 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In industrialised areas, teleost fish are often exposed to anthropogenic changes of the water quality. These often have negative effects on species with a narrow ecological range. Species with a wider ecological range, such as the three-spined stickleback (Gasterosteus aculeatus Linneaus, 1758), might benefit if water quality alteration reduces interspecific competition and/or parasite infection pressure. In the present study, we investigated sticklebacks in an inland brook, in which the inlet of warm and salty coal mine drainage water increases water temperature and changes the brook from freshwater to brackish (approx. 20 mS cm−1) conditions. We collected sticklebacks up- and downstream of the saltwater inlet (henceforth called freshwater and saltwater sites or habitats) in monthly intervals from April to October 2010, and monitored their body condition parameters and parasite infections. In particular during spring, the water temperature was higher (3.7–4.5°C) in the saltwater habitat and juvenile sticklebacks occurred earlier and grew faster compared to juveniles in the freshwater habitat. In the saltwater habitat, fewer parasite species were detected compared to the freshwater situation (7 vs. 10). Moreover, parasite index, which peaked in young-of-the-year sticklebacks in September, was lower in sticklebacks from the saltwater site. The present study suggests that changes of freshwater conditions by the inlet of warm and salty coal mine drainage water match the adaptive range of three-spined sticklebacks, which grew faster and had lower parasite burden in the altered habitat.
Introduction
In industrialised and intensively agriculturally used areas, water bodies and river systems are shaped by human interventions and often heavily altered compared to their natural structures. Accordingly, inhabiting fish species are confronted with numerous changes of their habitat such as flow regulation, dams and straightening of watercourses (Raeymaekers et al. Citation2009; Franssen et al. Citation2013), but also alterations of water quality, chemistry and temperature (Gravenmier et al. Citation2005; Candolin Citation2009; Cheek & Taylor Citation2015).
Such anthropogenic changes of water systems are generally considered detrimental for the inhabiting species, but teleost fish have evolved tolerance to various anthropogenic stressors (Schulte Citation2013). Some anthropogenic alterations of water conditions, such as increased salinity and/or temperature, might even be favourable for fish species with a wide physiological range.
In the present study, we investigated an inland brook, which is converted from fresh to brackish conditions by the inlet of warm and salty drainage water from a coal mine. The warm and salty load is toxic to many inhabiting freshwater organisms, including macrophytes, macrozoobenthos and the majority of the ichthyofauna, and thus leads to a drastic change in the ecosystem of the brook (Kaschek & Aschmeier Citation1993; Schimmer & Schindler Citation2000). However, three-spined stickleback (Gasterosteus aculeatus) are permanently present in both the saltwater-influenced and the freshwater parts of the brook (Scharsack et al. Citation2012).
Even though three-spined sticklebacks are a euryhaline fish species (Wootton Citation1984), they are still stressed by rapid salinity shifts (Li & Kueltz Citation2012). Both marine and freshwater sticklebacks are able to tolerate changes in salinity (Jakobsen & Klepaker Citation1985; Taugbøl et al. Citation2014), and specimens often migrate from marine to freshwater sites for spawning (Daniel Citation1985; Raeymaekers et al. Citation2005). Recently, resident stickleback populations have been identified which reproduce under marine, brackish and freshwater conditions (Kume et al. Citation2006; Raeymaekers et al. Citation2014). Although G. aculeatus are tolerant to variation in salinity, their adaptation to certain salinity levels was suggested to play a role in the segregation of stickleback populations (Campeau et al. Citation1984; DeFaveri & Merilä Citation2014).
A previous study, based on neutral microsatellite markers, included sticklebacks from the saltwater-influenced brook of the present study, and revealed that isolation by distance and by barriers, but not by habitat type (fresh vs. saltwater), explained most of the genetic variation of sticklebacks across different anthropogenically altered habitats (Scharsack et al. Citation2012). However, the saltwater impact of the brook started relatively recently (in the 1960s) and possible (genetic) segregation of salt/freshwater sticklebacks might not be obvious yet.
Three-spined sticklebacks have become an important model species to investigate effects of anthropogenic habitat alterations (Raeymaekers et al. Citation2008, Citation2009; Candolin Citation2009; Tuomainen et al. Citation2011; van der Sluijs et al. Citation2011; Candolin et al. Citation2014). Although G. aculeatus are hosts in many parasite life cycles with trophically transmitted stages (Kalbe et al. Citation2002), information on effects of anthropogenic habitat alterations on the parasite burden of sticklebacks is sparse (but see Heuschele & Candolin Citation2010).
For the present study, temperature and salinity might be relevant factors that influence the abundance of parasites. It is generally assumed that global warming increases parasite infection pressure on fish populations (Marcogliese Citation2008). Indeed, sticklebacks experimentally infected with the tapeworm Schistocephalus solidus had higher parasite burden at 20°C compared to 15°C (Macnab & Barber Citation2012). Also, in the wild, higher parasite burden was observed in sticklebacks collected from an area of an Icelandic lake with warm springs, compared to sticklebacks from cooler areas of the lake (Karvonen et al. Citation2013).
In the present study, sticklebacks (and their parasites) are exposed not only to temperature increase, but also to elevated salinity. It has been noted that naturally occurring salinity gradients from marine to brackish conditions are strong drivers of changes in parasite communities of fish (Thieltges et al. Citation2010). In three-spined sticklebacks, parasite communities are structured by geographical distance, but also by the local salinity conditions (Poulin et al. Citation2011). Thus, for the present study, changes of the parasite communities of sticklebacks due to the saltwater inlet might be expected. Here, only halotolerant parasites might be able to infest sticklebacks in the part of the brook that is influenced by saltwater. In addition, the saltwater-induced shift of the inhabiting invertebrate fauna (Kaschek & Aschmeier Citation1993; Schimmer & Schindler Citation2000) might deprive parasites with complex life cycles of intermediate hosts. This would also reduce infection pressure on inhabiting sticklebacks.
