Abstract
Members of the globally distributed bivalve family Ostreidae (oysters) have a significant role in marine ecosystems and include species of high economic importance. In this work, we report the occurrence of digenean parasites of the families Bucephalidae (Prosorhynchoides sp.) and Monorchiidae (Postmonorchis sp.) in Mediterranean native populations of Ostrea edulis (but not in the introduced Magallana gigas). Molecular detection was based on DNA sequencing of the ribosomal intergenic spacer 2 (ITS2) marker. The importance of detecting the presence of overlooked digenean parasites in Mediterranean oysters is discussed.
Introduction
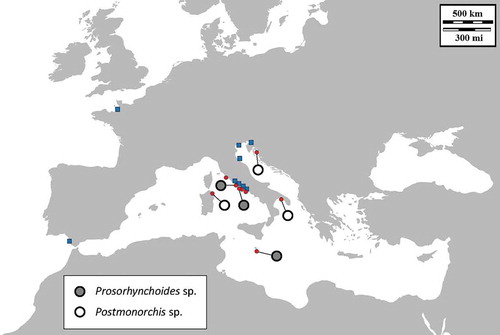
Oysters (family Ostreidae) are cosmopolitan sessile filter-feeder bivalves that play a remarkable ecological role in estuarine, intertidal and shallow-water ecosystems, through the mitigation of the excess of nutrients, algae and sediment. Among the c. 75 currently known species, many are of economic importance, being voluminously produced by the aquaculture industry. In Europe, oyster fisheries were historically based entirely on the autochthonous flat oyster Ostrea edulis Linnaeus, 1758, which occurs on hard substrata in estuarine and shallow coastal waters of the eastern Atlantic, from Scandinavia to North Africa, and into the Mediterranean Sea as far as the Black Sea (Yonge Citation1960). Oyster cultivation, based on the management of natural stocks, dates to the 17th century in Japan and earlier in China, and to Roman times in Europe, over 2000 years ago (Yonge Citation1960, Citation1970). A widespread decline occurred at the end of the 19th century (Gosling Citation2003) because of overfishing, habitat destruction and pollution (Orton Citation1937; Gosling Citation2003; Kirby Citation2004). Furthermore, the spread of a parasitic disease due to the haplosporidian protozoan Bonamia ostreae (Pichot et al. Citation1980), included in the International Aquatic Animal Health Code by the World Organisation for Animal Health (http://www.oie.int), led to massive mortality of European flat oysters in the last century (Renault et al. Citation1995). This has caused a shift to the rearing in Europe of the Pacific cupped oyster Magallana gigas Thunberg, 1793 (formely Crassostrea gigas; nomenclature after Salvi and Mariottini Citation2017; see also Bouchet and Marshall Citation2016), native to the Pacific coast of Asia, but introduced into North America, Australia, Europe and New Zealand. In 2004 M. gigas represented the bulk of farmed oyster world production (96.2%), whereas the production of the European flat oyster represented less than 0.11% of the total global production of all farmed oyster species (Svåsand et al. Citation2007). Today M. gigas is still among the most cultivated oysters, with a global annual production estimated at 583,464 tons, as compared to 2872 tons of O. edulis in 2015 (http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en). Monitoring of O. edulis and M. gigas populations for the presence (and putative overlapping) of parasite communities is necessary to set optimal aquaculture rearing practices and, thus, prevent or limit risks of cross-contamination (Mineur et al. Citation2014), as also specified by the World Organization for Animal Health – International Aquatic Animal Health Code (Alday-Sanz Citation2009). It is noteworthy that the co-presence in both oyster species of parasites, especially flatworms, is known in the natural environment (Aguirre-Macedo & Kennedy Citation1999). During laboratory experiments for a molecular phylogenetic study of Ostreidae (Salvi et al. Citation2014), the co-occurrence of Ostreidae-specific and non-target Polymerase Chain Reaction (PCR) products of the rRNA intergenic-spacer 2 (ITS2) was occasionally assessed as multiple bands in gel electrophoresis. These results suggested that the slightly degenerated ITS2 universal primers employed, originally designed by Oliverio and Mariottini (Citation2001) and herein named ITS2-3d* and ITS2-4r*, successfully amplified the target gene fragment both in oysters and in organisms associated with the analysed oyster tissues. Hence, we aimed to taxonomically identify the source of these ITS2-extra bands on a larger oyster sample by analysing a total of 36 specimens of O. edulis and M. gigas collected at 18 sites () over a period of 3 years (2014–2016; ).
