Abstract
Avian brood parasites do not raise their own young. Rather, they leave eggs and offspring in nests of other species. Finding potential nests to parasitize is mostly up to the female and requires enhanced spatial memory as the female must remember nest locations as well as whether each located nest is in the nest building, incubation or offspring provisioning stage. In many cases, enhancement of ecologically relevant spatial memory is associated with larger hippocampal volume compared to the sex, or a species, that does not have similar demand for spatial memory. Female brown-headed cowbirds (Molothrus ater), a parasitic species ubiquitous across North America, have greater hippocampus volume and new neuron recruitment as compared to male cowbirds and non-parasitic species. The present study reveals female cowbirds display greater new neuron recruitment compared to males specifically in the rostral hippocampal subdivision. It is still unclear, however, whether the function of new cells in the rostral hippocampus is related to sex differences in spatial memory. These data reveal the avian hippocampus exhibits subdivisions in new cell recruitment but it remains unclear whether these subdivisions serve distinct functions in spatial memory and other hippocampal functions.
Introduction
Ecologically relevant spatial memory is considerably variable across species, sexes and seasons across many vertebrates. Enhanced spatial ability is reflected in the volume of the hippocampus (Hp) such that an individual that requires better spatial memory may also exhibit a larger hippocampus, presumably because greater spatial memory requires a larger hippocampus (Rehkämper et al. Citation1988; Krebs et al. Citation1989; Sherry et al. Citation1989, Citation1993; Clayton Citation1996; Healy et al. Citation1996; Reboreda et al. Citation1996; Galea & McEwen Citation1999; Biegler et al. Citation2001; Lucas et al. Citation2004; Pravosudov et al. Citation2006; Burger et al. Citation2013). The Hp underlies the acquisition and retrieval of spatially related memories, and its importance in spatial memory is well demonstrated across taxonomic groups (Krebs et al. Citation1989; Burgess et al. Citation2002; Rodriguez et al. Citation2002; Broadbent et al. Citation2004).
Birds are an excellent system in which to understand the relationship of Hp and spatial memory as birds require spatial memory for food caching, homing, migration and nest searching (Rothstein et al. Citation1987; Krebs et al. Citation1989; Sherry & Vaccarino Citation1989; Sherry et al. Citation1989; Clayton Citation1995a,b; Healy et al. Citation1996; White et al. Citation2009; LaDage et al. Citation2010, Citation2011; Guigueno et al. Citation2014). For example, Hp lesions cause deficits in spatial orientation of homing pigeons (Bingman & Mench Citation1990) as well as in the acquisition of new spatial memories in black-capped chickadees (Parus atricapillus) and dark-eyed juncos (Junco hyemalis; Hampton & Shettleworth Citation1996). On the other hand, non-spatially related memory such as memory of color was not affected by Hp lesions (Hampton & Shettleworth Citation1996). Such neural adaptations have a clear link to behavioral adaptation, which generates an evolutionary framework in which to understand how variation in neural structures (such as the Hp) relate to ecologically relevant behaviors that enhance an animal’s fitness.
Spatial memory and Hp specialization are dependent on differences in the use of space and the animal’s reliance on cognitive skills to complete tasks that increase its fitness. A well-established example of this is the sex and species difference in spatial ability and Hp size in brown-headed cowbirds ((Molothrus ater (Boddaert, 1783); hereafter, cow bird)). The cowbird is an obligate brood parasite that does not build its own nests, incubate its own eggs, or provision its own young. Instead, the female cowbird searches for a nest of other species (i.e. the host) in which to lay her eggs. The host species is then left with a foreign nestling to provision until the young cowbird fledges the nest. Thus, the female cowbird must be proficient at spatial memory to remember potential host nest locations and how far along each nest is toward completion (Norman & Robertson Citation1975; Rothstein et al. Citation1987; White et al. Citation2009). By remembering whether the located nests are in the building, egg laying, incubation or nestling provisioning stages, the female cowbird significantly improves the odds of her young being raised to independence by the host (White et al. Citation2009). Female cowbirds also return to nest sites they have parasitized to evaluate potential egg rejection by the host (Hoover & Robinson Citation2007). Furthermore, the female cowbird must recognize and remember nests that are no longer available for parasitizing, indicating the female brood parasite must constantly update her memory of available host nests. When male and female cowbirds were trained to locate hidden food prior to testing their memory of these hidden food locations, results reveal that females trained on this spatial task made significantly fewer errors and took more direct paths to the location of stored food sites as compared to trained males (Guigueno et al. Citation2014). Even though this was a food finding task rather than a nest searching task, it does imply that the female has superior spatial memory relative to the male. Likewise, it has also been demonstrated that this increased demand for spatial ability is reflected in Hp volume and neuron recruitment, as female cowbirds have a larger Hp volume and more neurogenesis in comparison to males as well as closely related Icterid species that are not brood parasites (Sherry et al. Citation1993; Guigueno et al. Citation2016a). Overall, studies in the cowbird are consistent with the adaptive specialization hypothesis (ASH), which posits that selection can modify behavior as well as the underlying neural mechanisms if such modifications result in enhanced fitness (Krebs Citation1990).