In aquaculture systems it is well established that salinity and temperature, as well as food composition, influence the growth performance and development of fish (e.g. Karås & Klingsheim Citation1997; Rosenlund et al. Citation2004; Imsland et al. Citation2007; Slawski et al. Citation2011). In experiments with three-spined sticklebacks, their growth performance was strongly influenced by temperature and the availability of food (Robinson & Wardrop Citation2002), but depended on the adaptive range of the stickleback origin. Generally, sticklebacks grow slower at low temperatures (< 8°C) and have growth optima between 10 and 20°C (Guderley et al. Citation2001; Lefébure et al. Citation2011). In contrast, several earlier studies have shown that freshwater-inhabiting sticklebacks grow faster and experience a wider variation in growth as compared to those inhabiting saltwater (McGuigan et al. Citation2011; Robinson Citation2013). This would certainly alter temperature-related growth expectations. However, local adaptation to permanent conditions within the adaptive range may benefit the growth of sticklebacks.
We therefore expected that sticklebacks below the inlet of warm and salty coal mine drainage water would grow faster compared to freshwater sticklebacks, if food is available in sufficient amounts.
We hypothesised that the continuous inflow of saltwater, producing brackish-water conditions with elevated temperature, provides fitness advantages for sticklebacks caused by higher water temperature, less predation by fish and less interspecific competition. Furthermore, we assume that sticklebacks in the saltwater habitat have fewer parasites, due to reduced diversity of (freshwater) intermediate hosts (Kaschek & Aschmeier Citation1993; Schimmer & Schindler Citation2000) with their limited tolerance to salinity variation. We hypothesise that the changes of habitat conditions by the inlet of warm and salty coal mine drainage water might cause a lower parasite burden of the inhabiting sticklebacks.
Materials and methods
The study area
The freshwater inland brook Ibbenbürener Aa is subjected to a constant inlet of warm and salty drainage water from a coal mine, close to the town of Ibbenbüren, North Rhine-Westphalia, in the North-West of Germany (52.28286°N, 7.65309°E; Scharsack et al. Citation2012). The continuous inflow raises the water level of the brook and is associated with a rise in water temperature and a prominent increase in salinity compared to the upstream freshwater condition of the brook.
The sampling sites were selected for high similarity in the river bed morphology and profile. The freshwater sampling site was a stretch of 100 m, located right upstream of the inlet of the coal mine drainage water, and is subsequently referred to as the freshwater site or freshwater habitat. The saltwater sampling site (or saltwater habitat), also a stretch of 100 m, was located 2 km waterline distance downstream of the inlet of the salty drainage water, after a culvert of the brook under an artificial watercourse. The distance between sampling sites was chosen to be far enough to exclude regular migration of the sticklebacks between sampling sites.
Collection of sticklebacks, water parameters and accompanying fauna
Sticklebacks were collected from April–October 2010 at both sampling sites with hand nets at intervals of 30 days. The sampling proceeded upstream against the current along the 100-m stretch of each sampling site. Collected sticklebacks were transferred into 20-L buckets with water from their specific habitat and provided with aeration via a mobile pump. In the laboratory the sticklebacks were transferred to 70 × 45 × 33 cm plastic tanks filled with 40 L of water taken from the sampling sites, and screened within 48 h after catchment. Maintenance of sticklebacks for up to 48 h might have influenced the distribution of ectoparasites and the stomach contents. However, since stickleback from the two sampling sites were kept in separate tanks, overall differences in these parameters between habitats are still valid and are described below.
Water temperature and conductivity were measured at the sampling sites just before netting as well as in the saltwater inlet, using a multiple-purpose sensor (Cond 330i-sensor, WTW, Germany). To monitor the accompanying ichthyofauna, minnow traps were set at each of the sampling sites after the July, August, September and October samplings, for a period of 3 days.
Dissections and parasite screen
Dissection of the sticklebacks and the parasite screen were always done within 48 h after capture, as described by Kalbe et al. (Citation2002) with some modifications. Briefly: The sticklebacks were anaesthetised and killed with an overdose of MS222 (1.5 g L−1), and transferred to a petri dish with saline (0.64% w/v NaCl). The body surface of the fish was screened for ectoparasites using a binocular. For parasites, which were observed in huge numbers per fish, such as Gyrodactylus sp. or Trichodina sp., infection classes were determined (Class 1: 0–9 parasites, Class 2: 10–49 parasites, Class 3: 50–99 parasites, and Class 4: > 100 parasites per fish).
The number of lateral plates was counted on each fish. Thereafter, sticklebacks were dried on paper towels and measured for standard length (from the tip of the snout to the base of the caudal fin) to the nearest mm and weighed to the nearest mg. The head was removed by a vertical cut behind the operculum and the body cavity was opened by two lateral cuts towards the anus. The gill arches were dissected out from the head and transferred onto a trichinoscope with some drops of saline. The intestine, liver, spleen, kidney and swim bladder were dissected out. Liver and spleen were weighed to the nearest mg. All organs were layered on the trichinoscope with saline, squeezed and screened for endo-parasites under a microscope. The gonads were taken out and weighed to the nearest mg, and the sex of the fish was determined and recorded. The eyes were dissected and screened for parasites. A drop of blood from the gills and from the tail stalk was screened under a microscope for flagellates. Remains of food in the stomach were recovered with saline and determined under a microscope, to reveal potential differences in food items between the habitats.
Calculation of growth rates and body condition parameters
Growth of the fish is presented by two growth rates (Hopkins Citation1992; Lugert et al. Citation2014). The relative growth rate (RGR) displays the weight increase in percent from the first to the last sampling time point. RGR was calculated with the following equation:
with wi as the average initial weight per sampling site and wt as the individual weight of specimen collected at the last sampling time point.
The specific growth rate (SGR) relies on an exponential relationship between time and growth. The results are given in percent increase per day. SGR %/d was calculated as:
where Ln is the natural logarithm, t the time in days and wi and wt as above, to calculate SGRs over the whole sampling period. To calculate monthly SGRs, wi was the average weight per sampling site of the previous month and wt was the individual weight of the next month.
The body condition index (BCI) was calculated as a ratio of observed weight and weight expected from the observed length (Frischknecht Citation1993), with the following equation:
with wt as weight in g, L as length in cm and b as the exponent of the length–weight relationship (LeCren Citation1951; Frischknecht Citation1993). The b value for the present study was calculated with data of all sticklebacks analysed, and equalled 3.073.
The hepatosomatic index (HSI) was calculated with the equation:
with wL as the liver weight in mg and wt as above.
