 2–8 with superior group of five elongated broadly hooded chaetae (), and inferior group with five subspatulate chaetae posteriorly and two bayonet-type anteriorly. Subspatulate chaetae narrow, with long tip (). Thorax with 10 neuropodial uncini per torus, with a large tooth over the main fang and additional teeth of different sizes (). Abdominal neuropodial fascicles with modified, elongated narrowly hooded chaetae (). Abdominal notopodia with 9–10 avicular uncini, with main fang surmounted by three or four rows of teeth of different sizes, the first larger over the main fang, as in the thoracic uncini (). Intratorus variation absent.
Pre-pygidial depression not highly developed, formed by nine chaetigers without wings or distinct ridge, but hollowed and sometimes laterally swollen (, f and ). Uncini of the pre-pygidial depression not highly modified, but with more teeth of similar size over the main fang compared to that of abdominal segments anterior to the pre-pygidial depression (, ). Pygidium rounded, showing in most of the specimens a filiform appendix (). Tube incrusted with detritus and sand ().
Variation
All the type material and specimens collected in the British waters had a consistent staining pattern for the collar, and they had a poorly developed pre-pygidial depression (without a distinct ridge in the anterior edge). However, the specimens collected from the Gulf of Lion had highly variable staining pattern. These specimens can have a darker staining pattern of the collar coupled with a pre-pygidial depression similar to that of British material (–), or a staining pattern similar to that of British specimens, but with a more developed pre-pygidial depression (–).
Ecology and distribution
This species was collected from 10 to 80 m depth in coarse muddy sand bottoms. It is distributed in Mediterranean and British waters.
Etymology
The specific name ‘anceps’ is from Latin and refers both to the presence of some differences in generic definition from other Euchone species, and to the presence of high variability within some features.
Remarks
Due to the presence of a crenulated collar, at first the British material of P. anceps sp. nov. was reported by different authors as Chone cfr. collaris (SGS M-Scan Ltd Citation2012, Citation2014), and only after comparison to the Mediterranean material named E. cfr. pseudolimnicola. Indeed, it differs in several features from the true Chone collaris, now Dialychone collaris (Langerhans, Citation1881) (Tovar-Hernández Citation2008), including the shape of the collar with a discontinuous crenulation, the longer radiolar tips, the absence of peristomial eyes, the absence of paleate chaetae, and the presence of a pre-pygidial depression more developed and hollowed than in D. collaris. Mediterranean material from the Gulf of Salerno (Tyrrhenian Sea) was considered strongly similar to E. pseudolimnicola and at first named E. cfr. pseudolimnicola. The two taxa have a similar number of chaetigers, a similar number and morphology of radioles, and a filiform appearance of the body, an irregularly crenulated collar with a ventral cleft and a hollowed pre-pygidial depression without wings. However, numerous characters make this taxon different from E. pseudolimnicola. Euchone anceps sp. nov. is a larger species with a longer crown, and with more developed bilobed structures here interpreted as ventral sacs (, ). Moreover, the collar in E. pseudolimnicola is higher ventrally, with more developed lappets, and has a different staining pattern (–). In addition, the presently described species has narrower subspatulate chaetae in the inferior group. Finally, the two species show a different development of pre-pygidial depression. However, due to the high variability existing within the taxon, the best features separating it from E. pseudolimnicola are the development of ventral sacs and the staining pattern of the collar.