The avian Hp is not a center exclusively dedicated to processing spatial memory. It is well established that the Hp plays a critical role in affective and cognitive processes, including spatial memory, in both birds and mammals (Atoji & Wild Citation2006; Leuner & Gould Citation2010; Jinno Citation2011a), and these functional differences are represented in different subdivisions within the Hp (Atoji & Wild Citation2006; Atoji et al. Citation2016). The avian Hp is associated with cognitive skills including homing, food storage and retrieval, migration and imprinting (Sherry & Vaccarino Citation1989; Bingman & Mench Citation1990; Clayton Citation1995b; Broadbent et al. Citation2004; Pravosudov et al. Citation2006; LaDage et al. Citation2009), but is also part of the limbic network which underlies emotionally driven learning, motivation and memory of social signals (Cheng et al. Citation1999; Bailey et al. Citation2002; Cross et al. Citation2013). Therefore, it is not surprising that the avian Hp is divided into sub-regions, as is the case in the mammalian Hp. Although the avian and mammalian Hp are considerably different in neuroanatomical, cytoarchitectural and hodological characteristics (Székely Citation1999), the functions of some avian Hp subdivisions correspond to those of mammalian subdivisions (Atoji et al. Citation2016). In the avian Hp there are roughly seven subdivisions along the dorsal–ventral and lateral–medial extents (Székely Citation1999; Atoji et al. Citation2016) and some of these subdivisions are functionally distinct. For instance, sensory inputs from forebrain sensory regions enter specific Hp sub-regions and send output to regions regulating affective and motivational processes, including the septum and the hypothalamus (Atoji & Wild Citation2006). In addition, the avian hippocampus has a remarkable capacity for structural reorganization as is the case in other vertebrates, including mammals and fish (Zupanc Citation2006; Gu et al. Citation2012; Burger et al. Citation2014; Teles et al. Citation2016). However, even some measures of neural reorganization vary along the subdivisions of the avian Hp (Barnea & Nottebohm Citation1994).
Structural modification of pre-existing Hp neurons, such as changes in soma volume or dendritic arborization, as well as neuron death and replacement may occur in the Hp (van Praag et al. Citation2005; Leuner & Gould Citation2010; Tronel et al. Citation2010; McDonald & Kirn Citation2012; Garthe & Kempermann Citation2013). While it is not clear whether new neuron recruitment in avian Hp can result in net growth as it does in some studies of mammals (Bayer et al. Citation1982; Crespo et al. Citation1986), recruitment clearly makes up for cell loss in the avian Hp (Kirn & Nottebohm Citation1993; Scharff Citation2000), and the rostral sub-region of the Hp exhibits distinct neuron replacement patterns (Barnea & Nottebohm Citation1994). In the present study, the pattern of new neuron recruitment is measured in Hp using doublecortin (DCX), an endogenous protein that is commonly used as a marker of neurogenesis (Gleeson et al. Citation1999; Balthazart et al. Citation2008; LaDage et al. Citation2010; Melleu et al. Citation2013). DCX immunoreactivity (DCX-ir) was examined to quantify new neuron recruitment in the Hp of male and female brown-headed cowbirds. DCX is a microtubule-related protein that is expressed in neurons for approximately 20–30 d after the final mitotic division (Gleeson et al. Citation1999; Balthazart et al. Citation2008; LaDage et al. Citation2010; Melleu et al. Citation2013). It plays a critical role in the polymerization of microtubules that are used during neuronal migration and, therefore, it is a component of the machinery required for the migration of newly born neurons (Francis et al. Citation1999; Gleeson et al. Citation1999; Balthazart et al. Citation2008). DCX is quantified in the Hp and in a control brain region, the hyperpallium apicale (Ha). The Ha was chosen as a control region because there is no seasonal difference in the incorporation of new cells there (Sherry & Hoshooley Citation2010). DCX cell numbers were quantified in male and female cowbirds to determine whether sex-dependent differences in new neuron recruitment occur within specific Hp subdivisions in each sex, and whether new neuron recruitment in the Hp subdivisions is specific to the Hp in male and female cowbirds.
Materials and methods
Field capture of brown-headed cowbirds
Male (n = 6) and female (n = 7) cowbirds were captured during the breeding season from May to June in bait traps containing food and water at the Balcones Canyon Wildlife Refuge in Marble Falls, Texas (USA), in 2013. Birds were held in bait traps for less than 1 week before sacrifice via rapid decapitation to avoid or minimize the effects of captivity or restricted experience on the hippocampus (LaDage et al. Citation2009). Bait trap checks occurred twice per week and euthanasia occurred in the field immediately after collection from the trap. Euthanasia procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Hofstra University. Gonad measurements revealed all birds were adults with mature gonads.
The brains were quickly extracted and fixed in 0.5% acrolein solution (Alward et al. Citation2014; 9.5 mL 1M phosphate buffered saline (PBS) and 500 µL acrolein) for 2 h followed by cryoprotection in 30% sucrose before being stored at −80°C. Tissue was sectioned at 40 µm into four series of alternate sections, only one of which was used in this study. Sections were mounted to superfrost glass slides and stored at −20°C until immunocytochemistry (ICC) was conducted. Only adult birds as assessed by plumage coloration were used in this study (Ortega et al. Citation1996).
