Abstract
The distribution of four isopods – Idotea balthica basteri (Pallas, 1772), Idotea chelipes mediterranea Charfi-Cheikhrouha, 1996, Sphaeroma serratum (Fabricius, 1787), and Paracerceis sculpta (Holmes, 1904) – at Menzel Jemil (Bizerte Lagoon, North Tunisia) was analysed and then related to the variation of temperature, depth and plant biomass. The abundance of isopods showed monthly fluctuations with maxima in August and minima in December 2010. The most dominant species was I. b. basteri followed by S. serratum, with a spatial gradient in the distribution pattern of isopod species in the study area. The high density of juveniles in algae and seagrass highlights the important role played by this habitat as a nursery for species. Both dominant species were tolerant to temperature variations, with some preference values (19.73–27.73°C in I. b. basteri; up to 28°C in S. serratum) and salinity (36.7–38.6 psu in I. b. basteri; 39–39.2 psu in S. serratum). Multiple regression analysis between environmental variables and species abundance showed a positive relationship with biomass of Gracilaria bursa-pastoris and Ulva lactuca for the two species of Idotea and Sphaeroma, respectively, while abundance of P. sculpta was positively related to temperature. Biotic and environmental linking (BIOENV) analysis confirmed the greatest influence of temperature and vegetation biomass on the temporal distribution of these isopod species. A spatial distribution pattern according to successive depths was revealed.
Introduction
Isopods are one of the most diverse orders of crustaceans, living in a wide variety of environments from marine and fresh waters to terrestrial habitats. They are most commonly found in shallow marine waters (El-Shahawy & Desouky Citation2010). Marine isopods are normal constituents of the fauna inhabiting macroalgae (Healy & O’Neill Citation1984; Arrontes & Anadón Citation1990). They frequently receive less attention than amphipods in ecological and taxonomical studies, and knowledge of them is scarcer (Guerra-García et al. Citation2009).
Marine isopods have a wide variety of feeding habits, i.e. herbivory, scavengery, carnivory, omnivory and even foraminiferivory (Robertson & Mann Citation1980; Healy & O’Neill Citation1984; Gudmundsson et al. Citation2000; Orav-Kotta & Kotta Citation2004). Changes in food supply may influence the distribution of species and diversity patterns in the benthic environment. Food selection and habitat complexity may play an important role in the distribution and abundance of marine organisms such as peracarids (Duffy & Hay Citation1991; Edgar & Robertson Citation1992). Distribution and habitat preferences of isopods are poorly known (Holdich & Lincoln Citation1974) despite their great importance, both qualitative and quantitative, as main secondary and tertiary producers in marine macrobenthic assemblages. Isopods of southern Mediterranean lagoons are still relatively unexplored compared with those of northern lagoons (Giordani Soika Citation1950; Sconfietti Citation1988; Fava et al. Citation1992; Mancinelli Citation2010; Vincenzi et al. Citation2013; Longo & Mancinelli Citation2014).
Tunisian lagoons are extremely interesting due to their geographic distribution from north to south and their location in different climatic zones related to rainfall patterns. They cover a very large area, 110,000 hectares, and offer a wide range of biotopes since they are of different sizes and depths.
The presence of isopod species in the Tunisian lagoons was previously reported along shores of three northern coastal lagoons (Dridi & Prunus Citation1980), and in Bizerte (north) and Bougrara (south) lagoons (Zaouali Citation1980). Other restricted and fragmented occurrences of isopods were reported by several authors (Pantoustier & Prunus Citation1977; Prunus et al. Citation1978; Rezig Citation1979; Charfi-Cheikhrouha Citation1980, Citation1982, Citation1996; Zaouali Citation1981; Dridi Citation1989; Charfi-Cheikhrouha et al. Citation1998, Citation2000; Ben Souissi Citation2002; Ayari & Afli Citation2003; Diawara et al. Citation2008). Recently, more investigations have been conducted on reproductive strategies and population dynamics of Idotea balthica basteri associated with algae and seagrass in Bizerte Lagoon (Zaabar et al. Citation2014, Citation2015a, Citation2016).
To improve the knowledge of isopods in Tunisian wetlands, we identify the isopod species assemblage with algae and seagrass beds at Menzel Jemil in Bizerte Lagoon, study their spatial–temporal variation, analyse their distribution pattern and define the relationship between their abundance and the environmental factors.
Material and methods
Study area
The study site, located in the northeast of Bizerte Lagoon (37°14ʹ10.25”N, 9°54ʹ52.13”E), on a sandy mud bottom, was selected because of its abundance of isopods and its accessibility. According to Zaabar et al. (Citation2015b), the vegetal cover at Menzel Jemil mainly consists of Ulva lactuca (Linnaeus, 1753); during spring substantial numbers of Gracilaria bursa pastoris (S. G. Gmelin) P. C. Silva 1952 and Gracilariopsis longissima (S. G. Gmelin) M. Steentoft, L. M. Irvine and W. F. Farnham 1995 are present. Cladophora sp. is found occasionally, and the seagrass Cymodocea nodosa (Ucria) Ascherson, 1870 is present throughout the year, exhibiting a growth increase in summer. The plant biomass at Menzel Jemil is related to temperature reaching a minimum in January and a maximum in June (Zaabar et al. Citation2014).