Parasite infection data
For each parasite species, infection success was expressed as mean intensity (average number of parasites per infected fish) and prevalence (proportion of infected fish per sample). Additionally a parasite index was calculated for each parasite species (PIS) per stickleback as:
with Pt as the total number of parasites found on the stickleback and Pmax as the maximum number of this parasite per stickleback observed in the present study. An overall parasite index (PI) per fish was calculated as average of the index (PIS) calculated per parasite species.
Statistical analysis
Adult and juvenile sticklebacks were analysed as separate age classes. We only included samples where more than one stickleback was caught into the statistical analysis. Data that were not normally distributed were Box–Cox transformed to achieve normality. Due to the fact that stickleback samples from the respective cohorts (juvenile/adult) were not available in both habitats at each time point, a two-step analysis was performed: (1) data from samples where sticklebacks of the respective age classes were collected at both sampling sites were analysed with two-way analyses of variance (ANOVAs) with time and habitat as factors; (2) groups in which samples from additional samplings were available (e.g. juvenile stickleback in the saltwater in May) were additionally subjected to one-way ANOVAs (within cohort and habitat) to analyse changes over time. If ANOVAs were significant, significant differences between groups were analysed with post-hoc pairwise comparisons (Holm–Sidak). All analyses were performed using the statistical software Sigmaplot (Systat Software, Inc., 2010).
Results
Water temperature and conductivity
The flow rate of salty coal mine drainage in the connective ditch releasing the water into the brook (Ibbenbürener Aa) was about 0.4 m3 s−1, and daily or seasonal shifts of the flow rate were not observed throughout the present study. The temperature of the drainage water ranged from 15.9°C in April to 22.8°C in July, resulting in elevation of the water temperature of the brook by 5.5°C in April (1.6-fold), but only by 0.1°C in July (). The conductivity of the coal mine drainage water ranged from 39,700 to 48,900 µS cm−1, increasing that of the freshwater from < 1000 µS cm−1 to, at maximum 32,200 µS cm−1 (55.8 fold; ). Variations in the changes of conductivity in the saltwater habitat were attributed to seasonal variation in freshwater flow rates of the brook, with low flow rates in July and higher flow rates in August–September due to heavy rainfalls.
Table I. Water temperature and conductivity in the coal mine drainage water, above (fresh water) and 2 km below (salt water) the inlet of the drainage water and the fold change caused by the inlet. Samplings were performed monthly from April to October in 2010, each between 10 and 12 a.m.
Development of stickleback age classes
All sticklebacks investigated here had the leiurus form, with low (4–5) numbers of lateral plates, as typical for established freshwater populations of three-spined stickleback (Raeymaekers et al. Citation2005). Throughout the observation period only two age (size) classes of sticklebacks, namely juveniles (subsequently 0+) and adults (1+), were found. In April and May mostly adult (1+) sticklebacks, which were in breeding condition, were caught in the freshwater (n = 16 and 14) and in the saltwater (n = 20 and 21) habitats (see legend of for n per time points). Thereafter, catches of 1+ stickleback declined in both habitats, presumably caused by their death after the exhausting mating period, indicated by occasionally found cadavers. The decline of 1+ stickleback was earlier in the freshwater habitat, where only single 1+ stickleback were caught in June and August. In the saltwater habitat, numbers of 1+ sticklebacks declined from June (n = 10) to July (n = 5) to August (n = 1).
Figure 1. Body condition index (BCI, mean ± standard error) of adult 1+ (filled triangle) and juvenile 0+ (open triangle) sticklebacks from the 2010 samplings in (A) the freshwater habitat and (B) the saltwater habitat of the brook Ibbenbürener Aa; * = p < 0.05 between habitat at given time point, *) = p < 0.05 from previous time point.
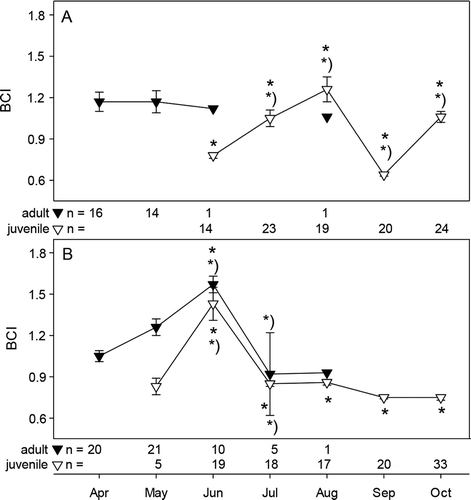
During the observation period a distinct shift of age (size) cohorts from 1+ adult stickleback to juvenile sticklebacks (0+) occurred in both habitats. While 0+ sticklebacks were present in the (warmer) saltwater habitat already in May (n = 5), the first 0+ sticklebacks (n = 15) occurred in the freshwater samplings in June (see legend of for n per time point).
Stickleback growth
Adult sticklebacks (1+) caught in April in the saltwater habitat were significantly larger and heavier than 1+ sticklebacks in the freshwater habitat (mean body length ± standard error: 64 ± 1 mm vs. 53 ± 3 mm; mean body weight: 3271 ± 178 mg vs. 2133 ± 302 mg). While 1+ sticklebacks did not increase in length and weight in the subsequent samples from the saltwater habitat, 1+ sticklebacks in the freshwater habitat grew significantly from April to May, up to 62 ± 2 mm in length and 3194 ± 300 mg in weight (to a similar range as saltwater 1+ in April).
Juvenile (0+) sticklebacks were caught earlier (May) in the saltwater habitat and were significantly heavier and longer at subsequent time points compared to freshwater 0+ sticklebacks (see for initial and final weights). The overall relative and specific growth rates (RGR and SGR) of juvenile sticklebacks from the salt water were higher compared to those of the freshwater sticklebacks (). This was biased by the earlier catch of still relatively small 0+ sticklebacks in the saltwater in May, which reached a higher average weight after 6 months compared to freshwater 0+ sticklebacks after 5 months at the end of the observation period (). However, the monthly SGR (%/d) was also generally higher in the saltwater compared to the freshwater habitat (). Negative SGR occurred from August to September in the freshwater and September to October in the saltwater habitat ().
