?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The population structure of Jujubinus striatus and Jujubinus exasperatus (Trochidae), common gastropod grazers of Posidonia oceanica leaves, was investigated in the seagrass bed off Lacco Ameno of Ischia (Gulf of Naples). Sampling was performed monthly, from July 1981 to June 1982, at five stations along a depth gradient (1, 3, 10, 15 and 25 m), using a hand-towed net. Throughout the study period, both populations were characterised by seasonal fluctuations in abundance, with a total of 550 individuals counted and measured. The two populations analysed showed different distributional trends along the depth gradient. Jujubinus striatus settled and occurred mainly at shallower stands (1–3 m depth), while J. exasperatus was found deeper (10–15 m). Both species are semelparous. Juveniles and small individuals (shell height < 4 mm) were mainly found during the late winter and early spring months. The survivorship curves of both Jujubinus species showed a very low mortality rate in the first few months after settlement. Demographic analysis suggests an indeterminate growth rate after sexual maturity, with a lifespan of about 22 months for J. striatus and 17 months for J. exasperatus. For both species the growth rate is fast during the first couple of months after settlement. The findings of this study, compared with earlier work, reveal a certain number of shared traits in the life cycle of the most common gastropod species living on the leaves of P. oceanica seagrass: semelparous reproduction, spring settlement, fast early growth and short lifespan (2 years maximum).
Introduction
Posidonia oceanica (L.) Delile represents the most common and widespread seagrass of the Mediterranean Sea. In the last 50 years, the biology and ecology of P. oceanica seagrass have been widely studied and, given its ecology-relevant services (e.g. new colonisable surfaces Casola et al. Citation1987; high primary production, Pergent et al. Citation1994; nursery, Guidetti Citation2000; etc.), it has been identified as the only priority marine habitat in the European Directive 92/43/EEC. The role played by P. oceanica seagrass beds is considered of paramount importance for the coastal Mediterranean food webs (Cinelli et al. Citation1984; Mazzella & Zupo Citation1995; Buia et al. Citation2000).
Gastropod molluscs are among the principal components of the vagile fauna living on the Posidonia leaf canopy (Scipione et al. Citation1983; Gambi et al. Citation1992; Mazzella et al. Citation1992). This fauna is mainly represented by herbivorous species, which graze on epiphytic diatoms, as well as on algal community, represented by filamentous and calcareous algae encrusting on the leaf surface of the plant (Peduzzi & Herndl Citation1986; Mazzella & Russo Citation1989; Gacia et al. Citation2009). The family Trochidae is a relevant taxonomic group of P. oceanica beds, which, together with the genus Gibbula the genus Jujubinus, is a principal representative. Nevertheless, Jujubinus species are also very frequent and abundant in different vegetated marine habitats, such as those formed by other important seagrass species (e.g. Zostera marina and Cymodocea nodosa: Terlizzi & Russo Citation1997; Rueda et al. Citation2009; Marina et al. Citation2012) and by photophilic macroalgae (Cystoseira spp. and Caulerpa prolifera: Chemello & Milazzo Citation2002; Rueda & Salas Citation2003; Pitacco et al. Citation2014).
To date, although several studies have been undertaken to investigate the molluscs inhabiting the Posidonia life stratum in terms of species composition, community ecology (e.g. temporal dynamics and diel variations) and trophic habits, studies on demography and population structure are still fairly scant.
Here, the two species Jujubinus striatus (Linnaeus, 1758) and Jujubinus exasperatus (Pennant, 1777) are considered and analysed for their respective monthly variations in abundance, population size and age structure, growth and survival rates.
As concerns J. striatus species living in Mediterranean Posidonia beds, relevant information has been provided by studies on feeding and the early life cycle, by Peduzzi and Herndl (Citation1986) and Aricò et al. (Citation1992) respectively. More recently Hily et al. (Citation2004) and Rueda et al. (Citation2008) have discussed the same two aspects in populations living in the Z. marina seagrass of the northern Europe and north-west Mediterranean seas, respectively. According to Rueda et al. (Citation2008), J. striatus represents the dominant epiphyte gastropod of the Zostera bed of the southern Spain, with a density of about 30 ind./m2 crawling on Zostera leaves. In this latter seagrass habitat, the species feeds preferentially on epiphytes growing on the apical part of the seagrass leaves, and has a long lifespan with several reproductive events during a year.