Sampling and laboratory procedures
Sampling was conducted monthly from October 2009 to September 2010, using a metal square of 0.25 m2 with three replicates at each depth of 20, 40, 60 and 80 cm. The surface was scraped, and the vegetation and associated macrofauna species, including isopods, were removed after washing the algae and seagrass in a plastic tray and sieving with 1-mm mesh, and were then transported to the laboratory.
The following environmental parameters were measured monthly in situ: water temperature (T), salinity (S), dissolved oxygen (O2), pH and turbidity (Tr), using a salinometer (WTW Cond 315i, SUNTEX, Weilheim, Germany), an oxymeter (WTW Oxi315i/SET, SUNTEX, Weilheim, Germany), a pH meter (pH 330i/SET, SUNTEX, Weilheim, Germany) and a type of microprocessor (WTW TURB 355IR, SUNTEX, Weilheim, Germany) calibrated beforehand.
In the laboratory, isopod specimens found at each depth were preserved in 70% alcohol, identified and counted. Individuals of each species were separated and sorted according to their differentiation state. Then, the plant biomass was estimated by weighing all plant matter collected from each species of algae and seagrass after being dried at 70°C for 48 h.
Data analysis
Species richness (S), total abundance (N), density (ind.m−2), Shannon index (H’; Shannon & Weaver Citation1963), evenness index (J’; Pielou Citation1969), frequency index (F%; Soyer Citation1970) and dominance (Di%; Bellan-Santini Citation1969) were calculated monthly. Possible differences in these parameters betwen months were tested by one-way analysis of variance (ANOVA), after verifying normality (Kolmogorov–Smimov) and homogeneity of variances (Levene). To investigate the spatial–temporal distribution of isopods, the same procedure was carried out for testing statistical differences in the values of diversity indices and abundance of the species according to depths and seasons, respectively.
To determine preferences of temperature and salinity, values measured monthly were plotted vs. the abundance of specimens obtained from the algae and seagrass.
To better establish temporal distribution patterns, multidimensional scaling (MDS) based on the Bray–Curtis similarity was applied to abundance. The quantitative data from monthly samples was analysed, grouped according to seasons and compared statistically using analysis of similarity (ANOSIM). The SIMPER (SIMilarity PERcentage) procedure was used to identify those species that contributed most to the dissimilarity between these groups of samples.
Relationships between environmental variables (temperature, dissolved oxygen, turbidity, plant biomass) and isopod assemblages were examined using multiple stepwise regression analyes. In these analyses, only environmental variables (independent variables) were accepted in each regression model (p < 0.05).
The faunal and environmental ordinations of samples were also studied using biotic and environmental linking (BIOENV) analysis. Previously, environmental variables showing high correlation (after Pearson test) were redundant and not considered. The BIOENV analysis resulted in different combinations of environmental variables that are highly correlated with the ordination of faunal samples. These multivariate analyses were carried out using the PRIMER v. 5 software package.
Results
Environmental variables
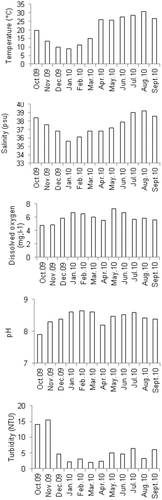
Regarding abiotic factors (Figure 1), the highest water temperature (30.86°C) and salinity (39.2 psu) were recorded in August; dissolved oxygen was high in May (7.3 mg.L−1), pH in February (8.65) and turbidity in November (15.44 NTU). Seasonal variations in the environmental parameters of Menzel Jemil (sampling site) outlined by ANOVA test were significantly different (p < 0.001). Pearson test showed that temperature and salinity were positively correlated (r = 0.763; p = 0.004); this was also the case for turbidity and pH (r = 0. 798; p = 0.004). Data showed a positive correlation between plant biomass and temperature (r = 0.868; p = 0.000).
Isopods and ecological analysis
A total of 4280 specimens belonging to two families, Idoteidae and Sphaeromatidea, and four species, Idotea balthica basteri, Idotea chelipes mediterranea, Sphaeroma serratum and Paracerceis sculpta, were identified.
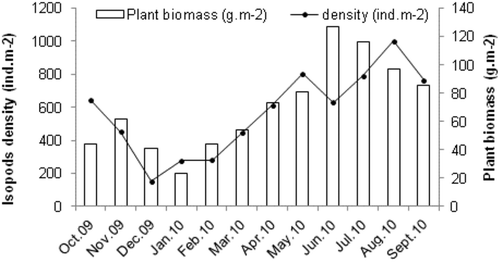
Monthly abundance () and density (Figure 2) of these species fluctuated, with maxima in August 2010 (625 specimens, 1000 ind.m−2), and minima in December 2009 (96 specimens, 153.6 ind.m−2), exhibiting a significant seasonal pattern (one-factor ANOVA; F = 49. 6, p < 0.001, F = 45. 84, p < 0.001, respectively). These densities () depended significantly on the plant biomass, which is related to temperature. However, temperature was also related to salinity; therefore, both parameters influence the density.
Table I. Monthly variation of isopod abundance and values of Shannon–Wiener (H′) and evenness indexes (J′) from October 2009 to September 2010. Di%, dominance; F%, frequency; N, total abundance of isopods; Ni, total abundance of species.
Considering species dominance, I. b. basteri was the most dominant species with 2610 individuals (61%), followed by S. serratum with 1516 individuals (35.4%), P. sculpta (2.4%) and I. c. mediterranea (1.2%).