DCX immunocytochemistry and quantitative analysis
ICC for DCX in the avian brain has been described (Balthazart et al. Citation2008; Yamamura et al. Citation2011). ICC in cowbird brain tissue was conducted and validated in Guigueno et al. (Citation2016a,b). Briefly, brain tissue was washed in 0.1% sodium borohydride to remove residual acrolein (Alward et al. Citation2014). Endogenous peroxidases were blocked in 20% normal horse serum (S-2000; Vector Laboratories) and 0.6% hydrogen peroxide in PBS. Tissue was incubated in primary antibody (1:200 dilution) at room temperature for 48 h. The primary antibody was raised against DCX in goat (sc-8066, Santa Cruz Biotechnology) and has been previously validated in many avian studies (Boseret et al. Citation2007; Balthazart et al. Citation2008; Fox et al. Citation2010; LaDage et al. Citation2010; Yamamura et al. Citation2011; Melleu et al. Citation2013; Alward et al. Citation2014; Guigueno et al. Citation2016a,b). The following day, tissue was incubated in secondary biotinylated anti-goat Immunoglobulin G (IgG) antibody (BA-9500, Vector Laboratories; 1:200) for 1 h at room temperature and with avidin-biotinylated peroxidase complex (Vectastain Elite ABC, PK-6100, Vector Laboratories) for 1 h at room temperature. DCX-specific peroxidase activity was visualized using 3,3-diaminobenzidine (DAB Peroxidase Substrate Kit, Vector Laboratories; ). Tissue sections were coverslipped using Permount (Fisher Scientific).
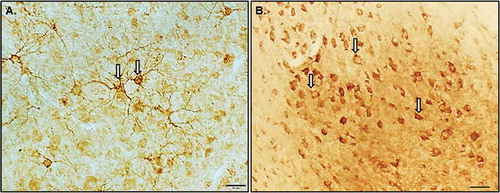
Figure 1. Photomicrographs illustrating the different doublecortin positive (DCX+) cell types observed in the cowbird hippocampus (Hp). As described in Boseret et al. (Citation2007), four types of DCX cells can be observed in the songbird brain: (1) densely stained round multipolar cells, (2) fusiform elongated cells, (3) weakly stained round cells with few immunolabelled processes detected, and (4) weakly stained cells associated with punctate structures. (A) Densely stained round multipolar cells with labelled neurites were observed only against the midline of the Hp near the ventricle dividing Hp from neostratium, and (B) weakly stained round cells with few immunolabelled processes detected were also observed in all sub-regions of Hp. The white arrows indicate the types of cells counted in this study. Photomicrographs were taken using a 40× objective. Scale bars = 20 µm.
Density of DCX+ cells () was quantified under bright field illumination on an Olympus Bx53 microscope equipped with an Olympus DP73 camera. Photomicrographs were taken using CellSens Standard software with a 20× objective ( shows 40× for clarity). The anatomical definitions of the Hp sub-regions examined here are based on the canary brain atlas by Stokes et al. (Citation1974). The hippocampal sections in which cells were quantified were photomicrographed using a 4× objective and imported into NIH ImageJ for surface area measurements. Surface area of the hippocampal sections used in this study was compared between male and female cowbirds to ensure there was no difference in the total area examined.
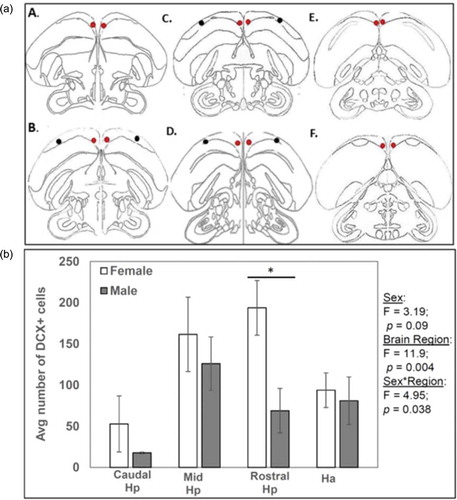
DCX cells were quantified along the rostral–caudal extent of Hp. The sections considered rostral, mid and caudal Hp are illustrated in ). For each of these subdivisions, three samples, each 1.66 mm × 1.24 mm in area, were collected from both the left and right hemispheres so that an average number of DCX+ cells could be calculated for each of the three Hp subdivisions. Three samples from the two Hp hemispheres resulted in six total samples for each Hp sub-region. These were used to generate an average DCX+ cell count per sub-region per subject. As a control, DCX+ cells were quantified in Ha, a structure lateral and adjacent to the Hp that has no seasonal difference in the incorporation of new cells (Sherry & Hoshooley Citation2010). The number of DCX cells in Ha (i.e. the control region) was quantified to provide a means of comparing DCX+ cells in the Hp to a nearby non-hippocampal region. The area used for quantification was positioned within the Hp using clearly defined neuroanatomical landmarks as described in Sherry and MacDougall-Shackleton (Citation2015). Manual cell counts of photomicrographs were done by an observer who was blind to the sex of the bird. An observer marked cells in NIH Image J to ensure cells were not counted twice. Cell counts from each sub-region were averaged to produce one value for each subject.