Table I. Geographic information of collection sites for all oyster specimens analysed in this study. Habitat/date of collection sites is reported (* indicates specimens collected in the Atlantic; all other samples were collected in the Mediterranean). The presence/absence of digenean parasites was confirmed by screening samples with the ITS2-DIG1/DIG2 PCR diagnostic tool. Voucher and ITS2 sequence accession numbers for both oysters and parasites are provided (for each locality one parasite specimen was vouchered and its sequence submitted to GenBank).
Materials and methods
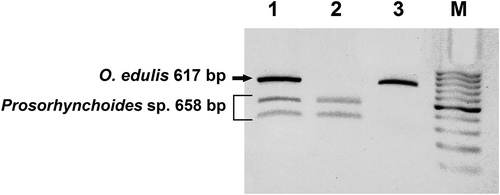
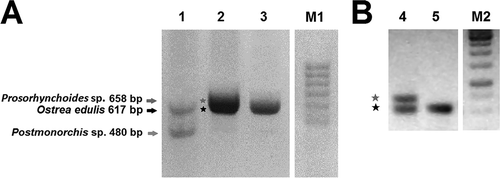
Genomic DNA was extracted using the “DNeasy Blood and Tissue Kit” (Qiagen, Hilden, Germany) from mantle tissues of each alcohol-preserved specimen of O. edulis and M. gigas. Before PCR amplification, quality and quantity of the extracted DNA were verified by 1% agarose gel electrophoresis runs. ITS2 amplifications were performed on all collected specimens of O. edulis (n = 18) and M. gigas (n = 18) using slightly degenerated ITS2 universal primers derived from the ones described by Oliverio and Mariottini (Citation2001) and named ITS2-3d* (5ʹ-GCATCGRTGAAGARCGCAG-3ʹ) and ITS2-4r* (5ʹ-AGTTTYTTYTCCTCCGCTTA-3ʹ), targeting the entire ITS2 region with the following thermal conditions: 3 min at 94°C, 35 cycles of 30 sec at 94°C, 30 sec at 50–55°C, 90 sec at 72°C, 6 min at 72°C for final extension. Oyster-specific ITS2 products were isolated, purified with the “Sure Clean” kit (Bioline) and sequenced by Macrogen Europe (Amsterdam, The Netherlands) (Genbank accession no. MF374290-MF374317). Co-amplified ITS2 extra bands (,)) having different molecular weights from those expected for O. edulis or M. gigas (i.e. ~600 and ~550 bp, respectively; see Salvi et al. Citation2014) were observed in some cases. These extra bands were isolated, purified with the “PCR Clean up Gel Extraction” kit (Macherey-Nagel) and sequenced by Macrogen Europe. Non-oyster organisms whose DNA was co-amplified in ITS2 PCRs were taxonomically identified as belonging to two different digenean genera (Postmonorchis Hopkins, 1841 and Prosorhynchoides Dollfus, 1929; see Results) through the National Center for Biotechnology Information (NCBI) BLASTn tool using default search parameter settings. GenBank matching sequences returning the highest values of “Max Score” (= highest alignment score or bit-score between the query sequence and the database sequence segment), “Query Coverage” (= percentage of the query length included in the aligned segments calculated over all segments) and “Identity” (percentage of similarity between the query and subject sequences over the length of the coverage area) served to outline the most plausible genera parasitising the collected oysters. The ITS2 sequences of these digeneans were used to design a novel primer pair to target more specifically both parasitic ITS2 sources: ITS2-DIG1 (5ʹ-AATGTGAACTGCGTACTG-3ʹ) and ITS2-DIG2 (5ʹ-AAGTTCAGCGGGTATTCA-3ʹ) (). These novel primers were used as a diagnostic PCR tool to screen the whole oyster sample (n = 36) of O. edulis and M. gigas to assess (or confirm) the presence of DNA from the two identified digeneans in the oysters’ mantle tissues used in DNA extractions [O. edulis ITS2 was then specifically amplified using ITS2-OED1 (5ʹ-AATGTGAATTGCAGGACA-3ʹ) and ITS2-OED2 (5ʹ-AAGTTCAGGGCGTAGTCT-3ʹ) () (see below and )]. Restriction enzyme digestion with BglII was carried on PCR products amplified with ITS2-3r*/4d*, ITS2-DIG1/DIG2 and ITS2-OED1/OED2 to confirm the presence of two distinct (but slightly overlapping) electrophoretic bands in the case of O. edulis parasitised by Prosorhynchoides sp (,)). A PCR-amplified sample with ITS2-3d/4r was cut with BglII restriction enzyme and produced three bands, as shown in (lane 1), due to the restriction site occurring exclusively in the Prosorhynchoides sp. DNA sequence (see Results and Discussion). The Prosorhynchoides sp. PCR product amplified with the specific primers ITS2-DIG1/DIG2 was also cut with the BglII restriction enzyme to produce the two corresponding gel bands (, lane 2). Digenean PCR products were obtained using the same thermal cycling protocol as reported above, then purified (using the “SureClean” Bioline Kit), sequenced and deposited in GenBank (Genbank Accession no. MF374318-MF374323).