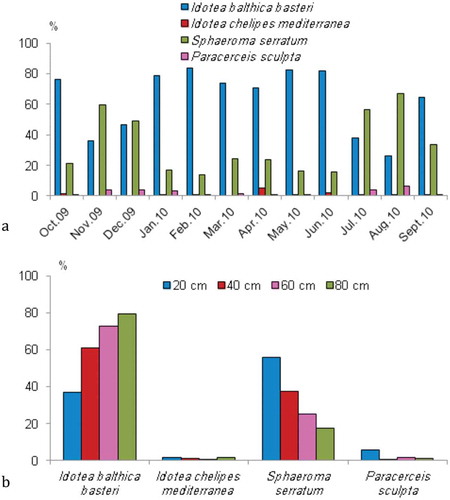
With respect to Soyer’s frequency (F) index, the four collected species were continuous (F ≥ 50%). Idotea balthica basteri and S. serratum were collected monthly (F = 100%, ) and many individuals of both species were juveniles. The non-indigenous species P. sculpta was also present in the algae and seagrass throughout the study year (F = 91.66%), except in June 2010. The presence of ovigerous females and juveniles in the vegetation revealed the establishment of the species in this area. Idotea chelipes mediterranea (F = 75%) was sparsely present, and totally absent from November 2009 to March 2010.
The Shannon–Wiener diversity index (H’) ranged between 0.74 bits in May and 1.25 bits in July 2010, with significant seasonal variation (one-factor ANOVA; F = 19.31, p < 0.001). The evenness (J’) index showed the same fluctuation, with a peak in December (0.76) and a low value in February (0.37). Significant seasonal differences were evident (one-factor ANOVA; F = 33.27; p < 0.001).
A temporal distribution pattern was also observed (Figure 3(a)): an increase of I. b. basteri in winter and spring (F = 8.2, p < 0.05) vs. the decrease of S. serratum during these seasons (F = 11.25, p < 0.05). Similarly I. c. mediterranea was more abundant in spring (F = 14.82, p < 0.05) than P. sculpta (F = 14.7, p ˂ 0.05).
A spatial distribution pattern according to successive depths was revealed for the four species ()), with evident differences: maximum abundance (79.69%) at 80 cm for I. b. basteri and 55.72% at 20 cm for S. serratum. No clear difference between I. c. mediterranea and P. sculpta was recorded, probably due to their reduced number. There is a spatial gradient in the distribution of isopod species in the study area. S. serratum was more related to the shoreline; its abundance decreased along the gradient depth, whereas the abundance of I. b. basteri was increasing (second author, pers. obs.). Based on the ANOVA test, S. serratum was more abundant at 20 cm depth (F = 1.09, p < 0.05), and I. b. basteri at 80 cm depth, than elsewhere (F = 0.62, p < 0.05).
Dynamics of two dominant species
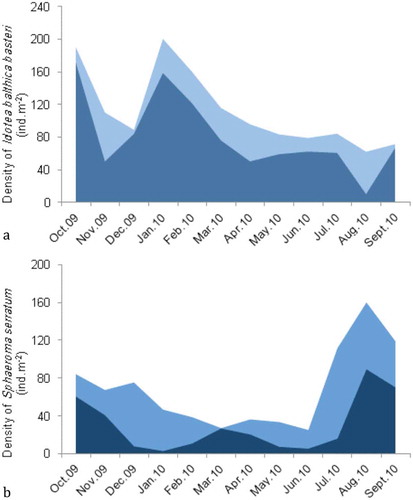
We focus on the two dominant species which determined the density of the total isopod assemblage. The monthly dynamics of the I. b. basteri population is displayed in Figure 4(a), where the juveniles are distinguished. Population density varied during the year, with two peaks in October and January (190.4 and 200.6 ind.m−2, respectively), and the lowest value (62.4 ind.m−2) was observed in August. The same pattern was recorded for juveniles, contributing more to the profile of population density in the algae and seagrass, which highlights the use of vegetation as a nursery ground.
Sphaeroma serratum was also present in the algae and seagrass throughout the year ()). Density values increased from July to October, with a maximum in August (160 ind.m−2). Similarly, the density of juveniles peaked in August, with a minimum in January (2.5 ind.m−2). In March, the bulk of the population was made up of juveniles, probably due to mortality of adults after their reproductive period.
Tolerance to temperature and salinity variation of two dominant species
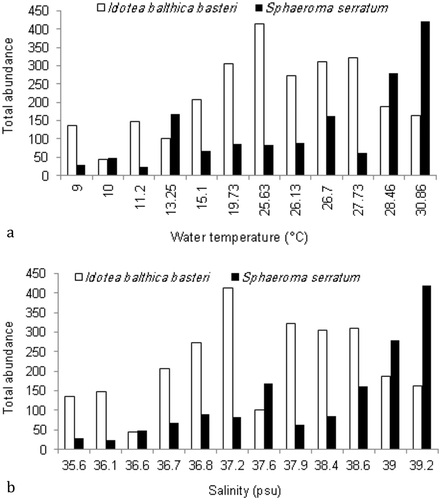
The abundance of the dominant species, I. b. basteri and S. serratum, exposed to specific temperature and salinity conditions was computed. Figure 5(a) indicates a wide tolerance to temperature 9–30.86°C for both species. The ANOVA test revealed that the greatest abundance of I. b. basteri occurred at a temperature range of 19.73–27.73°C (F = 40.12, p < 0.05), and the number of specimens decreased when the temperature reached 28.46°C. It was found that temperatures up to 28°C were preferred by the population of S. serratum (F = 9.76, p < 0.05).