1 a. Pre-pygidial depression present, posterior abdominal uncini modified from those in anterior abdomen2
b. Pre-pygidial depression absent, posterior abdominal uncini similar to those in anterior abdomen 20 (Chone)
2(1) a. Pre-pygidial depression simple flattened, always without lateral wings3
b. Pre-pygidial depression hollowed, often with lateral wings............................................10 (Euchone)
3(2) a. Uncini from anterior abdomen with a series of nearly uniform-sized teeth4 (Dialychone)
b. Uncini from anterior abdomen with a large tooth above main fang, followed by a series of smaller teeth. Narrow flanges reaching the tip of the radioles. Inferior thoracic chaetae paleate. A second glandular girdle on first abdominal chaetiger............Paradialychone gambiae
Mediterranean
4(3) a. Only a glandular girdle on the second chaetiger5
b. An additional glandular girdle on chaetiger 13Dialychone egyptica
Mediterranean
5(4) a. Collar with a crenulate marginDialychone collaris
Mediterranean
b. Collar with a smooth margin6
6(5) a. Collar high, dorsally covering the junction between peristomium and the base of the branchiae 7
b. Collar oblique, dorsally not covering the base of branchial crown8
7(6) a. Palmate membrane very low, maximum one-quarter of the radiolar length, difficult to detect. Collar very high, anterior peristomial lobe triangular, not exposed. Large speciesDialychone acustica
Mediterranean and Northeast Atlantic
b. Palmate membrane highly developed covering three-quarters of the radiolar length, and with flanges reaching the tip of the radioles. Peristomial lobe exposed and bilobed. Small species with superior thoracic chaetae long and narrowly hoodedDialychone longiseta
Mediterranean
8(6) a. Radioles with short free tips and several black spots along radioles. Peristomial lobe exposed triangularDialychone arenicola
Mediterranean
b. Radioles with long free tips and without black spots9
9(8) a. Anterior peristomial lobe not exposed and triangularDialychone usticensis
Mediterranean
b. Peristomial lobe exposed bilobedDialychone dunerificta*
Mediterranean and Northeast Atlantic
10(2) a. Margin of the collar crenulated11
b. Margin of the collar smooth13
11(10) a. Pre-pygidial depression without lateral wings. Ventral sacs present12
b. Pre-pygidial depression with well developed lateral wingsEuchone pararosea
Mediterranean
12(11) a. Ventral sacs highly developed, appearing as a fold under the collarEuchone anceps sp. nov.
Mediterranean and Northeast Atlantic
b. Ventral sacs smaller, covered by the collarEuchone pseudolimnicola
Mediterranean
13(10) a. Fewer than 10 abdominal chaetigers before the pre-pygidial depression, often small-sized species14
b. More than 10 abdominal chaetigers before the pre-pygidial depression, often large-sized species17
14(13) a. Pre-pygidial depression without lateral wings and formed by 9–12 chaetigers. Collar entire ventrallyEuchone limnicola
Northeast Atlantic
b. Pre-pygidial depression with lateral wings formed by fewer than nine chaetigers. Collar with a small ventral cleft15
15(14) a. Pre-pygidial depression formed by only three chaetigers. A second glandular girdle present in abdominal chaetigers.Euchone incolor
Northeast Atlantic and Mediterranean **
b. Pre-pygidial depression formed by 4–5 chaetigers16
16(15) a. Two types of uncini in anterior abdominal chaetigers. Euchone arenae
Northeast Atlantic
b. Only one type of uncini in anterior abdominal chaetigersEuchone southerni
Northeast Atlantic
17(13) a. Pre-pygidial depression formed by 8–12 chaetigers, large-sized species18
b. Pre-pygidial depression formed by 5–7 chaetigers, small-sized speciesE. rosea
Mediterranean and Northeast Atlantic
18(17) a. Abdominal chaetigers before the pre-pygidial depression numbering 11–15, and 10–12 chaetigers in the pre-pygidial depression E. rubrocincta
Mediterranean and Northeast Atlantic
b. Abdominal chaetigers before the pre-pygidial depression numbering 16–22, and 8–12 chaetigers in the pre-pygidial depression19
19(18) a. Abdominal chaetigers before the pre-pygidial depression numbering 21, and 8–10 chaetigers in the pre-pygidial depression. Inferior thoracic chaetae broadly hooded….. Euchone papillosa
Northeast Atlantic
b. Abdominal chaetigers before the pre-pygidial depression numbering 16–22, and 9–12 chaetigers in the pre-pygidial depression. Inferior thoracic chaetae narrowly hoodedEuchone analis
Northeast Atlantic
20(1) a. Collar high covering the base of the branchiae21
b. Collar oblique dorsally not covering the base of the branchiaeChone duneri
Northeast Atlantic
21(20) a. Tip of the radioles long, with very developed flanges. Peristomial lobe exposed and bilobedChone filicaudata*
Northeast Atlantic
b. Tip of the radioles short and flanged. Peristomial lobe not exposed, and triangular22
22(21) a. Collar with narrow gap dorsally. 23
b. Collar with broad gap dorsally. Methyl green pattern with clearer areas dorsally along the body and ventrally in the collarChone kroyeri
Northeast Atlantic
23(22) a. Methyl green pattern darker in the anterior part of the collarChone infundibuliformis
Northeast Atlantic
b. Collar very high with methyl green pattern lacking on the superior margin Chone fauveli
Northeast Atlantic
*Wasson et al. Citation2017.