As for J. exasperatus, the uniquely available information concerns diel variation of abundances, and food preferences for diatoms and encrusting algae growing on seagrass leaves (Russo et al. Citation1984a; Takada et al. Citation1999).
The objective of this study is to perform for the first time a demographic analysis on the populations of these very common seagrass-associated snails, using the heretofore unique set of data on molluscs collected monthly more than 30 years ago along a depth gradient in a P. oceanica bed (Russo et al. Citation1991). The present study represents the third step in the analysis of the data set, concerning the population ecology of the main species of gastropods living in a Mediterranean Posidonia oceanica seagrass beds, after those carried out on the Cerithiidae Bittium latreillii (Russo et al. Citation2002) and the Trochidae Gibbula ardens and G. umbilicaris (Donnarumma et al. Citation2016).
Materials and methods
Study area
Lacco Ameno Bay is located along the northwestern coast of the Ischia Island (Gulf of Naples, Italy; 40°45’30”N, 13°53’19”E). Here, a wide and continuous P. oceanica bed reached a depth of about 30 m. From the more superficial meadows (5 m) to the deepest one (30 m) the shoot density value changed from about 550 shoot/m2 to 37 shoot/m2 (Mazzella et al. Citation1989).
Sampling procedures
Samples of Jujubinus species were collected monthly from July 1981 to June 1982, at five stations located along a depth gradient (1, 3, 10, 15, 25 m). In each station, using a hand-towed net (400 μm mesh size) with a rectangular mouth of 40 × 20 cm, 60 consecutive strokes were performed on the Posidonia life canopy to allow the capture of whole vagile fauna composed by crawling and climbing species (Russo et al. Citation1985, Citation1986). Individuals of J. striatus and J. exasperatus, collected along a belt transect 50 m long, were separated under magnification and their shell height and width were measured.
Data analysis
For each station, monthly variation in abundance of J. striatus and J. exasperatus were obtained. Through measurements of shell height, size–frequency histograms (size classes of 1 mm) were generated.
For the identification of cohorts by resolution of the polymodal distribution into normal components, the Bhattacharya method (1967) was applied. Analyses were performed by means of the ELEFANT I routine of FISAT II (FAO-ICLARM Stock Assessment Tools II; Gayanilo et al. Citation1996).
It is likely that in the previous works, concerning the population ecology of other common gastropod species in P. oceanica beds (Russo et al. Citation2002; Donnarumma et al. Citation2016), time-specific survivorship curves (the best approximation of an age-specific survivorship curve) were constructed from a size–frequency distribution by plotting the relative density values of each size class (Dodd & Stanton Citation1990). Values were obtained by subtracting all the members of a single class from the total number of specimens, and repeating this procedure for each class. These members are the specimens that do not survive beyond that class. The equation for calculating points on the survivorship curve is:
where lx is the percentage of the population surviving at the end of class x, Nt is the total number of individuals in the population considered and (× Ni) is the sum of the number of individuals in all classes between 1 and x.
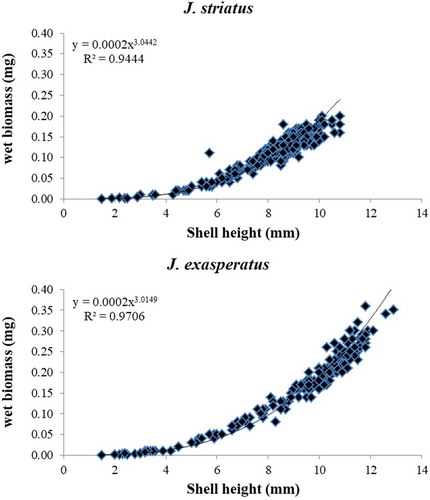
For each species, the individual wet weight was measured using a Mettler AE200 balance (0.001 mg precision), and then the biomass (mg) was compared with shell height to obtain a correlation curve.