Similarly, both species exhibited a tolerance for high values of salinity ()). Statistical analysis indicated a preference for 36.7–38.6 psu (F = 33.63, p < 0.05) and 39–39.2 psu (F = 6.89, p < 0.05) in the case of I. b. basteri and S. serratum, respectively.
Affinity between samples
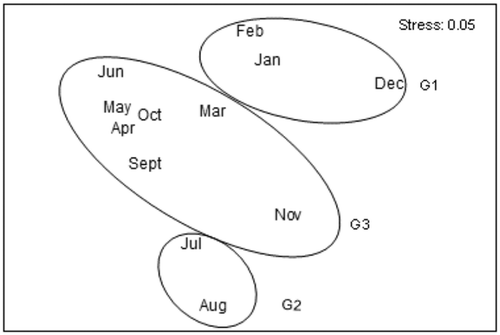
Multivariate analysis using fourth root transformed abundance data () revealed three distinct groups of samples corresponding to different periods of the year in MDS representation. Group I was made up of months from December to February (winter), in which abundance of isopod species and temperature were low. Group II included samples from July and August (summer) where Sphaeromatidea was abundant and temperature high. Group III, composed of the remaining months (autumn and spring), was located at the opposite side of the MDS ordination plot to the winter and summer months. The latter group results in an intermediate temperature with a little variation of I. b. basteri abundance. According to seasons, significant differences were found between the isopod assemblage (ANOSIM, R = 0.32; p = 0. 016). This was statistically confirmed when pairwise tests were applied. The highest dissimilarities of the SIMPER analysis were obtained between winter and summer (dissimilarity: 54.7%), autumn and winter (48.37%) and winter and spring months (44.48%). SIMPER analysis indicated that the species contributing the most to the difference between seasons were the two dominant species, I. b. basteri and S. serratum.
Linking isopod fauna and environmental variables
Using multiple stepwise regression analyses, the abundance values (N) of isopod species displayed a significant relationship with environmental variables ().
Table II. Multiple stepwise regression analysis of isopod abundance (N) in relation to environmental variables from October 2009 to September 2010. SE, standard error. Only environmental variables accepted in each regression model (p < 0.05) have been listed. G. long, Gracilariopsis longissima; G. b. pas, Gracilaria bursa-pastoris; U. lac, Ulva lactuca; t, t-statistic.
The abundance of I. b. basteri and I. c. mediterranea was related positively to biomass of Gracilaria bursa-pastoris and negatively to biomass of Gracilariopsis longissima. In contrast, abundance of Sphaeroma serratum and Paracerceis sculpta was related positively to biomass of Ulva lactuca, which was also positively related to temperature.
Using BIOENV as a different multivariate approach, the composition and structure of isopods displayed the highest correlation when combining two variables such as temperature–biomass of Ulva lactuca and temperature–biomass of Gracilaria bursa-pastoris, and three variables such as temperature–Ulva lactuca biomass–Gracilaria bursa-pastoris biomass ().
Table III. Results of biotic and environmental linking (BIOENV) analysis displaying the three highest Spearman rank correlations using square root quantitative data of isopods collected from October 2009 to September 2010. T, temperature (°C); U.lac, Ulva lactuca biomass (g.m−2); G.b.p, Gracilaria bursa-pastoris biomass (g.m−2).
Discussion
This paper is the first investigation of the influence of environmental variables on the distribution of marine isopods associated with algae and seagrass at Menzel Jemil (Bizerte Lagoon). Environmental conditions during this study were relatively comparable with those measured during the last few years (Harzallah Citation2003; Béjaoui et al. Citation2008; Ben Garali et al. Citation2010). In this Mediterranean lagoonal complex, environmental variables strongly fluctuated with seasonal variations related to precipitation, temperature and currents (Afli et al. Citation2009b). Therefore, only some invertebrate species were tolerant to the extreme salinity and temperature changes between winter and summer, and able to survive in Bizerte Lagoon (Afli et al. Citation2009a).
In the present study, four isopod species inhabiting algae and seagrass were examined and strong differences in their abundance and distribution were reported. Isopod richness associated with algae and seagrass at Menzel Jemil station was lower than that recorded in Bizerte Lagoon (10 species) by Dridi and Prunus (Citation1980) and Zaouali (Citation1980); this could be due to the increase in salinity after the construction of dams, reducing freshwater input from Ichkeul Lake during the last few decades.
Idotea balthica basteri was the most dominant species, particularly in spring and early summer inhabiting Gracilariopsis longissima, Gracilaria bursa-pastoris, Ulva lactuca and decaying seagrass. Despite many ecological changes in the Bizerte Lagoon in the last few decades (Afli et al. Citation2009a), I. b. basteri inhabiting algae and seagrass was still the most dominant species, while Idotea chelipes mediterranea has significantly decreased compared to the years 1980–1990 (second author pers. obs.). The abundance of I. balthica could be explained by its competition with I. chelipes for food resources and space (Korheina Citation1981). Kotta et al. (Citation2000) proposed that the decline of I. chelipes probably reflects the decreasing cover for benthic vegetation in the Gulf of Riga since other phytophilous species have declined. Ingólfsson and Agnarsson (Citation2003), studying dispersal and movements of isopods and amphipods during high tide, and Franke et al. (Citation2007) in their work about habitat selection and habitat segregation in I. balthica and I. emarginata, showed that I. balthica was the dominant species among surface-floating algae.