**Adriana Giangrande, personal observation.
Key to the “Chonea” group of genera and species from Northeast Atlantic and Mediterranean area
Discussion
Several problems arise when examining characters within the sabellid genera Euchone, Chone, Dialychone and Paradialychone. This is particularly true considering:
the internal structures of the crown, which need histological examination;
the uncinal shape variation, which remains a problematic feature in the definition of all these genera due to the difficulty in ascertaining the uncinal state (modified or not) (Giangrande et al. Citation2015) and the large variability within and/or between fascicles showed by several species (Banse Citation1972; Fitzhugh Citation1989; Bick & Randel Citation2005);
the presence of a pre-pygidial depression which in several Euchone, Dialychone and Paradialychone species often appears to be not clearly defined.
The presently described taxon E. anceps sp. nov., together with E. pseudolimnicola, shares with the genus Euchone the dorsal lips with radiolar appendages, a ventral cleft in the collar and the presence of a well-defined and hollowed pre-pygidial depression. However, the two species differs from the other Euchone species in the undefined ventral shields, and especially in a different dentition of both thoracic and abdominal uncini. This last feature appears very peculiar, with the presence of a second highly developed and asymmetric tooth over the main fang similar to that described for Paradialychone. Moreover, the poorly defined pre-pygidial depression lacking wings could suggest that E. anceps sp. nov. and E. pseudolimnicola are closely related to the genera Dialychone or Paradialychone, thus leading to a narrowing of the boundaries between genera of the “Chonea” group, and suggesting that generic definitions in this group need to be reexamined.
Due to the above considerations, specimens abundantly collected along the British coast have for a long time been identified as Chone cfr. collaris, and more recently as Euchone cfr. pseudolimnicola. A similar ambiguity in generic placement due to the mixed combination of morphological traits was also pointed out by Capa and Murray (Citation2015), who recently described a new taxon from Australian coasts as Dialychone ambigua Capa & Murray, Citation2015.
The cladistic analysis, performed to underline the relationships of the new taxon to the most plesiomorphic sabellid genera, revealed the genus Euchone to be not well defined. However, E. pseudolimnicola and E. cfr. pseudolimnicola (now E. anceps sp. nov.) appear more closely related to the other considered Euchone species than to Dialychone and Paradialychone. Therefore, we suggest maintaining both these taxa within the genus Euchone until a revision based on the examination of type material of all the Euchone species can be performed.
The pre-pygidial depression present in E. pseudolimnicola and E. anceps sp. nov. is simple but hollowed, while the pre-pygidial depression of Dialychone and Paradialychone appears only as a terminal flattened portion of the abdomen (simple depression). True lateral wings are lacking also in E. limnicola, a locally abundant species colonising marine to estuarine habitats along British and French coasts of the North Sea (Giangrande et al. Citation2015; Guyonnet & Borg Citation2015). This species, native to California on the Pacific Coast of North America, but introduced worldwide (South-Eastern Australia, New Zealand) (Wilson & McArthur Citation2008), still needs to be well defined with the examination of more material from different areas. The E. limnicola specimens here examined, indeed, show an entire ventral margin of the collar, whilst all the Euchone species up to now described have a more or less pronounced ventral cleft. This feature, however, is not unique to the Euchone genus, being present also in Jasmineira and Euchoneira, and in one species of Dialychone: D. arabica Tovar-Hernández & Dean, Citation2010.
Finally, peculiar to the new taxon and to E. pseudolimnicola is the presence of ‘ventral sacs’ which are highly developed, especially in E. anceps sp. nov. These structures have never been described within the plesiomorphic sabellid genera. Fitzhugh (Citation1989) described the parallel lamellae as an extension of the ventral lips along the anterior midline, which terminates near or between the mid-ventral cleft of the collar, or in two ventral vescicles (ventral sacs). Parallel lamellae usually characterise the most apomorphic sabellid genera and, according to the Fitzhugh analysis (Citation1989), in the most apomorphic area are present only in the genera Jasmineira and Panousea Rullier & Amoureux, Citation1970, arising between Myxicola Renier, Citation1804 and Potamethus Chamberlin, Citation1919. Therefore, the parallel lamellae were never described for any species of Chone or Euchone. Recently, however, Capa and Murray (Citation2015) report the presence of parallel lamellae in the two Euchone species they described, E. danieloi and E. glemnoi. We re-examined all the Euchone material available in our collection, finding that, although these structures are very difficult to check, probably most Euchone have parallel lamellae, and probably the presence of these structures is linked to the presence of a ventral cleft in the collar.