Results
Descriptive analysis of populations
A total of 550 individuals were counted and measured. Of these, 369 (67%) belonged to Jujubinus striatus and 181 to Jujubinus exasperatus. Monthly variations of abundance of the two Jujubinus species along the whole transect are shown in . Both species showed three main peaks, at the same periods through the year. For J. striatus, the highest peak occurred in June (51 individuals), the second in November (45 individuals), and the third in February (33 individuals), while the minimum value was found in December (16 individuals). For J. exasperatus, the occurrence of the three peaks was similar: a first peak in May–July (20.33 ± 1.15 individuals), a second in November (24 individuals) and a third in February (21 individuals), while the lowest abundance occurred in October (six individuals).
However, the two species showed different distribution patterns of dominances, constant over time, in relation to the depth gradient (). In fact, J. striatus had higher abundance values in the shallow stands of the P. oceanica bed (1–3 m), where 73% of total individuals were found. The species was particularly abundant during the summer (26 individuals at 1 m in June–July and 33 individuals at 3 m in August).
On the contrary, even though J. exasperatus was found in shallow stands (11% of the total specimens), its higher abundance occurred at intermediate depths (10–15 m), where 80% of total individuals were sampled. The species was particularly abundant during winter, with a maximum abundance of 20 individuals in February at a depth of 10 m. Both species also occurred at the deepest stand (25 m): J. striatus was represented by 5% of overall specimens, while J. exasperatus accounted for 9%.
Population structure
Annual variations in the size–frequency structure of J. striatus and J. exasperatus populations are shown in and , respectively. The shell height of J. striatus ranges from a minimum of 1.5 mm to a maximum of 10.8 mm. The shell height of J. exasperatus, in contrast, ranged from 1.5 to 12.9 mm. Juveniles and small individuals (less than 4 mm in shell height) of both Jujubinus species occurred mainly during the cold and early warm periods – January–June for J. striatus and January–May for J. exasperatus – while adults were recorded every month. However, even though juveniles were recorded over a long time, both species showed a well-structured second cohort of juveniles in March, joining the single cohort of adults present in the previous month. Also, the most frequent size classes over the whole year were 8–9 mm (16.9%) for J. striatus and 10–11 mm (19.35%) for J. exasperatus.
Growth patterns and survivorship curves
Growth curves of both Jujubinus species are shown in . Jujubinus striatus () mainly settled in March and showed a lifespan of about 22 months. The growth rate of shell height constantly increased from the month of settlement, when a modal size of juveniles is of about 2.75 mm, to July, when the species reached a modal size of 6.74 mm (shell height increment of about 0.99 mm/month). The growth rate was lower in the following months (about 0.14 mm/month). One year after settlement specimens reached a modal size of about 8 mm, while the maximum value recorded was 9.36 mm. Similarly, J. exasperatus settled in March, although it showed a shorter lifespan, of about 17 months (). The growth rate of shell height for this species also rapidly increased during the warm months, from a modal size of about 2 mm in the month of settlement to 9.50 mm in August (shell height increment of about 1.5 mm/month). After that, the growth rate was lower in the following cold months (increment of about 0.12 mm/month). One year after settlement specimens reached a modal size of about 10 mm, while the maximum value recorded was 10.92 mm.
Figure 1. Annual variations in abundance (no. individuals) of Jujubinus striatus and Jujubinus exasperatus for each month, obtained by pooling data from all sampled depths.
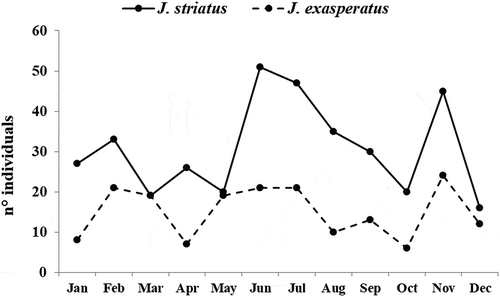
Figure 2. Monthly variations in abundance (no. ind./20 m2) of Jujubinus striatus and Jujubinus exasperatus, at each sampled depth.