The low abundance of P. sculpta with respect to Sphaeroma serratum could be related to its recent colonisation of this area. Paracerceis sculpta is the most acclimated isopod in the Tunisian brackish waters (Ounifi Ben Amor et al. Citation2010). According to Zibrowius (Citation1992), it was also the most widely ship-transported alien species in the world, and was recorded in the Mediterranean during the earlier part of the last century. The presence and establishment of this non-indigenous species, native to the northeast Pacific, in Bizerte Lagoon might be explained by biofouling on ships due to the proximity and connection of this lagoon to Bizerte Port. Furthermore, the position of Tunisia between the two Mediterranean basins will be a key area for understanding the influx of alien species in the whole Mediterranean Sea (Guidetti et al. Citation2010; Azzurro et al. Citation2014).
Temporal distribution of isopod species in Bizerte Lagoon might be due to a response to the same environmental factors (e.g. temperature, salinity, nutrients) causing the change and distribution in algae and seagrass cover on which animals live (Arrontes & Anadón Citation1990). There are several potential environmental factors that might influence a species’ distribution and abundance, including water temperature, salinity, light level, shelter from predators, and food resources (Dufour et al. Citation2007). Based on the recorded temporal distribution of Idotea and temperature and salinity fluctuations, we found that I. b. basteri, with a marine tendency, occurs at higher temperature and salinity than lagoonal species I. c. mediterranea, and contrary to Sphaeromatidea distribution. Similar patterns were observed by Leidenberger et al. (Citation2012) for Idotea distribution in the Baltic Sea. I. chelipes prefers warmer temperatures (winter temperatures in the Bizerte Lagoon) coinciding with its preferred habitat in lagoons and estuaries (Zettler Citation2000, Citation2001). On the other hand, I. chelipes was described as a brackish-water species, tolerating 3–30 psu (Łapucki & Normant Citation2008). Moreover, the decrease of I. c. mediterrranea over the last few decades in the Bizerte Lagoon could be explained by the increase of salinity due to the construction of dams in upstream Ichkeul Lake, causing a marinisation of the lagoon which becomes colonised by I. b. basteri. In addition, the Bizerte Lagoon ecosystem is currently destabilised by waste water coming from the bordering cities (Dellali et al. Citation2001) and shoreline industries, whereas I. c. mediterranea was a more sensitive species to pollution. Consequently, the rearing of this species in the laboratory is more difficult (experiment by second author).
On the other hand, I. b. basteri, S. serratum and P. sculpta, as well as other typically marine species, entered and populated the Bizerte Lagoon due to favourable environmental conditions: relatively low temperature, high salinity, good oxygenation, low turbidity, high insolation, and abundance of food (Alves Martins et al. Citation2015).
Among isopods, I. balthica and S. serratum were the most common species in the littoral zone of the Mar Piccolo basin (Ionian Sea, southern Italy); numerically dominant, they constituted an important food for many predators such as crustaceans, fish, and birds in this ecosystem (De Nicola Giudici & Guarino Citation1989).
In the study area, I. b. basteri was the most dominant species followed by S. serratum, exhibiting a spatial distribution pattern. According to Casagranda et al. (Citation2006), a similar distribution pattern was found in Lake Ichkeul. Indeed, I. chelipes was found in relatively deep stations with living vegetation and hard substrate (sand, gravel), whereas Sphaeroma hookeri mainly occurred at relatively shallow stations with plenty of detritus and silty substrate.
S. serratum usually lives in coastal marine or brackish waters, which are often subject to variations in salinity. It lives under stones close to the shore or among seaweed, in 5–80-cm-deep water (Prato et al. Citation2012).
Using multivariate analyses, Gracilaria bursa-pasteri biomass was the variable which better explained the temporal distribution of the Idotea genus, particularly I. b. basteri, the most investigated species in the Bizerte Lagoon, whereas the distribution of S. serratum and P. sculpta was related to Ulva lactuca biomass. Casagranda et al. (Citation2006) reported that the functional relationship between shredders (e.g. I. chelipes, S. hookeri) and macrophytes may be nutritional. This meadow also provides the shredders with a nursery and some refuge from wave action, predation, salinity and temperature variations. According to Raberg and Kautsky (Citation2008) I. balthica was the second most common crustacean meso-grazer besides Gammarus spp. on the Swedish coast, and its mean abundance appeared to be highest in the Cladophora glomerata zone.
The most dominant species in this study, I. balthica and S. serratum, were tolerant to some variations in temperature and salinity. Similar results have been reported for many other isopod species in a tropical coastal lagoon (Garcia-Guerrero & Hendrickx Citation2010). From their point of view, water and vegetation parameters may influence the seasonal distribution of isopods.
In summary, algae and seagrass in Bizerte Lagoon represent a habitat where isopods display spatial and temporal distributions. Moreover, this Mediterranean ecosystem, located between Ichkeul Lake and the Mediterranean Sea, under the influence on the one hand of fresh water which decreased after the construction of dams in upstream Ichkeul Lake, and marinisation of the lagoon due to climate change on the other hand, may favour the entrance of non-indigenous species. Finally, in order to increase knowledge about the dispersion of non-indigenous species in the Mediterranean lagoons, studies on the macrofauna of different coastal lagoons of Tunisia should be conducted regularly.