In E. pseudolimnicola and E. anceps sp. nov. not only parallel lamellae are present, but also the ventral sacs, though to different degrees of development. The presence of ventral sacs, which were not reported in the original description of E. pseudolimnicola, could be of high importance in sabellid phylogeny, even if in the present analysis their presence was not enough to separate these two taxa from the other Euchone species. Aside from differing development of the ventral sacs, these two species differ from each other in several features. Euchone anceps sp. nov. is a larger species with a longer crown, and has more broadly hooded chaetae in the superior thoracic fascicle, and narrow subspatulate chaetae in the inferior group. Also, it shows a different methyl green pattern in the collar and a less-developed pre-pygidial depression without a distinct ridge in the anterior edge.
These last two features are, however, highly variable in Mediterranean material from the Gulf of Lion, where specimens collected at 35–40 m depth are morphologically similar to the type material from Tyrrhenian Sea collected at 15 m depth; here the shallowest specimens present a darker staining pattern and a different development of the pre-pygidial depression. By contrast, the specimens from the British area, collected from 20 to 80 m depth, are morphologically indistinguishable from the type material. These populations could thus represent either a highly variable taxon or a complex of species. In conclusion, their status remains uncertain until additional morphological and molecular evidence is presented.
Finally, from the provided key for the genera included in the “Chonea” group, it is evident that the Northeast Atlantic region contains a higher number of species (16, of which 10 are exclusive to the area) compared to the Mediterranean region (14, of which nine are exclusive to the area). This is particularly interesting because usually the Mediterranean area is considered a biodiversity hotspot. Moreover, a low similarity between the two areas is observed (25%), with Chone present only in the Northeast Atlantic area, Paradialychone only in the Mediterranean, and Dialychone and Euchone more speciose in the Mediterranean and in the Northeast Atlantic area, respectively. As suggested by Giangrande and Licciano (Citation2004), this last point is probably linked to a taxonomic impediment, particularly for the genus Euchone, which is often neglected within Mediterranean works.
Acknowledgements
The specimens used in this paper were available from monitoring surveys. For the Tyrrhenian Sea type material we would like to thank M. Cristina Gambi (Zoological Station of Naples), who sent us the specimens from soft-bottom macrofaunal assemblages in the Gulf of Salerno. For the Shetland specimens we would like to thank Meriem Kayoueche-Reeve and the Sullom Voe Association Ltd, the funders of the Shetland Oil Terminal Environmental Advisory Group’s environmental monitoring programme for marine chemistry and macrobenthos. Thanks also go to Paul McIlwaine of the Centre for Environment Fisheries and Aquaculture Science (CEFAS) for the south-west England specimens, and to Celine Labrune Observatoire Océanologique de Banyuls/mer for the Mediterranean specimens from the Gulf of Lion. We wish to thank also Rhian Pugh from EMU Ltd who furnished us with specimens of Euchone limnicola from the Tees Estuary. The study was carried out using the facilities of the Experimental Research Centre of Biodiversity Organization and Ecosystem Functioning (BIOforIU) of the University of Salento.
References
- Banse K. 1972. Redescription of some species of Chone Krøyer and Euchone Malmgren, and three new species (Sabellidae, Polychaeta). Fishery Bulletin 70:459–495.
- Bick A, Randel N. 2005. Ontogenetic variations in characters of Euchone analis (Krøyer, 1856) (Polychaeta, Sabellidae, Sabellinae) from Spitsbergen, and new assignments of Oriopsis ingelorae Plate, 1995 and O. liefdefjordensis Plate, 1995. Acta Zoologica 86:145–157. DOI:10.1111/j.1463-6395.2005.00196.x.
- Capa M, Murray A. 2015. A taxonomic guide to the fan worms (Sabellidae, Annelida) of Lizard Island, Great Barrier Reef, Australia, including new species and new records. Zootaxa 4019:98–167. DOI:10.11646/zootaxa.4019.1.8.