Figure 2. Schematic drawings of coronal sections illustrating the regions examined along the rostral-caudal extent of the hippocampus (Hp). (a) A and B represent the range of sections included in the rostral Hp category. C and D represent the mid-Hp category, and E and F represent the caudal Hp category. Red dots represent areas quantified for Hp doublecortin immunoreactivity (DCX-ir) cell counts whereas black dots represent regions within the Ha (hyperpallium apicale; Ha) in which DCX cells were counted as a control. The illustrations are based on Stokes et al. (Citation1974). (b) Results of a multivariate analysis of variance (MANOVA) are represented with sex and Hp sub-region as well as Ha as explanatory factors. The average number of DCX+ cells was calculated from three 1.66 × 1.24 mm sampling frames for each hippocampal sub-region.
Boseret et al. (Citation2007) describes four DCX cell types found in bird brains. These include densely stained multipolar cells, fusiform elongated cells, weakly stained round cells with few immunostained processes, and weakly stained cells with small dots reminiscent of punctate structures. All of these cell types were found throughout the cowbird brain, including DCX cells with neurites. However, the most dominant stained cell type found in the Hp of this study is the weakly stained round cells with few immunostained processes. Furthermore, while both round and fusiform DCX cell types were detected in the cowbird brain, the round cells dominated in the Hp sub-regions. A similar pattern was reported by Guigueno et al. (Citation2016a) who also found very few fusiform cells in the hippocampus of cowbirds. Therefore, the results presented here do not distinguish between various cell morphologies.
Statistical analyses
Student t-test was used to compare the total surface area of the sections in which Hp was measured in males and females. The number of DCX+ cells was compared using multivariate analysis of variance (MANOVA) with sex and region (caudal Hp, mid Hp, rostral Hp and Ha) as explanatory factors as well as the interaction between these factors. This test generated pairwise comparisons to determine sex- and region-dependent differences in DCX+ cell counts.
Results
There were no significant differences between the sexes in the total surface area of the sections in which DCX was quantified (df = 11; t = 0.67; p = 0.51). Therefore, DCX+ cells were compared between sexes using multiple ANOVA, which revealed the amount of DCX+ cells is region dependent (; F3,7 = 11.9, p = 0.004), but no main effect of sex was identified (; F1,9 = 3.19, p = 0.09). However, there was a significant interaction between these two explanatory factors (; F3,7 = 4.95, p = 0.038), indicating sex differences in DCX+ cells exist in some sub-regions but not in others. Between-subject post-hoc comparisons in each of the brain sub-regions reveal the rostral Hp to be the only sub-region in which there is a sex difference in DCX+ cells (; F1,9 = 5.36; p = 0.046) whereas the caudal and mid-Hp as well as the control region (i.e. Ha) did not exhibit significant differences in DCX+ cells between the sexes (caudal: F1,9 = 0.77; p = 0.40; mid: F1,9 = 2.45; p = 0.63; Ha: F1,9 = 0.087; p = 0.77). Pairwise comparisons across Hp sub-regions in males and females combined reveal greater differences in DCX+ cell numbers between mid-Hp regions compared to caudal Hp (p = 0.002), greater differences in DCX+ cell numbers in rostral Hp regions compared to caudal Hp (p = 0.01) but no difference in DCX+ cells between caudal Hp and the control region, Ha (p = 0.42). Mid-Hp did not display more DCX+ compared to rostral Hp (p = 0.92). Mid-Hp did display greater DCX+ cell counts relative to Ha (p = 0.01), whereas rostral Hp did not display this difference (p = 0.08).
Discussion
One of the unique challenges faced by female obligate brood parasites is remembering the location of host nests as well as the time to completion of each located nest. One possible explanation for how these female birds excel at spatial skill is described by the adaptive specialization hypothesis (ASH). ASH posits that selection can modify behavior as well as the underlying neural mechanisms if such modifications result in enhanced fitness (Krebs et al. Citation1989; Sherry et al. Citation1989), and therefore it is possible that region-specific hippocampal neurogenesis fits this definition. A comparative study across species with stark differences in spatial memory is needed to investigate this possibility. However, there are multiple alternative explanations as to how modifications may occur in underlying mechanisms, especially neural mechanisms. In cowbirds, the species- and sex-dependent differences in spatial memory may be related to dispersion of existing neurons or new neuron recruitment. Examples include changes in dendritic arborization of existing neurons, addition of glial cells, and modification in the distance between neuron somata as well as new cell proliferation (Tronel et al. Citation2010; McDonald & Kirn Citation2012). These mechanisms could possibly contribute to renovating the hippocampus in such a way that it can manage the high demand for spatial memory required by the female cowbirds, a generalist brood parasite that discovers nest locations of roughly 200 different host species (Davies Citation2000). While the present study does not systematically investigate each possible alternative mechanism or the function of newly recruited neurons, the results demonstrate that topographical differences in sex-dependent neurogenesis may contribute to key neural modification that enhances fitness in cowbirds. This is similar to results reported by Guigueno et al. (Citation2016a), who found sex-dependent DCX+ in the Hp of cowbirds whereas no sex difference was demonstrated in a non-parasitic relative. The study presented here reveals that this sex-dependent difference is primarily within the rostral subdivision of the Hp of cowbirds.