Figure 2. (a) Electrophoresis analyses of double PCR products amplified with ITS2-3d*/4r* in Ostrea edulis from Ginosa (lane 1) and from Tor Paterno (lane 2). Specific oyster-product of Ostrea edulis from Tor Paterno obtained with ITS2-OED1/OED2 is shown (lane 3). M1 = marker (Hyperladder II, Bioline™). (b) Longer (+ 30 min at 80 V compared to the gel in Figure 2(a)) electrophoretic run of samples of lanes 2 (4) and 3 (5) of panel A samples. M2 = marker GeneRuler 1 kb DNA ladder (MBI Fermentas).
Results and discussion
We detected the presence of DNA from two distinct species of Digenea in mantle tissues of O. edulis, but not in those of M. gigas. In fact, electrophoresis of ITS2 products obtained with the primers ITS2-3d*/4r* () revealed the co-amplification in O. edulis of a 480-bp extra band in six specimens and of a 658-bp extra band in six other individuals (). BLASTn results indicated that the best match with the 480-bp extra-PCR product was the ITS2 sequence of an unidentified trematode ascribed to the genus Postmonorchis (Digenea: Monorchiidae; Accession no. KC603478; max score = 856, query cover = 98%, identity 99%), whereas the 658-bp PCR band matched best with the ITS2 sequence of Prosorhynchoides paralichthydis (Digenea: Buchephalidae; Accession no. KT273398; max score = 1068, query cover = 99%, identity 96%). Flatworm infection in the analysed oysters was then assessed by screening the whole sample with the novel primer pair () designed to target more specifically the ITS2 from the two identified digeneans.
Parasitism by digeneans has been already recorded in different oyster species (Millar Citation1963; Lee et al. Citation1996; Príncep et al. Citation1996; Aguirre-Macedo & Kennedy Citation1999). Bucephalid trematodes are known to be common parasites of commercially important molluscs (Cheng Citation1967) and cause parasitic castration in several bivalves (Lauckner Citation1983), including oysters (Cheng & Burton Citation1965; Feng & Canzonier Citation1970; Tripp Citation1973; Chun Citation1974; Mohan Citation1978). Infection by Bucephalus haimeanus was formerly reported in Mediterranean O. edulis (from the delta of the Ebro River in Spain), in which sporocysts were observed to invade the interstitial conjunctive tissues and to obstruct digestive gland tubes, affecting food absorption and ultimately causing host death (Príncep et al. Citation1996). Since the bucephaline genera Prosorhynchoides, Rhipidocotyle and Bucephalus as currently conceived are polyphyletic – as revealed by morphological and molecular data from ITS2 (D1–D3 region) and 28S rDNA markers (Nolan et al. Citation2015) – no conclusion can be drawn about the actual systematics of the trematode species associated with O. edulis in our study. However, our data revealed a bucephalid infection in O. edulis in the Mediterranean basin, as this occurred in the Italian and Maltese stocks (). Bucephalidae are a large cosmopolitan family with a life cycle including sporocysts and cercariae developing in bivalves (intermediate host), metacercariae encysting within the tissues of fishes (second intermediate host), and sexual adult stages inhabiting the digestive tract, and rarely other sites, in piscivorous teleosts (definitive host) (Overstreet & Curran Citation2002). The closest bucephalid to our targets retrieved in Genbank, i.e. Prosorhynchoides paralichthydis, parasitises the southern flounder Paralichthys lethostigma. It would be interesting to identify the Mediterranean definitive fish host – arguably a benthic species such as flat fishes – parasitised by the Prosorhynchoides species found in O. edulis, to improve our knowledge of the parasite life cycle and its potential impact on the health of oysters and fishes.