- Capa M, Nogueira JMM, Silva Rossi MC. 2011. Comparative internal structure of dorsal lips and radiolar appendages in Sabellidae (Polychaeta) and phylogenetic implications. Journal of Morphology 272:302–319. DOI:10.1002/jmor.10914.
- Chamberlin RV. 1919. The Annelida Polychaeta (Albatross Expeditions). Vol. 48. Memoirs of the Museum of Comparative Zoology at Harvard College, Cambridge. pp. 1–514.
- Claparède E. 1870. Les Annélides Chétopodes du Golfe de Naples. Seconde partie. Annélides sédentaires. Mémoires de la Société de physique et d’histoire naturelle de Genève 20:1–225. Available: http://www.archive.org/details/lesannlidesch02clap. Accessed Oct 2016 10.
- Cochrane SJ. 2003. Snowflakes and feather-dusters some challenges for soft-bottom fanworm systematics. Hydrobiologia 496:49–62. DOI:10.1023/a:1026168025573.
- Fitzhugh K. 1989. A systematic revision of the Sabellidae-Caobangiidae-Sabellongidae complex (Annelida: Polychaeta). Bulletin of the American Museum of Natural History 192:1–104.
- Fitzhugh K. 2002. Fan worm polychaetes (Sabellidae: Sabellinae) collected during the Thai-Danish BIOSHELF project. Phuket Marine Biological Center Special Publication 24:353–424.
- Fitzhugh K. 2003. A new species of Megalomma Johansson, 1927 (Polychaeta: Sabellidae: Sabellinae) from Taiwan, with comments on sabellid dorsal lip classification. Zoological Studies 42:106–134.
- Giangrande A, Licciano M. 2004. Factors i nfluencing latitudinal pattern of biodiversity: An example using Sabellidae (Annelida, Polychaeta). Biodiversity & Conservation 13:1633–1646. DOI:10.1023/B:BIOC.0000029327.63397.6b.
- Giangrande A, Licciano M. 2006. The genus Euchone (Polychaeta, Sabellidae) in the Mediterranean Sea, addition of two new species and discussion on some closely related taxa. Journal of Natural History 40:1301–1330. DOI:10.1080/00222930600901458.
- Giangrande A, Licciano M, Wasson B. 2015. Guide to identification of Sabellidae and Fabriciidae (Polychaeta) in north east Atlantic and Mediterranean waters. NMBAQC 2014 taxonomic workshop: Dove Marine Laboratory. Available: http://www.nmbaqcs.org/scheme-components/invertebrates/literature-and-taxonomic-keys/sabellid-guide/. Accessed Sep 2015 24.
- Gmelin JF. 1791. Vermes. In: Gmelin JF, editor. Caroli a Linnaei Systema Naturae per Regna Tria Naturae, Editio Decima Tertia, Aucta Reformata. Tome 1, Pars 6 (Vermes). Lipsiae: GE Beer. pp. 3021–3910.
- Guyonnet B, Borg D. 2015. Premier signalement de l’espèce introduite Euchone limnicola Reish, 1959 (Polychaeta: Sabellidae) sur les côtes françaises de la Mer du Nord (Grand Port Maritime de Dunkerque). Les Cahiers Naturalistes de l’Observatoire Marin 4:15–23.
- Hartmann-Schröder G. 1979. Die Polychaeten der “Atlantischen Kuppenfahrt” von FS “Meteor”(Fahrt 9 c, 1967). 1. Proben aus Schleppgeräten. Meteor Forschungs-Ergebnisse, Reihe D 31:63–90.
- Krøyer H. 1856. Meddelelser af en Afhandling Ormeslaegten Sabella Linn., isaer med Hensyn til dens nordiske Arter. Oversigt over det Kongelige Danske videnskabernes selskabs forhandlinger 1856:1–36.
- Langerhans P. 1880. Die wurmfauna Madeiras. II. Zeitschrift für wissenschaftliche Zoologie 33:271–316.
- Langerhans P. 1881. Die Wurmfauna von Madeira. III. Zeitschrift für wissenschaftliche Zoologie 34:87–143.