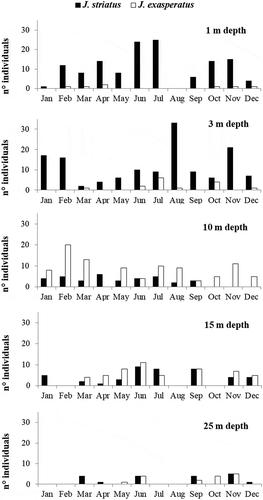
Figure 3. Size-frequency distributions of Jujubinus striatus, with the number of collected specimens (n), from the whole transect.
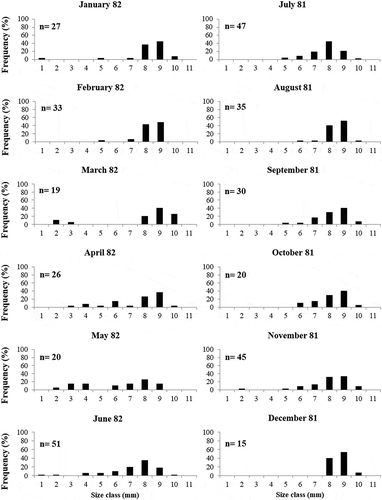
Figure 4. Size-frequency distributions of Jujubinus exasperatus, with the number of collected specimens (n), from the whole transect.
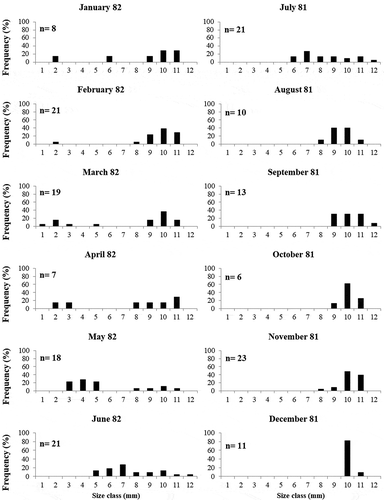
Figure 5. Growth curves for the identified cohorts of Jujubinus striatus (a) and Jujubinus exasperatus (b), obtained by considering pooled data for all sampled depths. The dots represent the modal size monthly of height–frequency distributions.
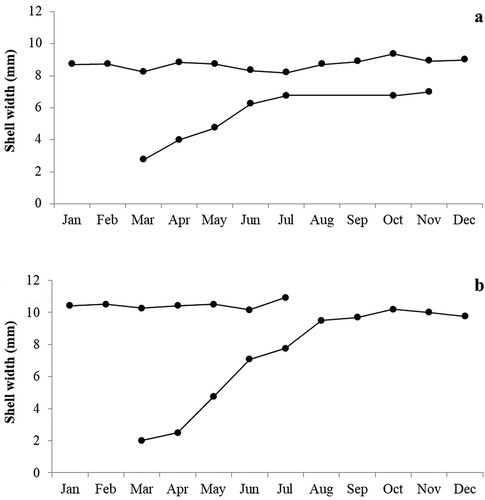
The survivorship curves of the two Jujubinus populations are shown in . In J. striatus, 70% of individuals survived to one year after settlement, reaching a shell height of 8 mm. In the following months, survival strongly decreased and only 3% of individuals reached a size greater than 10 mm. In contrast, only 45% of J. exasperatus individuals survived to one year after settlement, reaching a shell height of about 10 mm. In the following months survival gradually decreased, and only 1% of individuals obtained a size of more than 12 mm.
Figure 6. Survivorship curves for Jujubinus striatus and Jujubinus exasperatus, derived from size–frequency distribution of measured shells collected at all depths in 1 year of sampling (July 1981–June 1982).
The relationship between shell height (mm) and wet biomass (mg) was one of power-law in both the Jujubinus species (). The equation parameter of the curve was y = 0.0002x3.0442 (R2 = 0.9444) for J. striatus and y = 0.0002x3.0149 (R2 = 0.9706) for J. exasperatus.