Acknowledgements
The authors thank Prof. A. Ben Rhouma, English Inspector, for revising the English. This study was supported by the Research Unit of Bio-Ecology and Evolutionary Systematics (UR11ES11), Faculty of Sciences of Tunis, University Tunis El Manar.
References
- Afli A, Boufahja F, Sadraoui S, Ben Mustapha K, Aissa P, Mrabet R. 2009a. Functional organization of the benthic macrofauna in the Bizerte lagoon (SW Mediterranean Sea), semi-enclosed area subject to strong environmental/anhtropogenic variations. Cahiers de Biologie Marine 50:105–117.
- Afli A, Chakroun R, Ayari R, Aissa P. 2009b. Seasonal and spatial variability of the community and trophic structure of the benthic macrofauna within Tunisian lagoonal and marine coastal areas (south-western Mediterranean). Journal of Coastal Research 25:140–149.
- Alves Martins MV, Zaaboub N, Aleya L, Frontalini F, Pereira E, Miranda P, Mane M, Rocha F, Laut L, El Bour M. 2015. Environmental quality assessment of Bizerte Lagoon (Tunisia) using living Foraminifera assemblages and a multiproxy approach. PLoS ONE 10:e0137250. DOI:10.1371/journal.pone.0137250.
- Arrontes J, Anadón R. 1990. Distribution of intertidal isopods in relation to geographical changes in macroalgal cover in the Bay of Biscay. Journal of the Marine Biological Association of the United Kingdom 70:283–293. DOI:10.1017/S0025315400035402.
- Ayari R, Afli A. 2003. Bionomie benthique du petit golfe de Tunis. Bulletin de l’Institut National des Sciences de Technologie de la Mer de Salammbô 30:79–87.
- Azzurro E, Ben Souissi J, Boughedir W, Cast Riota L, Deidun A, Falautano M, Ghanem R, Zammit-Mangion M, Andaloro F. 2014. The Sicily Strait: A transnational observatory for monotoring the advance of non indigenous species. Biologia Marina Mediterranea 21:105–106.
- Béjaoui B, Harzallah A, Moussa M, Chapelle A, Solidoro C. 2008. Analysis of hydrobiological pattern in the Bizerte lagoon (Tunisia). Estuarine, Coastal and Shelf Science 80:121–129. DOI:10.1016/j.ecss.2008.07.011.
- Bellan-Santini D. 1969. Contribution a l’étude des peuplements infralittoraux sur substrat rocheux (Etude qualitative et quantitative de la frange supérieure)- Travaux de Recherche Station Marine d’Endoume. France 63:9–294.
- Ben Garali A, Ouakad M, Gueddari M. 2010. Geochemistry and ionic interaction in the Bizerte Lagoon waters (Northern Tunisia). Journal of Oceanography and Marine Science 2:1–9.
- Ben Souissi J. 2002. Impact de la pollution sur les communautés macrobenthiques du lac sud de Tunis avant sa restauration environnementale. Thèse de doctorat, Faculté des Sciences de Tunis, Tunisie. 267 pp.
- Casagranda C, Dridi MS, Boudouresque CF. 2006. Abundance, population structure and production of macroinvertebrate shredders in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Estuarine, Coastal and Shelf Science 66:437–446. DOI:10.1016/j.ecss.2005.10.005.
- Charfi-Cheikhrouha F. 1980. Recherches systématiques, biologiques et expérimentales sur les Idotées de Tunisie (Isopodes Valvifères). Thèse de doctorat. Spécialité Biologie Marine, Université de Tunis. 193 pp.
- Charfi-Cheikhrouha F. 1982. Etude du cycle annuel d’Idotea chelipes (Pallas) du lac de Tunis (Isopodes Valvifères). Revue de la Faculté des Sciences de Tunis 2:101–113.
- Charfi-Cheikhrouha F. 1996. Idotea chelipes (Pallas, 1766), espèce polytypique: Description de ses sous-espèces (Isopodes Valvifères). Crustaceana 69:187–203. DOI:10.1163/156854096X00501.
- Charfi-Cheikhrouha F, Laulier M, Hamelin E, Mocquard J-P. 1998. Genetic differentiation and evolutionary process of speciation in the Idotea chelipes complex (Crustacea, Isopoda). Genetics Selection Evolution 30:289–303. DOI:10.1186/1297-9686-30-3-289.
- Charfi-Cheikhrouha F, Zghidi W, Ould Yarba L, Trilles JP. 2000. Le Cymothoidae (Isopodes parasites de poissons) des côtes tunisiennes: Ecologie et indices parasitologiques. Systematic Parasitology 46:143–150. DOI:10.1023/A:1006336516776.
- De Nicola Giudici M, Guarino SM. 1989. Effects of chronic exposure to cadmium or copper on Idothea baltica (Crustacea, Isopoda). Marine Pollution Bulletin 20:69–73. DOI:10.1016/0025-326X(89)90229-4.
- Dellali M, Elbour M, Aissa P. 2001. Evaluation de la pollution bactérienne dans la lagune de Bizerte: Résultats préliminaires. Journal de Recherche Océanographique 26:18–28.
- Diawara M, Zouari-Tlig S, Rabaoui L, Ben Hassine OK. 2008. Impact of management on the diversity of macrobenthic communities in Tunis north lagoon: Systematics. Cahiers de Biologie Marine 49:1–16.
- Dridi MS 1989. Sur l’espèce Cymodoce emarginata (Leach) en Tunisie. Etude systématique et écologique. Revue de la Faculté des Sciences de Tunis 4(D):111–128.