Larval stages of digeneans of the family Monorchiidae have been described mostly in bivalves of the Atlantic and Pacific oceans (Carella et al. Citation2013). Only recently, metacercariae of Postmonorchis were detected in tissues of the Mediterranean wedge clam Donax trunculus Linnaeus, 1758 collected along the Italian Tyrrhenian coast (Carella et al. Citation2013). This pathogen invades several molluscan tissues such as gills, labial palps, mantle, gut, kidney epithelium, and foot, triggering a strong inflammatory response in the host (Carella et al. Citation2013). The adult stages of Postmonorchis may occur in Sciaenidae (presumably Umbrina sp. in Mediterranean waters), but further data are required to identify the definitive host species (Carella et al. Citation2013). In our study, a DNA source of a parasite plausibly referred to Postmonorchis sp. was detected for the first time in oysters (; ). This result would suggest the co-occurrence of this trematode in both Ostrea and Donax, consistent with previous observations indicating that digeneans parasitising oysters are commonly shared also with distantly related bivalve families (Lauckner Citation1983). On the other hand, no parasites were detected in any of our 18 specimens of M. gigas collected over a wide geographic range (; ), although a further sampling effort would be needed to confirm the absence of bucephalids and monorchids in non-autochthonous populations of M. gigas. In this regard, it is relevant to remark that digeneans are known to parasitise the native Indo-Pacific M. gigas, and particularly the human intestinal trematode Gymnophalloides seoi (Digenea: Gymnophallidae) is commonly transmitted by this oyster species in Korea (Lee et al. Citation1996; Pyo et al. Citation2013). It is possible that the successful thriving of the invasive M. gigas is related to a lack of parasites in the invaded areas, as expected under the enemy release hypothesis (ERH: Torchin et al. Citation2001; Keane & Crawley Citation2002; Mitchell & Power Citation2003). However, it is reasonable to assume that with time host-shifts by bucephalids and Postmonorchis sp. trematodes can potentially occur, so a risk of cross-infection between O. edulis and M. gigas cannot be excluded in the Mediterranean waters.
Conclusions
The growth of aquaculture – including oyster farming – has been accompanied by the emergence of new and transboundary diseases, stimulating epidemiological studies of aquatic animal pathogens. However, studies evaluating the occurrence and the impact of pathogens in wild aquatic animal populations are still sparse compared to those considering farmed species (Peeler & Taylor Citation2011). As a by-product of a molecular phylogenetic study on Ostreidae through a universal barcode marker (ITS2) (Salvi et al. Citation2014), it was possible to detect overlooked digenean parasites in wild Mediterranean populations of O. edulis. Notably, putative co-amplification of host/parasite bands were also occasionally observed during molecular phylogenetic studies on Mactridae and Donacidae (e.g. Mactra and Donax Salvi D., personal observation), suggesting that an ITS2 host/parasite DNA barcode approach may contribute in uncovering a still-hidden digenean diversity in benthic communities.
The identification of Bucephalidae and Monorchidae in the Mediterranean populations of flat oyster encourages future efforts in exploring their epidemiological consequences on such economically important molluscan species. Histopathological, taxonomical and ecological analyses on a larger collection of samples are certainly required to better characterise these digenean infections and to link the infection prevalence with environmental parameters and seasonality. These will allow evaluating the impact of these parasites on oyster health and fitness, the potential risks of cross-contamination to other oyster species (e.g. M. gigas) and, ultimately, the level of biosecurity and surveillance necessary to avoid the putative emergence of food-borne diseases.