The DCX+ cell pattern within the Hp reveals a topographic distribution in both male and female cowbirds along the rostral–caudal extent. Doublecortin cells are low in caudal Hp but significantly increase in both sexes in the mid- and rostral sub-regions of Hp. DCX+ cell counts across the entire extent of the Hp (i.e. caudal, mid and rostral sub-regions) reveal significant sex-related differences, specifically in the rostral Hp. Moreover, the increase in DCX+ cells in the rostral subdivision of Hp does not occur in Ha (i.e. the control region) or in males, indicating the rostral Hp exhibits sex- and region-specific neuronal recruitment. The average DCX cell count in Ha is greater in comparison to the caudal Hp, indicating that this sub-region displays little specificity in neuronal recruitment. Such gradients in new neuron recruitment across the Hp have also been reported in other bird species, including food storing birds (Barnea & Nottebohm Citation1994). For instance, cell proliferation in free ranging black capped chickadees is greater in rostral Hp as compared to mid or caudal Hp regions, and the caudal Hp in these birds contained the lowest number of new neurons as compared to the other Hp sub-regions (Barnea & Nottebohm Citation1994). A functional topography of the Hp has been described in mammals for some time now and this functional topography is mirrored by differences in adult neurogenesis (Jinno Citation2011a,b). For instance, dorsal Hp is involved in spatial memory, whereas ventral Hp is related to emotion, anxiety and social memory, and these functional differences are mirrored by adult neurogenesis patterns along the ventral–dorsal extent (Jinno Citation2011b). For instance, numbers of DCX+ cells were significantly higher in the suprapyramidal region of the dorsal hippocampus (dHp) compared to other subdivisions (Jinno Citation2011b). The topography of cell proliferation in mammalian Hp suggests dorsal Hp might be responsible for spatial learning and memory, whereas ventral Hp might be associated with emotional processes. Although it is not clear how this functional topography relates to the function of the Hp subdivisions in birds, unpublished studies in cowbirds reveal that activity-dependent gene expression in the dorsal Hp of juvenile cowbirds represents social memory of song only after repeated exposure to heterospecific and conspecific songs (K. S. Lynch, unpublished data). The pattern of activity-dependent gene expression reflected results previously reported in the caudalmedial mesopallium (CMM) of the auditory forebrain (Lynch et al. Citation2017), suggesting the dHp and CMM serve similar roles in social memory of previously experienced songs. Together, these studies in the cowbird and mammal Hp highlights the importance of considering topographical differences when evaluating changes in adult neurogenesis or function.
Adult neurogenesis is regulated by internal and external factors such as species, sex, singing activity, social environment, environmental conditions, hormones, seasonality, photoperiod, stress, aging and activity levels (Patel et al. Citation1997; Adar et al. Citation2008; Day et al. Citation2008; LaDage et al. Citation2009, Citation2010, Citation2011; Delgado-Gonzalez et al. Citation2011; Yamamura et al. Citation2011; Maruska et al. Citation2012; Onksen et al. Citation2012; Alward et al. Citation2014). In food caching bird species neuron recruitment is also regulated by experiences with food caching and retrieval (Clayton & Krebs Citation1994; Clayton Citation1995a, Citation1996; Patel et al. Citation1997). Even short, limited experience with hoarding can initiate cell proliferation in these species (Patel et al. Citation1997). These converging results reveal that seasonal changes in spatial memory demands and performing spatial memory tasks regulate neuroanatomical plasticity in the avian Hp. However, it is still unclear whether the regulatory mechanism of this seasonal change in the brain is related to photoperiod, experience or hormonal changes that coincide with changes in season and/or experience. Restricting experiences such as food caching or nest searching reduces Hp plasticity, including new cell recruitment (Day et al. Citation2008; LaDage et al. Citation2009). Here, captivity, even within natural conditions, may have restricted experiences that would lead to greater DCX+ cells, and therefore it is not possible to know the absolute extent of new cell recruitment that can happen without any captivity. In this case, it is females that are primarily restricted from performing key behaviors during captivity that may lead to new cell recruitment, yet this study still reveals that relative to males, there are greater numbers of DCX+ cells in the rostral Hp, regardless of restricted experience. However, if stress regulates neuron recruitment and there are sex differences in stress, this may account for sex differences in sub-regional DCX differences as well.
Whether newly added neurons are functional in the Hp or what their function may be is also not clear. In mammals, the addition of new neurons underlies a process of net growth (Bayer et al. Citation1982), whereas in other animals, particularly birds, new neuron recruitment makes up for cell loss, with no net gain in neuron numbers rather than net addition of neurons (Kirn & Nottebohm Citation1993; Scharff Citation2000). Thus, it is not clear whether these neurons contribute to sex-difference in Hp volumes in cowbirds, but they may contribute to the formation of new spatial memories that is required to remember the location of nests and the stage of each located nest. It is possible that neuronal architecture of Hp undergoes enduring changes by long-term memories and these memories are eventually replaced to create space for new, updated memories. Thus, this exploratory study simply defines the sub-region of the Hp in which sex-dependent new neuron recruitment occurs. It remains to be tested whether new neuron recruitment changes are directly related to associated changes in hormonal milieu that are associated with changing seasons and experiences, and what the function of these new neurons is in relation to spatial memory.