- Dridi MS, Prunus G. 1980. Analyse qualitative et quantitative du peuplement en isopodes et amphipodes dans les milieux lagunaires du nord de la Tunisie. Bulletin de l’Office National des Pêches, Tunisie 4:17–25.
- Duffy JE, Hay ME. 1991. Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology 72:1286–1298. DOI:10.2307/1941102.
- Dufour CM, Engels NM, Burns CW. 2007. Distribution, substrate preference and habitat enhancement of the isopod Austridotea lacustris in Tomahawk Lagoon, Otago, New Zealand. New Zealand Journal of Marine and Freshwater Research 41:299–307. DOI:10.1080/00288330709509917.
- Edgar GJ, Robertson AI. 1992. The influence of seagrass structure on the distribution and abundance of mobile epifauna: Pattern and process in a Western Australian Amphibolis bed. Journal of Experimental Marine Biology and Ecology 160:13–31. DOI:10.1016/0022-0981(92)90107-L.
- El-Shahawy IS, Desouky ARY. 2010. Myripristis murdjan (Beryciformes: Holocentridae) a new host record for Cymothoa indica (Crustacea, Isopoda, Cymothoidae). Acta Adriatica 51:103110.
- Fava G, Zangaglia A, Cervelli M. 1992. Ecology of Idotea balthica (Pallas) populations in the lagoon of Venice. Oceanologica Acta 15:651–660.
- Franke H-D, Gutow L, Janke M. 2007. Flexible habitat selection and interactive habitat segregation in the marine congeners Idotea baltica and Idotea emarginata (Crustacea, Isopoda). Marine Biology 150:929–939. DOI:10.1007/s00227-006-0421-2.
- Garcia-Guerrero M, Hendrickx ME. 2010. Distribution of Isopods (Peracarida, Isopoda) associated with prop roots of Rhizophora mangle in a tropical coastal lagoon, southeastern gulf of California, Mexico. Crustaceana 76:1153–1169. DOI:10.1163/156854003773123393.
- Giordani Soika A. 1950. Tanaidacei e gli Isopodi marini della Laguna di Venezia. Archivio Oceanography Limnology 7:215–238.
- Gudmundsson G, Von Schmalensee M, Svavarsson J. 2000. Are foraminifers (Protozoa) important food for small isopods (Crustacea) in the deep-sea? Deep Sea Research Particle I: Oceanographic Research Papers 47:2093–2109. DOI:10.1016/S0967-0637(00)00013-3.
- Guerra-García JM, Ros M, Sánchez JA. 2009. Isopods, tanaids and cumaceans (Crustacea, Peracarida) associated to the seaweed Stypocaulon scoparium in the Iberian Peninsula. Zooogica Baetica 20:35–48.
- Guidetti P, Giardina F, Azzurro E. 2010. A new record of Cephalopholis taeniops in the Mediterranean Sea, with considerations on the Sicily channel as a biogeographical crossroad of exotic fish. Marine Biodiversity Records 3:e13. DOI:10.1017/S1755267210000023.
- Harzallah A. 2003. Transport des polluants dans la lagune de Bizerte simulé par un modèle de circulation de l’eau. Bulletin de l’Institut National des Sciences de Technologie de la Mer de Salammbô 30:115–133.
- Healy B, O’Neill M. 1984. The life cycle and population dynamics of Idotea pelagica and I. granulosa (Isopoda: Valvifera) in south-east Ireland. Journal of the Marine Biological Association of the United Kingdom 64:21–33. DOI:10.1017/S0025315400059610.
- Holdich DM, Lincoln RJ. 1974. Distribution and habitat preferences of British marine isopods: A survey scheme. Field Studies 4:97–104.
- Ingólfsson A, Agnarsson I. 2003. Amphipods and isopods in the rocky intertidal: Dispersal and movements during high tide. Marine Biology 143:859–866. DOI:10.1007/s00227-003-1132-6.
- Korheina K. 1981. Environment and coexistence of Idotea species in the Southern Baltic. PhD Thesis, Lund, Sweden.
- Kotta J, Lauringson V, Martin G, Simn M, Kotta I, Herkul K, Ojaveer H. 2000. Gulf of Riga and Parnu Bay. In: Schiewer U, editor. Ecology of baltic coastal waters. Ecology Studies. Vol. 197. Berlin, Heidelberg: Springer- Varlag. pp. 217–244.
- Łapucki T, Normant M. 2008. Physiological responses to salinity changes of the isopod Idotea chelipes from the Baltic brackish waters. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 149:299–305. DOI:10.1016/j.cbpa.2008.01.009.
- Leidenberger S, Harding K, Jonsson PR. 2012. Ecology and distribution of the isopod genus Idotea in the Baltic Sea: Key species in a changing environment. Journal of Crustacean Biology 32:359–381. DOI:10.1163/193724012X626485.
- Longo E, Mancinelli G. 2014. Size at the onset of maturity (SOM) revealed in length-weight relationships of brackish amphipods and isopods: An information theory approach. Estuarine, Coastal and Shelf Science 136:119–128. DOI:10.1016/j.ecss.2013.11.013.
- Mancinelli G. 2010. Intraspecific, size-dependent variation in the movement behaviour of a brackish-water isopod: A resource-free laboratory experiment. Marine and Freshwater Behaviour and Physiology 43:321–337. DOI:10.1080/10236244.2010.512728.