Table II. Growth performance of juvenile sticklebacks in the Ibbenbürener Aa over the study period from April to October 2010: initial and final body wet weight (mean ± standard error), absolute growth, and relative and specific growth rates (RGR and SGR) of juvenile sticklebacks from both habitats over the whole observation period. Numbers of investigated specimens are given in parentheses (n).
Table III. Specific growth rates (SGR, %/d) of juvenile sticklebacks between monthly sampling intervals in the Ibbenbürener Aa in 2010. Numbers of investigated specimens are given in parentheses (n).
Body condition parameters
The body condition indices (BCI) of adult 1+ sticklebacks from the freshwater habitat did not change significantly during their main occurrence from April to May ( )). By contrast, the BCI of 1+ sticklebacks from the saltwater started at a similar level in April, but thereafter increased significantly with a peak in June, followed by a sharp decline in July ( )). The BCIs of juvenile (0+) sticklebacks differed significantly between the fresh- and the saltwater habitats throughout the observation period, as indicated by the maxima and minima at different time points ( ) and (b)). While the BCI of juvenile 0+ sticklebacks in the saltwater habitat had peaked already in June, at the same time as in saltwater adults, the BCI of freshwater 0+ sticklebacks increased until August, dropped significantly in September and recovered fast until October ().
The HSI of 1+ sticklebacks in the freshwater habitat increased from April to May, while it remained unchanged in saltwater adults until June and dropped thereafter (data not shown). The HSI of 0+ sticklebacks followed similar trends as the BCI with an early peak in June in saltwater 0+ and a sharp drop in September in freshwater 0+ sticklebacks (data not shown, for redundancy with BCI).
Parasites
In total 11 parasite species were detected in sticklebacks from the two habitats. With 10 species, parasite diversity was higher in the freshwater habitat, compared to the saltwater habitat where seven species were discovered. One parasite (Raphidascaris acus) was exclusively found in the saltwater habitat (). Glochidia (Unio sp.), leeches (Piscicola geometra), thorny-headed worms (Acanthocephalus lucii) and the eel swim bladder nematode (Anguillicola crassus), which uses sticklebacks as paratenic hosts (Thomas & Ollevier Citation1992), were exclusively found in freshwater sticklebacks. The most abundant parasite species in both habitats were Trichodina sp. and Gyrodactylus sp., both ectoparasites with monoxenic life cycles. A single parasite species, namely Contracaecum sp., occurred in both habitats at all sampling times. It also is the only parasite showing higher intensity in the saltwater habitat in the majority of samples.
Table IV. Parasite infection. Prevalence (%) and mean intensity (± standard error) of parasite species in the saltwater (salt) and freshwater (fresh) habitats of the Ibbenbürener Aa in 2010.
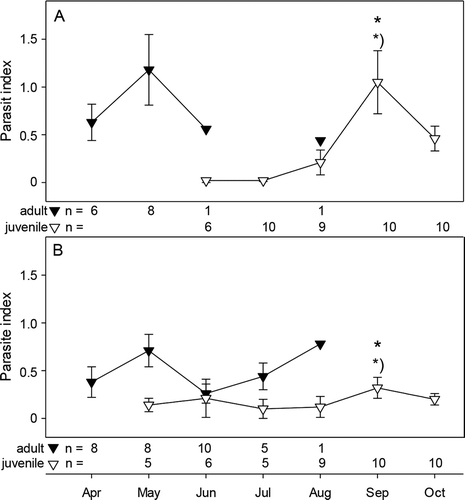
In both habitats prominent seasonal variation of parasite infections was observed, with high diversity, prevalence and mean intensities in May and September (). This was also reflected in the parasite index (PI), with a first peak in May in the adult 1+ sticklebacks and the second peak in September in 0+ juveniles (). In both peaks, the PI was higher in the freshwater habitat, but this was significant only between the juvenile sticklebacks in September.
Figure 2. Parasite index of adult (black triangle) and juvenile (open triangle) sticklebacks (mean ± standard error) collected in the Ibbenbürener Aa in 2010 from (A) the freshwater habitat and (B) the saltwater habitat; * = p < 0.05 between habitat at given time point, *) = p < 0.05 from previous time point.
Stickleback stomach content
Sticklebacks from the fresh- and saltwater habitats showed distinct differences in their stomach contents. Sticklebacks from the salt water, independent of their age and size, mainly fed on larvae of Chironomus sp. cf. halophilus, of which head capsules remained in the stomachs. The larvae had been observed previously in the riverbed of the saltwater-influenced part of the brook, in high numbers (Schimmer & Schindler Citation2000). There were also remains of crustaceans, presumably Gammarus tigrinus, which favours brackish conditions, but also immigrated into freshwater systems of North Rhine Westphalia (Kaschek & Aschmeier Citation1993).
The diet of the sticklebacks caught in the freshwater habitat above the inlet comprised a higher variety of prey items. Here the number of chironomid larvae was negligible and many more remains from other (undetermined) insect species were found. The amount of remains from crustaceans was similar in fresh and salt water, but most likely belonged to Gammarus pulex or Gammarus roeseli, which both inhabit the freshwater habitat, but not the saltwater habitat (Schimmer & Schindler Citation2000).
Accompanying ichthyofauna
Parallel sampling with minnow traps in the freshwater and saltwater habitats revealed almost exclusively three-spined sticklebacks (G. aculeatus) in the saltwater habitat, with the exception of one roach (Rutilus rutilus), which might have drifted down from an upstream lake (Aasee, Ibbenbüren) due to heavy rainfall in August. In the freshwater habitat, other fish species typical for lowland brooks, such as nine-spined stickleback (Pungitius pungitius, n = 1), river perch (Perca fluviatilis, n = 3), ruffe (Gymnocaphalus cernua, n = 3) and stone loach (Barbatula barbatula, n = 3), were caught.
Discussion
In the present study, three-spined sticklebacks (G. aculeatus) were investigated in an inland brook, where water conditions are changed prominently by a constant inflow of warm and salty coal mine drainage water. We hypothesised that euryhaline sticklebacks might grow faster and have fewer parasites in such an anthropogenically caused situation, and compared sticklebacks from upstream (fresh water) and downstream of the inflow (salt water) for body condition parameters and development of age (size) classes, and for parasite infections, from spring to autumn 2010.