Discussion
On the basis of unpublished data (M. Oliverio & P. Mariottini, personal communication) it seems that both species actually represent complexes of cryptic species. In this case, the validity of the present study should be considered strictly limited to the geographic area where samples were collected.
In the P. oceanica seagrass bed of Lacco Ameno d’Ischia, both populations (J. striatus and J. exasperatus) were characterised by a marked seasonal fluctuation in abundance throughout the year, a pattern which is consistent with that observed in the same year and the same study site for Gibbula spp. (Donnarumma et al. Citation2016). Such a trend is likely to be due to the food availability represented by algal cover of Posidonia leaves, characterised by an annual life cycle, starting at the end of summer–autumn (September–October) (Mazzella & Ott Citation1984; Pergent & Pergent-Martini Citation1991). Indeed, J. striatus reaches its maximum density during the summer (from June to August; ), when the maximum growth of filamentous epiphytic algae – representing the main food of this species – occurs on Posidonia leaves (Russo et al. Citation1984a; Hily et al. Citation2004). Conversely, the maximum density of J. exasperatus occurred during the winter (February), consistent with the higher abundance of diatoms and encrusting macroalgae, which represent the early successional stage of epiphytic colonisation on P. oceanica leaves (Mazzella & Russo Citation1989) and the main food items of the species (Takada et al. Citation1999).
In addition to trophic habits, the quali-quantitative variation of water movement along the depth gradient represents a further important environmental factor in structuring the mollusc community of Posidonia beds (Russo et al. Citation1991). Indeed, even though the two examined trochid species were present from the shallowest to the deepest Posidonia bed, peaks of abundance occurred at well-defined bathymetric levels: J. striatus mainly occurred in the shallowest stand (1–3 m), while J. exasperatus mainly occurred at the intermediate level of the Posidonia bed (10–15 m). Consistently with the present data, Russo et al. (Citation1984b) observed a decrease in the quantitative dominance of trochid and rissoid gastropods with increasing depth, and therefore considered these taxonomic groups as part of the “fundamental stock” of the mollusc community of the shallow P. oceanica beds (Russo et al. Citation1984a; Russo & Patti Citation2005).
Furthermore, this distribution pattern is well explained by the “morpho-functional characteristics of gastropod shells” by Russo (Citation1989), who identified three main assemblage structures, strongly related to the quali-quantitative changes in water movement occurring with depth (Riedl Citation1971). A first assemblage, living in the shallow prairie and subjected to wave action, is mainly represented by the largest gastropod species, with a solid shell and a large adhesive foot strongly attached to the leaves. A second assemblage, living in the intermediate level of the prairie, where the wave action is of minor importance, is mainly represented by species with an elongated shell and a slender, poorly adhesive foot. Finally, a third assemblage, living in the deep prairie, is represented by small species with a rounded shell and adhesive foot. On the basis of these latter observations, the difference between the two Jujubinus species of about 2 mm in maximum shell height is consistent with their distribution along the depth gradient: J. striatus is particularly abundant in the shallow stand and reaches a maximum shell height of 10.8 mm; J. exasperatus is mainly present at a greater depth and reaches a maximum shell height of 12.9 mm.
The two species show different growth patterns after a rather short benthic larval phase (12 day after fertilisation of eggs in J. striatus; Aricò et al. Citation1992), with a life cycle of 22 months for J. striatus and 17 months for J. exasperatus.
Rueda et al. (Citation2008) investigated the reproductive cycle of J. striatus at Cañuelo Bay, in southern Spain. They found in a Zostera bed only two sexually mature specimens at 2.2 mm. They also found 241,712 individuals with shell height ranging from 2.2 to 6.8 mm. Our data set ranges from 1.5 to 10.8 mm in a Posidonia bed. There are evident differences in maximum size of specimens between the compared Zostera and Posidonia populations (4 mm: an increment of about 60% of maximum size). On this basis, a shift of the minimum reproductive size, which is possibly higher in the Posidonia specimens, cannot be excluded. In fact, Frank (Citation1969) and Hughes (Citation1986) suggest that, when a gastropod species reaches sexual maturity, the growth stops (determinate growth), or it continues but more and more slowly (indeterminate growth).