Acknowledgements
The author thanks Balcones Wildlife Refuge for support in the field and Texas EcoLabs for financial support of this project. The author would like to thank many undergraduate and graduate students and one high school student for assistance in the field and the lab, including Nathan Wilkens, Inderdeep Jaswal, Jamie Scala and Anthony Bogden. The author also thanks Tim LeFevre for his assistance with animal care. Permit approval numbers include: IACUC approval 13/14-18; Texas state scientific collecting permit SPR-0313-035; federal scientific collecting permit MB96705A-1; and Balcones Wildlife Refuge research and monitoring special use permit 1018-0102.
References
- Adar E, Nottebohm F, Barnea A. 2008. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. Journal of Neuroscience 14:5394–5400. doi:10.1523/JNEUROSCI.5706-07.2008
- Alward BA, Mayes WD, Peng K, Stevenson TJ, Balthazart J, Ball GF. 2014. Dissociable effects of social context on song and doublecortin immunoreactivity in male canaries. European Journal of Neuroscience 40:2941–2947. doi:10.1111/ejn.2014.40.issue-6
- Atoji Y, Sarkar S, Wild JM. 2016. Proposed homology of the dorsomedial subdivision and V-shaped layer of the avian hippocampus to Ammon’s horn and dentate gyrus, respectively. Hippocampus 26:1608–1617. doi:10.1002/hipo.v26.12
- Atoji Y, Wild JM. 2006. Anatomy of the avian hippocampal formation. Review of Neuroscience 17:3–15. doi:10.1515/REVNEURO.2006.17.1-2.3
- Bailey DJ, Rosebush JC, Wade J. 2002. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. Journal of Neurobiology 52:43–51. doi:10.1002/(ISSN)1097-4695
- Balthazart J, Boseret G, Konkle AT, Hurley LL, Ball GF. 2008. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. European Journal of Neuroscience 27:801–817. doi:10.1111/j.1460-9568.2008.06059.x
- Barnea A, Nottebohm F. 1994. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proceedings of the National Academy of Science USA 91:11217–11221. doi:10.1073/pnas.91.23.11217
- Bayer SA, Yackel JW, Puri PS. 1982. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216:890–892. doi:10.1126/science.7079742
- Biegler R, McGregor A, Krebs JR, Healy SD. 2001. A larger hippocampus is associated with longer-lasting spatial memory. Proceedings of the National Academy of Science USA 98:6941–6944. doi:10.1073/pnas.121034798
- Bingman VP, Mench JA. 1990. Homing behavior of hippocampus and parahippocampus lesioned pigeons following short-distance releases. Behavioral Brain Research 40:227–238. doi:10.1016/0166-4328(90)90079-T
- Boseret G, Ball GF, Balthazart J. 2007. The microtubule-associated protein doublecortin is broadly expressed in the telencephalon of adult canaries. Journal of Chemical Neuroanatomy 33:140–154. doi:10.1016/j.jchemneu.2007.02.002
- Broadbent NJ, Squire LR, Clark RE. 2004. Spatial memory, recognition memory, and the hippocampus. Proceedings of the National Academy of Science USA 101:14515–14520. doi:10.1073/pnas.0406344101
- Burger DK, Gulbrandsen T, Saucier DM, Iwaniuk AN. 2014. The effects of season and sex on dentate gyrus size and neurogenesis in a wild rodent, Richardson’s ground squirrel (Urocitellus richardsonii). Neuroscience 11:240–251. doi:10.1016/j.neuroscience.2014.04.067
- Burger DK, Saucier JM, Iwaniuk AN, Saucier DM. 2013. Seasonal and sex differences in the hippocampus of a wild rodent. Behavioral Brain Research 236:131–138. doi:10.1016/j.bbr.2012.08.044
- Burgess N, Maguire EA, O’Keefe J. 2002. The human hippocampus and spatial and episodic memory. Neuron 35:625–641. doi:10.1016/S0896-6273(02)00830-9
- Cheng M, Chaiken M, Zuo M, Miller H. 1999. Nucleus taenia of the amygdala of birds: Anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris). Brain Behavior Evolution 53:243–270. doi:10.1159/000006597
- Clayton NS. 1995a. The neuroethological development of food-storing memory: A case of use it or lose it!. Behavioral Brain Research 70:95–102. doi:10.1016/0166-4328(95)00133-E
- Clayton NS. 1995b. Comparative studies of food-storing, memory, and the hippocampal formation in parids. Hippocampus 5:499–510. doi:10.1002/(ISSN)1098-1063
- Clayton NS. 1996. Development of food-storing and the hippocampus in juvenile marsh tits (Parus palustris). Behavioral Brain Research 74:153–159. doi:10.1016/0166-4328(95)00049-6
- Clayton NS, Krebs JR. 1994. Hippocampal growth and attrition in birds affected by experience. Proceedings of the National Academy of Science USA 91:7410–7414. doi:10.1073/pnas.91.16.7410
- Crespo D, Stanfield BB, Cowan WM. 1986. Evidence that late-generated granule cells do not simply replace earlier formed neurons in the rat dentate gyrus. Experimental Brain Research 62:541–548. doi:10.1007/BF00236032
- Cross DJ, Marzluff JM, Palmquist I, Minoshima S, Shimizu T, Miyaoka R. 2013. Distinct neural circuits underlie assessment of a diversity of natural dangers by American crows. Proceedings of the Royal Society Biological Sciences 280:20131046. doi:10.1098/rspb.2013.1046
- Davies NB. 2000. Cuckoos, cowbirds and Other Cheats. London: T & AD Poyser Ltd.