Three-spined stickleback adults (1+) in breeding condition and juveniles (0+) were caught in considerable numbers in the freshwater and saltwater sites, indicating that (stable) reproducing stickleback populations are present at both sampling sites. In subsequent years G. aculeatus of both sexes in breeding condition were caught regularly in the salt-polluted part of the brook, supporting this assumption (J. P. Scharsack, personal observation).
Stickleback growth
Adult (1+) sticklebacks caught in April, at the beginning of the sampling period, were larger in the saltwater habitat compared to the freshwater site, and juvenile (0+) sticklebacks occurred 1 month earlier in the salt water. This suggests that sticklebacks in the saltwater habitat had grown faster over winter and early spring and matured and mated earlier, presumably due to the higher water temperature downstream of the inlet of warm and salty coal mine drainage water. The relative and specific growth rates (RGR and SGR) of 0+ sticklebacks were higher in the saltwater habitat. The overall RGRs and SGRs were biased by the earlier occurrence of 0+ sticklebacks in the saltwater, but also the monthly SGR of 0+ sticklebacks were mostly higher in saltwater 0+ sticklebacks. This suggests that growth conditions were more favourable for sticklebacks in the warmer water. A negative growth impact due to higher salinity, as observed in other studies (McGuigan et al. Citation2011; Robinson Citation2013), could not be documented. Also, a larger variation in growth in fresh water as described by Robinson (Citation2013) was not supported by our data. This might have several causes. On the one hand, salinity differences in the study area are not as distinct as between freshwater and true marine habitats with approximately 35 ppt. salinity. Accordingly, growth deficits related to salinity might be overlain by the overall benefit of temperature. Also, the parasite burden and the associated loss in energy might favour growth conditions in the salt water.
Juvenile sticklebacks in the fresh water grew generally slower than the juveniles in the salt water, which might be attributed to colder water temperature, inter- and intraspecific competition for food, and higher parasite burden. Fish species other than three-spined stickleback, which are likely competitors for food (Göcke et al. Citation2013) and/or predators of three-spined sticklebacks, were caught almost exclusively in the freshwater part of the brook. Negative SGR from August to September in the freshwater habitat and September to October in the saltwater habitat might be attributed to erosion of food items and elevated physical activity, due to high water current caused by heavy rain falls in August.
The strong increase of the body condition index (BCI) of 1+ and 0+ saltwater sticklebacks until June supports the assumption that food (and temperature) conditions were more favourable in the salt water, at least in the early season. We are aware that increasing gonad weight in gravid females can potentially bias BCI and HIS values. However, as sex ratio was mainly even at sampling times and between locations, this would hold true for both locations likewise. As spawning starts earlier in the saltwater, biased BCI due to gonads in May and June would indicate an elongated reproduction cycle with multiple spawnings, which in return shows greater fitness of the animals.
The drop of BCI in the salt water after June suggests that conditions in salt water became less favourable here, possibly caused by resource limitation due to intraspecific competition for food (Wootton Citation1984). The main food sources of juvenile sticklebacks in the saltwater habitat were the numerous chironomid larvae, which matured into adult dipterans in late summer and thereby decreased the food availability for the saltwater sticklebacks.
In addition, elevated temperature might have contributed to the reduction in BCI of saltwater sticklebacks. Temperature experiments have shown that sticklebacks grow less and have lower BCI at temperatures above 20°C, presumably caused by higher metabolic rates and reduced energy storage (Guderley et al. Citation2001; Lefébure et al. Citation2011; Dittmar et al. Citation2014; Shama & Wegner Citation2014). In the present study, temperatures > 20°C were observed in July in both habitats, but negative effects on BCI might have been more prominent in the salt water due to the constant inlet of the warm drainage water. Interestingly, the drop of BCI in the fresh water in September coincided with a peak in the parasite index, which was more prominent in freshcompared to salt water. Higher parasite burden might have contributed to the reduction of BCI in the fresh water.
Parasite infections
Typical saltwater (marine) parasite species, which might have been introduced by bird migration to the saltwater site, were not observed. However, many marine parasites do not tolerate brackish conditions (Moller Citation1978) as present in the saltwater part of the brook. In the saltwater-influenced site of the brook, the diversity and frequency of (freshwater) parasite species was lower compared to in the freshwater habitat, which might be explained by the intolerance to increased salinity of the majority of parasite species detected here, as well as by the absence of appropriate intermediate hosts.
In sticklebacks from the freshwater habitat a total of 10 parasite species were detected, while only six of these species were present in the saltwater habitat. The four species not detected in the salt water were glochidia, Piscicola geometra, Acanthocephalus lucii and Anguillicoloides crassus. Glochidia of unionid mussels cannot tolerate salinity (Gillis Citation2011) and were absent in the saltwater habitat. The host-unspecific freshwater leech P. geometra can tolerate brackish conditions (Hajduk & Hajduk Citation1984), but only for short times, and adverse effects of salinity on this parasite species are more prominent at elevated temperatures (Moller Citation1978), which presumably explains its absence in the saltwater habitat. Correspondingly, the prevalence of blood flagellates (Trypanosoma sp.), which are transmitted by fish-biting leeches, was higher in the fresh water. Occurrence of a Trypanosoma sp.-infected stickleback in the saltwater habitat in September might have been caused by drift from the freshwater habitat due to high current.
The thorny-headed worm A. lucii depends on Asselus aquaticus as first intermediate host (Kracke Citation1975), which is an obligatory freshwater organism, and consequently A. lucii was absent in the saltwater habitat. The swim bladder nematode A. crassus was detected only once in the fresh water. Its infection is presumably not bound to freshwater conditions, since it uses a number of crustacean species as first and fish as paratenic hosts, among those the three-spined stickleback (Thomas & Ollevier Citation1992; Krobbach et al. Citation2007).
The only parasite that was observed exclusively in the saltwater habitat was Raphidascaris acus. This endoparasite parasitises several fish species, and its intermediate host is either a copepod or an insect nymph (Moravec Citation1970). Given the high number of chironomid larvae in the salt water and the fact that sticklebacks in the salt water mainly fed on this prey, an infection with R. acus seems possible.