- Orav-Kotta H, Kotta J. 2004. Food and habitat choice of the isopod Idotea baltica in the northeastern Baltic Sea. Hydrobiologia 514:79–85. DOI:10.1023/B:hydr.0000018208.72394.09.
- Ounifi Ben Amor K, Ben Salem M, Ben Souissi J. 2010. Sphaeroma walker Stebbing, 1905 (Crustacea, Isopoda, Sphaeromatidae) introduced and established in Tunisia waters. Rapport Commission International pour l’exploration scientifique de la Mer Méditerranée 39: 615 pp.
- Pantoustier G, Prunus G. 1977. Cycle biologique de l’isopode Jaera hopeana dans les milieux marins et lagunaires du Nord-Est de la Tunisie. Marine Biology 43:361–367. DOI:10.1007/BF00396929.
- Pielou EC. 1969. An introduction to mathematical ecology. New York: Wiley Interscience. pp. 286.
- Prato E, Danieli A, Maffia M, Biandolino F. 2012. Lipid contents and fatty acid compositions of Idotea balthica and Sphaeroma serratum (Crustacea: Isopoda) as indicators of food sources. Zoological Studies 51:38–50.
- Prunus G, Dridi MS, Sapoure B. 1978. Les peuplements littoraux et leur intérêt écologique dans les milieux lagunaires du Nord de la Tunisie. Bulletin de l’office National des Pêches Tunisie 2:227–234.
- Raberg S, Kautsky L. 2008. Grazer identity is crucial for facilitating growth of the perennial brown alga Fucus vesiculosus. Marine Ecology Progress Series 361:111–118. DOI:10.3354/meps07428.
- Rezig M. 1979. Etude comparée du cycle biologique de quelques espèces du genre Sphaeroma (Isopodes flabellifères) en Tunisie. Bulletin de l’Institut National Scientifique et Technique d’Océanographie et de Pêche Salammbô, Tunisie 6:93–121.
- Robertson AI, Mann KH. 1980. The role of isopods and amphipods in the initial fragmentation of eelgrass detritus in Nova Scotia, Canada. Marine Biology 59:63–69. DOI:10.1007/BF00396983.
- Sconfietti R. 1988. Researches on spatial distribution of amphipods, isopods and tanaids (Peracarida) in a Mediterranean estuary (River Dese, Lagoon of Venice). Crustaceana 55:193–201. DOI:10.1163/156854088X00528.
- Shannon CE, Weaver W. 1963. The mathematical theory of communication. Urbana: Illinois University press. pp. 117.
- Soyer J. 1970. Bionomie benthique du plateau continental de la côte catalane Française. III. Les Peuplements de Copépodes Harpacticoïdes (Crustacea). Vie Milieu 21:377–511.
- Vincenzi C, Lanzafame C, Colombo M, Caccia MG, Abbiati M, Ponti M. 2013. Alien species in the northern Adriatic lagoons: Paracerceis sculpta (Isopoda: Sphaeromatidae). Rapport de la Commission International de la Mer Méditerranée 40.
- Zaabar W, Achouri MS, Charfi-Cheikhrouha F. 2014. Life cycle and population dynamics of Idotea balthica basteri (Pallas, 1772), Isopoda Valvifera from the Bizerte lagoon (Southern Mediterranean Sea – Tunisia). Marine Ecology 35:367–376. DOI:10.1111/maec.2014.35.issue-3.
- Zaabar W, Achouri MS, Charfi-Cheikhrouha F. 2015a. Reproductive and growth strategies of Idotea balthica basteri (Pallas, 1772) population in the Bizerte lagoon (Tunisia, Southern Mediterranean). Marine Ecology 36:585–594. DOI:10.1111/maec.2015.36.issue-3.
- Zaabar W, Charfi-Cheikhrouha F, Achouri MS. 2016. The influence of environmental factors on the population structure and reproductive biology of Idotea balthica basteri (Isopoda, Valvifera) of the Bizerte Lagoon (Northern Tunisia). Open Journal of Ecology 6:206–218. DOI:10.4236/oje.2016.64021.
- Zaabar W, Zakhama-Sraieb R, Charfi-Cheikhrouha F, Achouri MS. 2015b. Abundance and diversity of amphipods (Crustacea: Peracarida) on shallow algae and seagrass in lagoonal ecosystem of the Mediterranean Tunisian coast. Zoological Studies 54:38. DOI:10.1186/s40555-015-0113-z.
- Zaouali J. 1980. Flore et faune benthiques de deux lagunes tunisiennes: Le lac de Bizerte (Tunisie septentrionale) et la mer de Bougrara (Tunisie méridionale). Bulletin d’Office Nationale des Pêches, Tunisie 4:196–200.
- Zaouali J. 1981. Evolution des peuplements floristiques et faunistiques (invertébrés) dans le lac Nord de Tunis (Mois de mai et juillet 1980). Rapport de la Commission International de la Mer Méditerranée 27:185–187.
- Zettler M. 2000. Biologische Artenvielfalt in Küstengewässern der Ostsee am Beispiel der Krebse (Malacostraca). Deutsche Gesellschaft für Limnologie (DGL) Tagungsbericht 1999 (Rostock) Bd 1:414–418.
- Zettler M. 2001. Some malacostracan crustacean assemblages in the southern and western Batlic Sea. Rostocker Meeresbiologische Beiträge 9:127–143.
- Zibrowius H. 1992. Ongoing modification of the Mediterranean fauna and flora by the establishment of exotic species. Mésogée 51:83–107.