- Day LB, Guerra M, Schlinger BA, Rothstein SI. 2008. Sex differences in the effects of captivity on hippocampus size in brown-headed cowbirds (Molothrus ater obscurus). Behavioral Neuroscience 122:527–534. doi:10.1037/0735-7044.122.3.527
- Delgado-Gonzalez FJ, Gonzalez-Granero S, Trujillo-Trujillo CM, García-Verdugo JM, Damas-Hernandez MC. 2011. Study of adult neurogenesis in the Gallotia galloti lizard during different seasons. Brain Research 16:50–58. doi:10.1016/j.brainres.2011.03.027
- Fox RA, Roth II TC, LaDage LD, Pravosudov VV. 2010. No effect of social group composition or size on hippocampal formation morphology and neurogenesis in mountain chickadees (Poecile gambeli). Developmental Neurobiology 70:538–547.
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. 1999. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 23:247–256. doi:10.1016/S0896-6273(00)80777-1
- Galea LA, McEwen BS. 1999. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89:955–964. doi:10.1016/S0306-4522(98)00345-5
- Garthe A, Kempermann G. 2013. An old test for new neurons: Refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Frontiers in Neuroscience 7:1–10.
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. 1999. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271.
- Gu Y, Janoschka S, Ge S. 2012. Neurogenesis and hippocampal plasticity in adult brain. Current Topics in Behavioral Neuroscience 15:31–48.
- Guigueno MF, MacDougall-Shackleton SA, Sherry DF. 2016a. Sex and seasonal differences in hippocampal volume and neurogenesis in brood-parasitic brown-headed cowbirds (Molothrus ater). Developmental Neurobiology 76:1275–1290.
- Guigueno MF, Sherry DF, MacDougall-Shackleton SA. 2016b. Sex and seasonal differences in neurogenesis and volume of the song-control system are associated with song in brood-parasitic and non-brood-parasitic icterid songbirds. Developmental Neurobiology 76:1226–1240.
- Guigueno MF, Snow DA, MacDougall-Shackleton SA, Sherry DF. 2014. Female cowbirds have more accurate spatial memory than males. Biology Letters 10:20140026.
- Hampton RR, Shettleworth SJ. 1996. Hippocampal lesions impair memory for location but not color in passerine birds. Behavioral Neuroscience 110:831–835.
- Healy SD, Gwinner E, Krebs JR. 1996. Hippocampal volume in migratory and non-migratory warblers: Effects of age and experience. Behavioral Brain Research 8:61–68.
- Hoover JP, Robinson SK. 2007. Retaliatory mafia behavior by a parasitic cowbird favors host acceptance of parasitic eggs. Proceedings of the National Academy of Sciences USA 104:4479–4483.
- Jinno S. 2011a. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. Journal of Comparative Neurology 519:451–466.
- Jinno S. 2011b. Topographic differences in adult neurogenesis in the mouse hippocampus: A stereology-based study using endogenous markers. Hippocampus 21:467–480.
- Kirn JR, Nottebohm F. 1993. Direct evidence for loss and replacement of projection neurons in adult canary brain. Journal of Neuroscience 13:1654–1663.
- Krebs JR. 1990. Food-storing birds: Adaptive specialization in brain and behaviour? Philosophical Transactions of the Royal Society B: Biological Sciences 2:153–160.
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. 1989. Hippocampal specialization of food-storing birds. Behavior Brain Research 86:1388–1392.
- LaDage LD, Roth TC, Fox RA, Pravosudov VV. 2009. Effects of captivity and memory-based experiences on the hippocampus in mountain chickadees. Behavioral Neuroscience 123: 284–291.
- LaDage LD, Roth 2nd TC, Fox RA, Pravosudov VV. 2010. Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proceedings of the Royal Society: Biological Sciences 277:1071–1079.
- LaDage LD, Roth 2nd TC, Pravosudov VV. 2011. Hippocampal neurogenesis is associated with migratory behaviour in adult but not juvenile sparrows (Zonotrichia leucophrys ssp.). Proceedings of the Royal Society: Biological Sciences 278:138–143.
- Leuner B, Gould E. 2010. Structural plasticity and hippocampal function. Annual Review of Psychology 61:111–140.