- Licciano M, Giangrande A, Gambi MC. 2009. A new genus of Sabellidae (Annelida, Polychaeta) from Antarctica, with discussion of relationships among plesiomorphic genera within Sabellinae. Zootaxa 2226:28–42. DOI:10.11646/%25x.
- Lorenti M, Gambi MC, Guglielmo R, Patti FP, Scipione MB, Zupo V, Buia MC. 2011. Soft bottom macrofaunal assemblages in the Gulf of Salerno, Tyrrhenian Sea, Italy, affected by the invasive seaweed Caulerpa racemosa var. cylindracea. Marine Ecology 3:320–334. DOI:10.1111/j.1439-0485.2011.00472.x.
- Maddison DR, Maddison WP. 2005. MacClade 4: Analysis of phylogeny and character evolution. Version 4.08a. Available: http://macclade.org. Accessed Oct 2015 20.
- Malmgren AJ. 1866. Nordiska Hafs-Annulater. Öfversigt af Königlich Vetenskapsakademiens förhandlingar, Stockholm 22:355–410.
- Nishi E, Tanaka K, Tovar-Hernández M, Giangrande A. 2009. Dialychone, Jasmineira and Paradialychone (Annelida: Polychaeta: Sabellidae) from Japan and adjacent waters, including four new species descriptions. Zootaxa 2167:1–24. DOI:10.11646/%25x.
- Nogueira JMM, Fitzhugh K, Rossi S, Cappellani M. 2010. A new genus and new species of fan worms (Polychaeta: Sabellidae) from Atlantic and Pacific Oceans-the formal treatment of taxon names as explanatory hypotheses. Zootaxa 2603:1–52. DOI:10.11646/%25x.
- Reish DJ. 1959. A new species of Sabellidae (Annelida, Polychaeta) from southern California. Annals and Magazine of Natural History (Series 13) 2:717–719. DOI:10.1080/00222935908655757.
- Renier SA. 1804. Prospetto della Classe dei Vermi nominati e ordinati secondo il sistema di Bosc. Zoologische Jahrbucher. Abteilung Für Systematik Jena 64:41–110.
- Rullier F, Amoureux L. 1970. Nouvelle contribution a l’étude de la faune des Annélides Polychètes du Maroc. Bulletin de la Société des Sciences Naturelles et Physiques du Maroc 49:109–142.
- Selim SA, Rzhavsky AV, Britayev ТА. 2012. Dialychone and Paradialychone (Polychaeta: Sabellidae) from the Mediterranean Coast of Egypt with description of Dialychone egyptica sp.n. Invertebrate Zoology 9:105–114.
- SGS M-Scan Ltd. 2012. Chemical and macrobenthic monitoring in Sullom Voe Sediments- 2012. Report to SOTEAG from SGS M-Scan Ltd, 1211/23366. Available: http://www.soteag.org.uk. Accessed Jan 2016 12.
- SGS M-Scan Ltd. 2014. Chemical and macrobenthic monitoring in Sullom Voe Sediments- 2014. Report to SOTEAG from SGS M-Scan Ltd, 141/24728. Available: http://www.soteag.org.uk/files/2015/05/SGS-M-Scan-2014-Chemical-and-macrobenthic-monitoring-in-Sullom-Voe-sediments_final.pdf. Accessed Jan 2016 12.
- Swofford DL 2002. PAUP*. Phylogenetic analysis using parsimony (* and other methods) v.4. Sunderland, MA: Sinaurer Associates.
- Tovar-Hernández MA. 2008. Phylogeny of Chone Kröyer, 1856 (Poychaeta: Sabellidae) and related genera. Journal of Natural History 42:2193–2226. DOI:10.1080/00222930802254714.
- Tovar-Hernández MA, Dean H. 2010. Four new species of fan worms (Polychaeta: Sabellidae) from Worldwide Localities. Scientia Marina 74:815–826. DOI:10.1080/00222930802254714.
- Wasson B, Giangrande A, Tovar-Hernández MA. 2017. Re-establishment of Chone filicaudata Southern, 1914 from material collected from British coasts, and the first British record of the Mediterranean species Dialychone dunerificta (Tovar Hernández et al. 2007). Cahiers de Biologie Marine.
- Wilson R, McArthur M. 2008. Fan worm Euchone limnicola. Australia: Pest and Diseases Image Library. Available: http://www.padil.gov.au. Accessed Jul 2016 14.