Endoparasites that were present at all sampling time points and in both habitats were nematodes of the genus Contracaecum sp. It also is the only parasite showing higher intensity in the saltwater habitat in the majority of samples. Parasites of this genus are present in stickleback and other fish species over a wide salinity range, from marine to fresh water (Dartnall & Walkey Citation1979; Køie Citation1999), and were tolerant to experimental variation of salinity (Moller Citation1978). Their dominant presence might be explained by their distinct life stages and intermediate hosts, namely copepods. Copepods act as intermediate hosts for a variety of fish parasites. Parasites using this intermediate host are therefore able to infect even young individuals who feed on comparatively small prey items. As their final host is a bird, Contraceacum sp. can easily be transferred over large areas and into multiple habitats. We assume that higher infection pressure in the saltwater site is a result of limited variance in prey items for juvenile sticklebacks. The infection chance and pressure accordingly rises as fish mainly fed on potential intermediate hosts. The general assumption that global warming increases parasite infection pressure on fish populations can accordingly be linked to specific parasites, since they, or their intermediate hosts, can tolerate or even benefit from such a temperature increase. Accordingly, shifts in the food web due to temperature changes, as in the present study, can favour parasite infection and increase parasite pressure.
Ectoparasites of the genus Gyrodactylus sp. are well known for their tolerance to different levels of salinity (Dartnall & Walkey Citation1979; Marcogliese Citation1995), and were regularly recorded at both sampling sites during the present study. The ectoparasite Trichodina sp. is moderately tolerant to increased salinity (Dartnall Citation1974) and, in the present study, was detected more regularly and in higher frequencies in sticklebacks from the freshwater habitat. Overall, the shift from freshwater to saltwater (brackish) conditions seems to reduce parasite infection pressure for inhabiting sticklebacks, which might contribute to the higher growth rates of sticklebacks in the saltwater habitat.
General discussion
Although sticklebacks in the saltwater site seem to have advantages regarding growth and parasite burden, we are uncertain whether a true distinction between specific habitat ecotypes permanently exists. The impression of distinct ecotypes evolving on the basis of adaptive divergence was not supported by a population genetics study using a neutral microsatellite marker (Scharsack et al. Citation2012). Although sticklebacks have a very short generation interval, and are known for their potential for fast adaptive divergence, the saltwater impact of the brook started relatively recently, and possible divergence of salt/freshwater sticklebacks might not be obvious yet.
We suppose that migratory movements within the entire area lead to a steady mix in population structure. However, animals hatching in certain habitats, like the studied saltwater site, gain advantages as they hatch earlier and benefit from warmer temperatures, abundance of food, less predation by fish and lower parasite burden. We accordingly assume that survival rates in these juveniles are considerably higher. As individual fish from such strong and big cohorts grow, they might seek new territories and migrate into surrounding habitats due to resulting resource limitations (Wootton Citation1984). It might also be possible that some adult individuals are triggered to migrate from the saltwater into the freshwater habitat with the beginning of the breeding season, just as their coastal relatives do (Daniel Citation1985; Raeymaekers et al. Citation2005).
In contrast, the present study might also suggest that adult individuals actively migrate downstream during wintertime as temperature is more favourable. As sticklebacks also inhabit almost all water bodies of the region, it seems likely that heavy rainfalls, such as those recorded in August–September, with increased flowrates, frequently drift individuals downstream, where they establish. It is yet unknown whether migration and habitat switch is actively used by sticklebacks, to benefit from (seasonal) advantages of the respective habitats (e.g. lower parasite pressure, less intraspecific competition).
Summary
In summary, prominent differences in body condition and parasite load were observed between sticklebacks from above and below the inlet of warm and salty drainage water from a coal mine. In the salt water, sticklebacks reproduced earlier and grew faster compared to those in the freshwater site above the inlet. Furthermore, due to the changed water conditions, the parasite infection pressure differs between the habitats, resulting in lower parasite burden of sticklebacks in the saltwater habitat. Although the inflow of warm and salty coal mine drainage water is detrimental for many inhabiting freshwater species, the euryhaline three-spined stickleback seems to take advantage of higher temperatures, less interspecific competition and lower parasite infection pressure in the salt-polluted part of the brook.
Acknowledgements
We are grateful to the angling club of the town Ibbenbüren for their support of the present study and the catching permits. The present study was funded by the German Science Foundation (DFG, grant # SCHA 1257/2-1). The sampling was conducted with the approval of the local animal welfare authorities.
Additional information
Funding
References
- Campeau S , Guderley H , Fitzgerald G. 1984. Salinity tolerances and preferences of fry of two species of sympatric sticklebacks: Possible mechanisms of habitat segregation. Canadian Journal of Zoology 62:1048–1051. DOI:10.1139/z84-150.
- Candolin U. 2009. Population responses to anthropogenic disturbance: Lessons from three-spined sticklebacks Gasterosteus aculeatus in eutrophic habitats. Journal of Fish Biology 75:2108–2121. DOI:10.1111/jfb.2009.75.issue-8.
- Candolin U , Nieminen A , Nyman J . 2014. Indirect effects of human-induced environmental change on offspring production mediated by behavioural responses. Oecologia 174:87–97. DOI:10.1007/s00442-013-2752-2.
- Cheek CA , Taylor CM . 2015. Salinity and geomorphology drive long-term changes to local and regional fish assemblage attributes in the lower Pecos River, Texas. Ecology of Freshwater Fish 3:340–351.
- Daniel W . 1985. Questions on the migratory behavior of the threespine stickleback Gasterosteus aculeatus . Faunistisch-Oekologische Mitteilungen 5:419–430.
- Dartnall HJ . 1974. The salinity tolerance of trichodinids (Protozoa) parasitic on the three-spined stickleback Gasterosteus aculeatus . Journal of Zoology 172:207–214. DOI:10.1111/j.1469-7998.1974.tb04102.x.
- Dartnall HJG , Walkey M . 1979. Parasites of marine sticklebacks. Journal of Fish Biology 14:471–474. DOI:10.1111/jfb.1979.14.issue-5.
- DeFaveri J , Merilä J . 2014. Local adaptation to salinity in the three-spined stickleback? Journal of Evolutionary Biology 27:290–302. DOI:10.1111/jeb.2014.27.issue-2.