Conflict of interest statement
The authors declare that there are no competing interests.
Acknowledgements
We thank Dr Marialetizia Fioravanti (Università di Bologna) for suggestions on the first draft of the manuscript. The authors are greatly indebted to Mrs Rosetta Ponzo-Leonetti for the revision of the English text.
Additional information
Funding
References
- Aguirre-Macedo ML, Kennedy CR. 1999. Patterns in metazoan parasite communities of some oyster species. Journal of Helminthology 73:283–288.
- Alday-Sanz V. 2009. Directive 88/2006: European Union animal health requirements for aquaculture animals and products thereof. In: Bondad-Reantaso MG, Arthur JR, Subasinghe RP, editors. Strengthening aquaculture health management in Bosnia and Herzegovina. Rome, Italy: FAO Fisheries and Aquaculture Technical Paper No. 524. pp. 17–25.
- Bouchet P, Marshall B. 2016. Magallana Salvi & Mariottini. In: MolluscaBase, 2016 World Register of Marine Species. Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=836032 on 2017-01-22. Accessed Oct 2017 14.
- Carella F, Culurgioni J, Aceto S, Fichi G, Pretto T, Luise D, Gustinelli A, De Vico G. 2013. Postmonorchis sp. inq. (Digenea: Monorchiidae) metacercariae infecting natural beds of wedge clam Donax trunculus in Italy. Diseases of Aquatic Organisms 106:163–172. doi:10.3354/dao02650
- Cheng TC. 1967. Marine molluscs as hosts for symbiosis. With a review of known parasites of commercially important species. Advances in Marine Biology 5:1–424.
- Cheng TC, Burton RW. 1965. Relationships between Bucephalus sp. and Crassostrea virginica: Histopathology and sites of infection. Chesapeake Science 6:3–16. doi:10.2307/1350618
- Chun SK. 1974. Histopathology and localities infected by the Bucephalus sp. in oysters on the southern coast of Korea. Publications of Marine Laboratories. Pusan Fisheries Collection 7:77–86.
- Feng SY, Canzonier WJ. 1970. Humoral responses in the American oyster Crassostrea virginica infected with Bucephalus sp. and Minchinia nelsoni. In: A symposium on diseases of fishes and shellfishes. American Fisheries Society Special Publication 5:497–510.
- Gosling E. 2003. Bivalve molluscs biology, ecology and culture. Oxford: Fishing News Books.
- Keane R, Crawley MJ. 2002. Exotic plant invasions and the Enemy Release Hypothesis. Trends in Ecological Evolution 17:164–170. doi:10.1016/S0169-5347(02)02499-0
- Kirby MX. 2004. Fishing down the coast: Historical expansion and collapse of oyster fisheries along continental margins. Proceedings of the National Academy of Sciences U.S.A 101:13096–13099. doi:10.1073/pnas.0405150101
- Lauckner G. 1983. Diseases of Mollusca: Bivalvia. In: Kinne O, editor. Diseases of marine animals, Vol. II. Introduction, Bivalvia to Scaphopoda. Hamburg, Germany: Biologische Anstalt Helgoland. pp. 477–961.