- Lucas JR, Brodin A, de Kort SR, Clayton NS. 2004. Does hippocampal size correlate with the degree of caching specialization? Proceedings of the Royal Society: Biological Sciences 7:2423–2429.
- Lynch KS, Gaglio A, Tyler E, Coculo J, Louder MI, Hauber ME. 2017. A neural basis for password-based species recognition in an avian brood parasite. Journal of Experimental Biology 220:2345–2353.
- Maruska KP, Carpenter RE, Fernald RD. 2012. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. Journal of Comparative Neurology 520:3471–3491.
- McDonald KS, Kirn JR. 2012. Anatomical plasticity in the adult zebra finch song system. Journal of Comparative Neurology 520:3673–3686.
- Melleu FF, Santos TS, Lino-de-Oliveira C, Marino-Neto J. 2013. Distribution and characterization of doublecortin-expressing cells and fibers in the brain of the adult pigeon (Columba livia). Journal of Chemical Neuroanatomy 47:57–70.
- Norman RF, Robertson RJ. 1975. Nest-searching behavior in the brown-headed cowbird. Auk 92:610–611.
- Onksen JL, Briand LA, Galante RJ, Pack A, Blendy JA. 2012. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behavior 11:529–538.
- Ortega CP, Ortega JC, Backensto SA, Rapp CA. 1996. Improved methods for aging second-year and after-second-year male brown-headed cowbirds. Journal of Field Ornithology 67:542–548.
- Patel SN, Clayton NS, Krebs JR. 1997. Spatial learning induces neurogenesis in the avian brain. Behavior Brain Research 89:115–128.
- Pravosudov VV, Kitaysky AS, Omanska A. 2006. The relationship between migratory behaviour, memory and the hippocampus: An intraspecific comparison. Proceedings of the Royal Society: Biological Sciences 273:2641–2649.
- Reboreda JC, Clayton NS, Kacelnik A. 1996. Species and sex differences in hippocampus size in parasitic and non-parasitic cowbirds. Neuroreport 31:505–508.
- Rehkämper G, Haase E, Frahm HD. 1988. Allometric comparison of brain weight and brain structure volumes in different breeds of the domestic pigeon, Columba livia f.d. (fantails, homing pigeons, strassers). Brain Behavior and Evolution 31:141–149.
- Rodriguez F, Lopez JC, Vargas JP, Gomez Y, Broglio C, Salas C. 2002. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. Journal of Neuroscience 22:2894–2903.
- Rothstein SI, Yokel DA, Fleischer RC. 1987. Social dominance, mating and spacing systems, female fecundity, and vocal dialects in captive and free-ranging brown-headed cowbirds. In: Johnston RF, editor. Current ornithology. Vol. 3. New York, NY: Plenum. pp. 127–185.
- Scharff C. 2000. Chasing fate and function of new neurons in adult brains. Current Opinion in Neurobiology 10:774–783.
- Sherry DF, Forbes MR, Khurgel M, Ivy GO. 1993. Females have a larger hippocampus than males in the brood-parasitic brown-headed cowbird. Proceedings of the National Academy of Science 90:7839–7843.
- Sherry DF, Hoshooley JS. 2010. Seasonal hippocampal plasticity in food-storing birds. Philisophical Transactions of Royal Society London B Biologic Sciences 365:933–943.
- Sherry DF, MacDougall-Shackleton SA. 2015. Seasonal change in the avian hippocampus. Frontiers in Neuroendocrinology 37:158–167.
- Sherry DF, Vaccarino AL. 1989. Hippocampus and memory for food caches in black capped chickadees. Behavioral Neuroscience 103:308–318.
- Sherry DF, Vaccarino AL, Buckenham K, Herz RS. 1989. The hippocampal complex of food-storing birds. Brain Behavior Evolution 34:308–317.
- Stokes TM, Leonard CM, Nottebohm F. 1974. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. Journal of Comparative Neurology 156:337–374.
- Székely AD. 1999. The avian hippocampal formation: Subdivisions and connectivity. Behavior Brain Research 98:219–225.
- Teles MC, Cardoso SD, Oliveira RF. 2016. Social plasticity relies on different neuroplasticity mechanisms across the brain social decision-making network in zebrafish. Frontiers Behavioral Neuroscience 16:16. doi:10.3389/fnbeh.2016.00016
- Tronel S, Fabre A, Charrier V, Oliet SH, Gage FH, Abrous DN. 2010. Spatial learning sculpts the dendritic arbor of adult-born hippocampal neurons. Proceedings of the National Academy of Science 107:7963–7968.
- van Praag H, Shubert T, Zhao C, Gage FH. 2005. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience 25:8680–8685.
- White DF, Ho L, Freed-Brown G. 2009. Counting chicks before they hatch: Female cowbirds can time readiness of a host nest for parasitism. Psychological Science 20:1140–1145.
- Yamamura T, Barker JM, Balthazart J, Ball GF. 2011. Androgens and estrogens synergistically regulate the expression of doublecortin and enhance neuronal recruitment in the song system of adult female canaries. Journal of Neuroscience 31:9649–9657.
- Zupanc GK. 2006. Neurogenesis and neuronal regeneration in the adult fish brain. Journal of Comparative Physiology A 192:649–670.