Jujubinus striatus shows indeterminate growth, with a first phase of fast growth to the modal size of 6.74 mm (March–July; shell height increment of about 0.99 mm/month), and a second phase of very slow growth to the end of life (mean increment of about 0.14 mm/month). Similarly, J. exasperatus shows indeterminate growth, with a first phase of fast growth in the first 6 months after settlement (March–August; shell height increment of about 1.5 mm/month), and a second phase of slower growth (increment of about 0.12 mm/month). This type of growth, faster during the spring–summer period than in winter, has been also reported for B. latreillii and G. ardens (Russo et al. Citation2002; Donnarumma et al. Citation2016).
The first year of life after settlement of the two Jujubinus species is characterised by a high population survivorship. J. striatus shows a higher survival percentage (70%) than J. exasperatus (45%), while among the other gastropods associated with P. oceanica beds, the highest survivorship rate at the first year was recorded for G. ardens (90%) (Donnarumma et al. Citation2016).
Fretter and Graham (Citation1962) claim that marine snails show different types of reproductive strategies (semelparous or iteroparous), in relation to the environmental conditions (e.g. reduced resources availability), similar to what has been observed in many freshwater gastropods (Pinel-Alloul & Magnin Citation1971; Calow Citation1978). Our data, indeed, suggest that Jujubinus species are semelparous, with two age classes in the period of settlement, similarly to other gastropod species investigated in P. oceanica seagrass beds (Russo et al. Citation2002; Donnarumma et al. Citation2016).
In conclusion, the findings of this study, compared with previous works, reveal a certain number of shared traits in the life cycle of most common gastropod species living on the leaves of P. oceanica seagrass – semelparous reproduction, spring settlement, fast early growth and short lifespan (2 years maximum) – providing insight into the ecological features of species characterising the biodiversity and the functioning of the overall Posidonia oceanica ecosystem.
Acknowledgements
This study was partially performed during GFR’s stay at the Benthic Ecology Laboratory of the Stazione Zoologica “Anton Dohrn”, Ischia Island.
References
- Aricò S, Russo GF, De Nicola M. 1992. Osservazioni sulle prime fasi di sviluppo di Jujubinus striatus (L.) (Archaeogastropoda, Trochidae). Oebalia 17:325–328.
- Bhattacharya CG. 1967. A simple method of resolution of a distribution into Gaussian components. Biometrics 23:115–135.
- Buia MC, Gambi MC, Zupo V. 2000. Structure and functioning of Mediterranean seagrass ecosystems: An overview. Biologia Marina Mediterranea 7:167–190.
- Calow P. 1978. Evolution of life-cycle strategies in freshwater gastropods. Malacologia 17:351–364.
- Casola E, Scardi M, Mazzella L, Fresi E. 1987. Structure of the epiphytic community of Posidonia oceanica leaves in a shallow meadow. Marine Ecology 8:285–296.
- Chemello R, Milazzo M. 2002. Effect of algal architecture on associated fauna: Some evidence from phytal molluscs. Marine Biology 140:981–990.
- Cinelli F, Cormaci M, Furnari G, Mazzella L. 1984. Epiphytic macroflora of Posidonia oceanica (L.) Delile leaves around the island of Ischia (Gulf of Naples). In: Boudouresque CF, Jeudy De Grissac A, Olivier J, editors. Ist International Workshop on Posidonia oceanica beds. Marseille: G.I.S. Posidonie publ., Fr. 1:91–99.
- Dodd RJ, Stanton RJ. 1990. Paleoecology: Concepts and applications. New York: John Wiley & Sons.
- Donnarumma L, Bruno R, Terlizzi A, Russo GF. 2016. Population ecology of Gibbula umbilicaris and Gibbula ardens (Gastropoda, Trochidae) in a Posidonia oceanica seagrass bed. Italian Journal of Zoology 83:103–112.
- Frank PW. 1969. Growth rates and longevity of some gastropod mollusks on the coral reef at Heron Island. Oecologia 2:232–250.