- Lee SH, Sohn WM, Hong SJ, Huh S, Seo M, Choi MH, Chai JY. 1996. A nationwide survey of naturally produced oysters for infection with Gymnophalloides seoi metacercariae. The Korean Journal of Parasitology 34:107–112. doi:10.3347/kjp.1996.34.2.107
- Millar RH. 1963. Oyster killed by trematode parasites. Nature 197:616. doi:10.1038/197616b0
- Mineur F, Le Roux A, Maggs CA, Verlaque M. 2014. Positive feedback loop between introductions of non-native marine species and cultivation of oysters in Europe. Conservation Biology 28:1667–1676. doi:10.1111/cobi.12363
- Mitchell CE, Power AG. 2003. Release of invasive plants from fungal and viral pathogens. Nature 421:625–627. doi:10.1038/nature01317
- Mohan JM. 1978. Observations on the larval trematode Bucephalus sp. parasitic in the oyster Crassostrea madrasenis. Journal of Invertebrate Pathology 32:381–383. doi:10.1016/0022-2011(78)90204-5
- Nolan MJ, Curran SS, Miller TL, Cutmore SC, Cantacessi C, Cribbd TH. 2015. Dollfustrema durum n. sp. and Heterobucephalopsis perardua n. sp. (Digenea: Bucephalidae) from the giant moray eel, Gymnothorax javanicus (Bleeker) (Anguilliformes: Muraenidae), and proposal of the Heterobucephalopsinae n. subfam. Parasitology International 64:559–570. doi:10.1016/j.parint.2015.07.003
- Oliverio M, Mariottini P. 2001. Contrasting morphological and molecular variation in Coralliophila meyendorffii (Muricidae, Coralliophilinae). Journal of Molluscan Studies 67:243–246. doi:10.1093/mollus/67.2.243
- Orton JH. 1937. Oyster biology and oyster culture. London: Arnold.
- Overstreet RM, Curran SS. 2002. Superfamily bucephaloidea poche 1907. In: Gibson DI, Jones A, Bray RA, editors. Keys to the Trematoda. Vol. 1. Wallingford, U.K.: CABI Publishing. pp. 67–110.
- Peeler EJ, Taylor NG. 2011. The application of epidemiology in aquatic animal health opportunities and challenges. Veterinary Research 11:94. doi:10.1186/1297-9716-42-94
- Pichot Y, Comps M, Tige G, Grizel H, Rabouin MA. 1980. Recherches sur Bonamia ostreae gen. n., sp. n., parasite nouveau de l’huître plate Ostrea edulis L. Revue des travaux de l’Institut des pêches maritimes 43:131–140.
- Príncep M, Bigas M, Durfort M. 1996. Incidence of Bucephallus haimeanus (Lacaze-Duthiers, 1854) (Trematoda, Digenea) in the digestive gland of Ostrea edulis Linné. Iberus 14:211–220.
- Pyo KH, Kang EY, Hwang YS, Jun HC, Sohn WM, Cho SH, Lee WJ, Chai JY, Shin EH. 2013. Species identification of medically important trematodes in aquatic food samples using PCR-RFLP targeting 18S rRNA. Foodborne Pathogen Diseases 10:290–292. doi:10.1089/fpd.2012.1299
- Renault T, Cochennec N, Grizel H. 1995. Bonamia ostreae, parasite of the European flat oyster, Ostrea edulis, does not experimentally infect the Japanese oyster, Crassostrea gigas. Bulletin of the European Association of Fish Pathologists 15:78–80.
- Salvi D, Macali A, Mariottini P. 2014. Molecular phylogenetics and systematics of the bivalve family Ostreidae based on rRNA sequence-structure models and multilocus species tree. PLoS One 9:e108696. doi:10.1371/journal.pone.0108696
- Salvi D, Mariottini P. 2017. Molecular taxonomy in 2D: A novel ITS2 rRNA sequence-structure approach guides the description of the oysters’ subfamily Saccostreinae and the genus Magallana (Bivalvia: Ostreidae). Zoological Journal of the Linnean Society 179:263–276.
- Svåsand T, Crosetti D, García-Vázquez E, Verspoor E 2007. Genetic impact of aquaculture activities on native populations. Genimpact final scientific report (EU contract n. RICA–CT–2005–022802). Available: http://genimpact.imr.no. Accessed Nov 2017 28.
- Torchin ML, Lafferty KD, Kuris AM. 2001. Release from parasites as natural enemies: Increased performance of a globally introduced marine crab. Biological Invasions 3:333–345. doi:10.1023/A:1015855019360
- Tripp MR. 1973. Hermaphroditism in Bucephalus infected oysters. Journal of Invertebrate Pathology 21:321–322. doi:10.1016/0022-2011(73)90221-8
- Yonge CM. 1960. Oysters. London: Collins.
- Yonge CM. 1970. Oyster cultivation. Underwater Journal 2:138–144.