- Dittmar J , Janssen H , Kuske A , Kurtz J , Scharsack JP . 2014. Heat and immunity: An experimental heat wave alters immune functions in three-spined sticklebacks (Gasterosteus aculeatus). Journal of Animal Ecology 83:744–757. DOI:10.1111/1365-2656.12175.
- Franssen NR , Harris J , Clark SR , Schaefer JF , Stewart LK . 2013. Shared and unique morphological responses of stream fishes to anthropogenic habitat alteration. Proceedings of the Royal Society B: Biological Sciences 280:20122715. DOI:10.1098/rspb.2012.2715.
- Frischknecht M . 1993. The breeding colouration of male three-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigour. Evolutionary Ecology 7:439–450. DOI:10.1007/BF01237640.
- Gillis PL . 2011. Assessing the toxicity of sodium chloride to the glochidia of freshwater mussels: Implications for salinization of surface waters. Environmental Pollution 159:1702–1708. DOI:10.1016/j.envpol.2011.02.032.
- Göcke C , Kaschek N , Meyer EI . 2013. Diet of fishes in a detritus-based sandy lowland brook. Limnologica – Ecology and Management of Inland Waters 43:451–459. DOI:10.1016/j.limno.2013.02.002.
- Gravenmier JJ , Johnston DW , Arnold WR . 2005. Acute toxicity of vanadium to the threespine stickleback, Gasterosteus aculeatus . Environmental Toxicology 20:18–22. DOI:10.1002/(ISSN)1522-7278.
- Guderley H , Leroy PH , Gagné A . 2001. Thermal acclimation, growth, and burst swimming of threespine stickleback: Enzymatic correlates and influence of photoperiod. Physiological and Biochemical Zoology 74:66–74. DOI:10.1086/319313.
- Hajduk D , Hajduk Z . 1984. The hirudinean fauna of the brackish lake Resko Przymorskie Poland. Wiadomosci Parazytologiczne 30:643–648.
- Heuschele J , Candolin U . 2010. Reversed parasite-mediated selection in sticklebacks from eutrophied habitats. Behavioral Ecology and Sociobiology 64:1229–1237. DOI:10.1007/s00265-010-0937-9.
- Hopkins KD . 1992. Reporting fish growth: A review of the basics. Journal of the World Aquaculture Society 23:173–179. DOI:10.1111/jwas.1992.23.issue-3.
- Imsland AK , Schram E , Roth B , Schelvis-Smit R , Kloet K . 2007. Improving growth in juvenile turbot (Scophthalmus maximus Rafinesque) by rearing fish in switched temperature regimes. Aquaculture International 15:403–407. DOI:10.1007/s10499-007-9099-9.
- Jakobsen P , Klepaker T . 1985. Freshwater tolerance in the trachurus form of three-spined stickleback Gasterosteus aculeatus in West Norway. Fauna Norvegica Series A 6:9–12.
- Kalbe M , Wegner KM , Reusch TBH . 2002. Dispersion patterns of parasites in 0+ year three-spined sticklebacks: A cross population comparison. Journal of Fish Biology 60:1529–1542. DOI:10.1111/jfb.2002.60.issue-6.
- Karås P , Klingsheim V . 1997. Effects of temperature and salinity on embryonic development of turbot (Scophthalmus maximus L.) from the North Sea, and comparisons with Baltic populations. Helgoländer Meeresuntersuchungen 51:241–247. DOI:10.1007/BF02908710.
- Karvonen A , Kristjánsson BK , Skúlason S , Lanki M , Rellstab C , Jokela J . 2013. Water temperature, not fish morph, determines parasite infections of sympatric Icelandic threespine sticklebacks (Gasterosteus aculeatus). Ecology and Evolution 3:1507–1517. DOI:10.1002/ece3.2013.3.issue-6.
- Kaschek N , Aschmeier C . 1993. Gewässergütebericht der Stadt Ibbenbüren 1992/93, 2 Bände. Münster: Institut für Spezielle Zoologie.
- Køie M . 1999. Metazoan parasites of flounder Platichthys flesus (L.) along a transect from the southwestern to the northeastern Baltic Sea. Ices Journal of Marine Science 56:157–163. DOI:10.1006/jmsc.1999.0463.
- Kracke HJ . 1975. Zur Parasitenfauna von Stichlingen (Gasterosteidaei; Pisces). Wissenschaftliche Arbeit zur Erlangung der Doktorwürde, University of Münster. Doctoral thesis, University of Münster.
- Krobbach CK , Kalbe M , Kurtz J , Scharsack JP . 2007. Infectivity of two nematode parasites, Camallanus lacustris and Anguillicola crassus, in a paratenic host, the three-spined stickleback Gasterosteus aculeatus . Diseases of Aquatic Organisms 74:119–126. DOI:10.3354/dao074119.
- Kume M , Kuwahara T , Arai T , Okamoto M , Goto A . 2006. A part of the Japan Sea form of the threespine stickleback, Gasterosteus aculeatus, spawns in the seawater tidal pools of western Hokkaido Island, Japan. Environmental Biology of Fishes 77:169–175. DOI:10.1007/s10641-006-9068-6.
- LeCren ED . 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). The Journal of Animal Ecology 20:201–219. DOI:10.2307/1540.
- Lefébure R , Larsson S , Byström P . 2011. A temperature-dependent growth model for the three-spined stickleback Gasterosteus aculeatus. Journal of Fish Biology 79:1815–1827. DOI:10.1111/jfb.2011.79.issue-7.
- Li J , Kueltz D . 2012. Response of a marine population of three-spine sticklebacks (Gasterosteus aculeatus) to salinity stress. Faseb Journal 26(1 Supplement):1070.5.–1070.5.
- Lugert V , Thaller G , Tetens J , Schulz C , Krieter J . 2014. A review on fish growth calculation: Multiple functions in fish production and their specific application. Reviews in Aquaculture 6:1–13.
- Macnab V , Barber I . 2012. Some (worms) like it hot: Fish parasites grow faster in warmer water, and alter host thermal preferences. Global Change Biology 18:1540–1548. DOI:10.1111/j.1365-2486.2011.02595.x.