- Fretter V, Graham A. 1962. British prosobranch molluscs. Their functional anatomy and ecology. London: The Royal Society. 820 pp.
- Gacia E, Costalago D, Prado P, Piorno D, Tomas F. 2009. Mesograzers in Posidonia oceanica meadows: An update of data on gastropod-epiphyte-seagrass interactions. Botanica Marina 52:439–447.
- Gambi MC, Lorenti M, Russo GF, Scipione MB, Zupo V. 1992. Depth and seasonal distribution of some groups of the vagile fauna of the Posidonia oceanica leaf stratum: Structural and trophic analyses. P.S.Z.N.I. Marine Ecology 13:17–39.
- Gayanilo FC, Sparre P, Pauly D. 1996. The FAO-ICLARM stock assessment tools. User’s manual. No. 8. Rome: Computerized Information Series: Fisheries (FAO). 126 pp.
- Guidetti P. 2000. Differences among fish assemblages associated with nearshore Posidonia oceanica seagrass beds, rocky–Algal reefs and unvegetated sand habitats in the Adriatic Sea. Estuarine, Coastal and Shelf Science 50:515–529.
- Hily C, Connan S, Raffin C, Wyllie-Echeverria S. 2004. In vitro experimental assessment of the grazing pressure of two gastropods on Zostera marina L. ephiphytic algae. Aquatic Botany 78:183–195.
- Hughes RN. 1986. Laboratory observations on the feeding behaviour, reproduction and morphology of Galeodea echinophora (Gastropoda: Cassidae). Zoological Journal of the Linnean Society 86:355–365.
- Marina P, Urra J, Rueda JL, Salas C. 2012. Composition and structure of the molluscan assemblage associated with a Cymodocea nodosa bed in south-eastern Spain: Seasonal and diel variation. Helgoland Marine Research 66:585–599.
- Mazzella L, Buia MC, Gambi MC, Lorenti M, Gf R, Scipione MB, Zupo V. 1992. Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: A review. In: John DM, Hawkins SJ, Price JH, editors. Plant-animal interactions in the marine benthos. Oxford: Clarendon Press. pp. 165–187.
- Mazzella L, Ott JA. 1984. Seasonal changes in some features of Posidonia oceanica (L.) Delile leaves and epiphytes at different depths. In: Boudouresque CF, Jeudy de Grissac A, Olivier J, editors. Ist International Workshop on Posidonia oceanica beds. Marseille, France: G.I.S. Posidonie publ., Fr. 1: 119–127.
- Mazzella L, Russo GF. 1989. Grazing effect of two Gibbula species (Mollusca, Archeogastropoda) on the epiphytic community of Posidonia oceanica leaves. Aquatic Botany 35:357–373.
- Mazzella L, Scipione MB, Buia MC. 1989. Spatio-temporal distribution of algal and animal communities in a Posidonia oceanica meadow. P.S.Z.N.I. Marine Ecology 10:107–129.
- Mazzella L, Zupo V. 1995. Reti trofiche e flussi di energia nei sistemi a fanerogame marine. Plant Biosystem 129:337–350.
- Peduzzi P, Herndl GJ. 1986. Role of bacteria in decomposition of fecal pellets egested by the epiphyte-grazing gastropod Gibbula umbilicaris. Marine Biology 92:417–424.
- Pergent G, Pergent-Martini C. 1991. Leaf renewal cycle and primary production of Posidonia oceanica in the bay of Lacco Ameno (Ischia, Italy) using lepidochronological analysis. Aquatic Botany 42:49–66.
- Pergent G, Romero J, Pergent-Martini C, Mateo MA, Boudouresque CF. 1994. Primary production, stocks and fluxes in the Mediterranean seagrass Posidonia oceanica. Marine Ecology-Progress Series 106:139–149.
- Pinel-Alloul B, Magnin E. 1971. Cycle vital et croissance de Bithynia tentaculata L. (Mollusca, Gastropoda, Prosobranchia) du lac St-Louis près de Montréal. Canadian Journal of Zoology 49:759–766.
- Pitacco V, Orlando-Bonaca M, Mavrič B, Popović A, Lipej L. 2014. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (Northern Adriatic Sea). Mediterranean Marine Science 15:225–238.
- Riedl R. 1971. Water movement: Animals. Marine Ecology 1:1124–1156.
- Rueda JL, Gofas S, Urra J, Salas C. 2009. A highly diverse molluscan assemblage associated with eelgrass beds (Zostera marina L.) in the Alboran Sea: Micro-habitat preference, feeding guilds and biogeographical distribution. Scientia Marina 73:679–700.
- Rueda JL, Marina P, Salas C, Urra J. 2008. Jujubinus striatus (Linnaeus, 1758) (Gastropoda: Trochidae) from a deep Zostera marina bed in southern Spain (Alboran Sea): Aspects of ecology and biology. Journal of Molluscan Studies 74:345–354.
- Rueda JL, Salas C. 2003. Seasonal variation of a molluscan assemblage living in a Caulerpa prolifera meadow within the inner Bay of Cádiz (SW Spain). Estuarine, Coastal and Shelf Science 57:909–918.
- Russo GF. 1989. La scelta dei descrittori morfo-funzionali nell’analisi dei sistemi bentonici: Un approccio con la componente malacologica di una prateria a Posidonia oceanica. Oebalia N.S. 15:213–228.
- Russo GF, Fraschetti S, Terlizzi A. 2002. Population ecology and production of Bittium latreillii (Gastropoda, Cerithidae) in a Posidonia oceanica seagrass bed. Italian Journal of Zoology 69:215–222.
- Russo GF, Fresi E, Scardi M. 1986. Il campionamento della malacofauna marina di fondo mobile: Confronto tra metodiche (la draga “Charcot” e la benna “SmithMcIntyre”) in rapporto alla zonazione del syntaxon. Lavori S.I.M., Società Italiana Di Malacologia 22:7–14.
- Russo GF, Fresi E, Vinci D. 1985. The hand-towed net method for direct sampling in Posidonia oceanica beds. Rapport Commission International Mer Méditerranée 29:175–177.
- Russo GF, Fresi E, Vinci D, Chessa LA. 1984a. Mollusk syntaxon of foliar stratum along a depth gradient in a Posidonia oceanica (L.) Delile meadow: Seasonal variability. In: Boudouresque CF, Jeudy De Grissac A, Olivier J, editors. Ist international workshop on Posidonia oceanica beds. Marseille: G.I.S. Posidonie. pp. 311–318.
- Russo GF, Fresi E, Vinci D, Chessa LA. 1984b. Malacofauna di strato foliare delle praterie di Posidonia oceanica (L.) Delile intorno all’isola d’Ischia (Golfo di Napoli): Analisi strutturale del popolamento estivo in rapporto alla profondità ed alla esposizione. Nova Thalassia 6:655–661.
- Russo GF, Patti FP. 2005. Early life history of two closely related gastropods, Rissoa auriscalpium and Rissoa italiensis (Caenogastropoda: Rissoidae). Marine Biology 147:429–437.
- Russo GF, Vinci D, Fresi E, Scardi M. 1991. Mollusc syntaxon of foliar stratum along a depth gradient in a Posidonia oceanica bed: III. A year’s cycle at Ischia Island. Posidonia Newsletter 4:15–25.
- Scipione MB, Fresi E, Wittmann KJ. 1983. The vagile fauna of Posidonia oceanica (L.) Delile foliar stratum: A community approach. Rapport Commission International Mer Méditerranée 28:141–142.
- Takada Y, Russo GF, Mazzella L. 1999. Activity patterns and habitat preferences of two herbivorous gastropods (Gibbula umbilicaris and Jujubinus exasperatus) on leaves of the Mediterranean seagrass Posidonia oceanica. Benthos Research 54:71–80.
- Terlizzi A, Russo GF. 1997. The molluscan taxocoene of differently-exposed Cymodocea nodosa beds: Year-long structural pattern and sampling methods. Bollettino Malacologico 33:77–82.