- Marcogliese DJ . 1995. Comparison of parasites of mummichogs and sticklebacks from brackish and fresh-water ponds on Sable Island, Nova-Scotia. American Midland Naturalist 133:333–343. DOI:10.2307/2426398.
- Marcogliese DJ . 2008. The impact of climate change on the parasites and infectious diseases of aquatic animals. Revue Scientifique et Technique-Office International des Epizooties 27:467–484. DOI:10.20506/rst.27.2.1820.
- McGuigan K , Nishimura N , Currey M , Hurwit D , Cresko WA . 2011. Cryptic genetic variation and body size evolution in threespine stickleback. Evolution 65:1203–1211. DOI:10.1111/evo.2011.65.issue-4.
- Moller H . 1978. The effects of salinity and temperature on the development and survival of fish parasites. Journal of Fish Biology 12:311–323. DOI:10.1111/jfb.1978.12.issue-4.
- Moravec F . 1970. On life history of Raphidascaris acus (Bloch, 1779) in natural environment of river Bystrice, Czechoslovakia. Journal of Fish Biology 2:313–322. DOI:10.1111/j.1095-8649.1970.tb03290.x.
- Poulin R , Blanar CA , Thieltges DW , Marcogliese DJ . 2011. The biogeography of parasitism in sticklebacks: Distance, habitat differences and the similarity in parasite occurrence and abundance. Ecography 34:540–551. DOI:10.1111/j.1600-0587.2010.06826.x.
- Raeymaekers JAM , Konijnendijk N , Larmuseau MHD , Hellemans B , De Meester L , Volckaert FAM . 2014. A gene with major phenotypic effects as a target for selection vs. homogenizing gene flow. Molecular Ecology 23:162–181. DOI:10.1111/mec.12582.
- Raeymaekers JAM , Maes GE , Audenaert E , Volckaert FAM . 2005. Detecting Holocene divergence in the anadromous-freshwater three-spined stickleback (Gasterosteus aculeatus) system. Molecular Ecology 14:1001–1014. DOI:10.1111/j.1365-294X.2005.02456.x.
- Raeymaekers JAM , Maes GE , Geldof S , Hontis I , Nackaerts K , Volckaert FAM . 2008. Modeling genetic connectivity in sticklebacks as a guideline for river restoration. Evolutionary Applications 1:475–488. DOI:10.1111/j.1752-4571.2008.00019.x.
- Raeymaekers JAM , Raeymaekers D , Koizumi I , Geldof S , Volckaert FAM . 2009. Guidelines for restoring connectivity around water mills: A population genetic approach to the management of riverine fish. Journal of Applied Ecology 46:562–571. DOI:10.1111/j.1365-2664.2009.01652.x.
- Robinson B , Wardrop S . 2002. Experimentally manipulated growth rate in threespine sticklebacks: Assessing trade offs with developmental stability. Environmental Biology of Fishes 63:67–78. DOI:10.1023/A:1013820101348.
- Robinson BW . 2013. Evolution of growth by genetic accommodation in Icelandic freshwater stickleback. Proceedings of the Royal Society B: Biological Sciences 280:20132197. DOI:10.1098/rspb.2013.2197.
- Rosenlund G , Karlsen O , Tveit K , Mangor-Jensen A , Hemre G-I . 2004. Effect of feed composition and feeding frequency on growth, feed utilization and nutrient retention in juvenile Atlantic cod, Gadus morhua L. Aquaculture Nutrition 10:371–378. DOI:10.1111/anu.2004.10.issue-6.
- Scharsack JP , Schweyen H , Schmidt AM , Dittmar J , Reusch TBH , Kurtz J . 2012. Population genetic dynamics of three-spined sticklebacks (Gasterosteus aculeatus) in anthropogenic altered habitats. Ecology and Evolution 2:1122–1143. DOI:10.1002/ece3.232.
- Schimmer H , Schindler A . 2000. Die Güteentwicklung der Ems von 1969 bis 1999. Ministerium für Umwelt und Naturschutz, Landwirtschaft und Verbraucherschutz des Landes Nordrhein-Westfalen. Special report. The Ministry for Environment, Agriculture, Conservation and Consumer Protection of the State of North Rhine-Westphalia.
- Schulte PM . 2013. Evolution of tolerance to multiple interacting stressors in fish. Integrative and Comparative Biology 53:E191–E191.
- Shama LNS , Wegner KM . 2014. Grandparental effects in marine sticklebacks: Transgenerational plasticity across multiple generations. Journal of Evolutionary Biology 27:2297–2307. DOI:10.1111/jeb.12490.
- Slawski H , Adem H , Tressel RP , Wysujack K , Kotzamanis Y , Schulz C . 2011. Replacement of fish meal with rapeseed protein concentrate in diets fed to turbot (Psetta maxima L.). Zuchtungskunde 83:451–460.
- Taugbøl A , Arntsen T , Østbye K , Vøllestad LA , Laudet V . 2014. Small changes in gene expression of targeted osmoregulatory genes when exposing marine and freshwater threespine stickleback (Gasterosteus aculeatus) to abrupt salinity transfers. Plos ONE 9:e106894–e106894. DOI:10.1371/journal.pone.0106894.
- Thieltges DW , Dolch T , Krakau M , Poulin R . 2010. Salinity gradient shapes distance decay of similarity among parasite communities in three marine fishes. Journal of Fish Biology 76:1806–1814. DOI:10.1111/jfb.2010.76.issue-7.
- Thomas K , Ollevier F . 1992. Pratenic hosts of the swimbladder nematode Anguillicola crassus . Diseases of Aquatic Organisms 13:165–174. DOI:10.3354/dao013165.
- Tuomainen U , Sylvin E , Candolin U . 2011. Adaptive phenotypic differentiation of courtship in response to recent anthropogenic disturbance. Evolutionary Ecology Research 13:697–710.
- van der Sluijs I , Gray SM , Amorim MCP , Barber I , Candolin U , Hendry AP , Krahe R , Maan ME , Utne-Palm AC , Wagner H-J , Wong BBM . 2011. Communication in troubled waters: Responses of fish communication systems to changing environments. Evolutionary Ecology 25:623–640. DOI:10.1007/s10682-010-9450-x.
- Wootton RJ . 1984. A functional biology of sticklebacks. Berkeley: University of California Press.
