Abstract
In the last few decades, macrobenthic community structures and their species abundances have shown significant changes in the Mediterranean Sea, whose causes were attributed to anthropogenic activities and to global warming effects. The Mediterranean sponges have shown a peculiar sensitivity to these changes: the populations of some species showed significant decreases, while others, more thermophilous, increased. Therefore, sponges may be a good proxy for evaluating the effects of environmental changes. Thanks to the observations conducted by Sarà about 55 years ago, a comparative analysis of the sponge populations present within two semi-submerged caves in the Ligurian Sea was possible. The two sponge assemblages re-studied in 2016 showed an increase in terms of specific richness and a significant change in their structural aspects, since the three-dimensional growth forms were mostly replaced by two-dimensional ones, a process observed also in other littoral communities. Consequently, the sponge communities inside the semi-submerged caves may be considered poorly resilient: the massive sponges were hit by the positive thermal anomalies occurring in the Ligurian Sea in the last decade and were replaced by encrusting forms, within a possible phase of cave recolonisation.
Introduction
In the last few decades, Mediterranean hard-bottom macrobenthic communities have undergone significant structural changes in terms of species richness and abundance, whose causes were attributed to anthropogenic activities and/or global warming effects (Cerrano et al. Citation2000, Citation2005; Pérez et al. Citation2000; Coma et al. Citation2004; Linares et al. Citation2005; Thibaut et al. Citation2005; Airoldi & Beck Citation2007; Garrabou et al. Citation2009; Claudet & Fraschetti Citation2010; Coll et al. Citation2010; Fraschetti et al. Citation2011; Sala et al. Citation2012; Rossi Citation2013).
Also in the Ligurian Sea, recurrent water-temperature anomalies and periodical mucilaginous proliferations caused often multiphyletic mass mortalities, leading to radical changes in the coastal communities (Bavestrello et al. Citation1994; Cerrano et al. Citation2000; Morri & Bianchi Citation2001; Bianchi & Morri Citation2004; Schiaparelli et al. Citation2007; Garrabou et al. Citation2009; Cattaneo-Vietti et al. Citation2010; Parravicini et al. Citation2010; Bertolino et al. Citation2016; Betti et al. Citation2017; Bianchi et al. Citation2017; Longobardi et al. Citation2017; Cattaneo-Vietti Citation2018). Moreover, some boreal species disappeared and others, more thermophilous, arose (Bianchi et al. Citation2001; Cerrano et al. Citation2006; Puce et al. Citation2009; Roghi et al. Citation2010; Parravicini et al. Citation2013, Citation2015; Gatti et al. Citation2015), with a significant spread of warm-water native species (WWS) and subtropical non-indigenous species (NIS) which has led to a “meridionalisation”, if not to a “tropicalisation”, of the Ligurian Sea (Bianchi et al. Citation2017).
In this age of changes, the Mediterranean sponges have shown a peculiar sensitivity to global warming effects (Bianchi et al. Citation2014a,b; Bertolino et al. Citation2016). Along the vertical cliffs of the Portofino Promontory (Ligurian Sea), the frequencies of massive and/or erect demosponges ((Calyx nicaensis (Risso, 1826), Spongia (Spongia) officinalis Linnaeus, 1759, S. (Spongia) lamella (Schulze, 1879), Petrosia (Petrosia) ficiformis (Poiret, 1789), Chondrosia reniformis Nardo, 1847, Axinella spp.)) have shown, in the last few decades, a significant decrease, while those with encrusting growth patterns, e.g. Spirastrella cunctatrix Schmidt, 1868 and Crambe crambe (Schmidt, 1862), have experienced significant expansion (Bertolino et al. Citation2016; Betti et al. Citation2017). Moreover, a calcarean sponge of tropical origin, Paraleucilla magna Klautau, Monteiro and Borojevic, 2004, appeared in the Ligurian waters for the first time in 2012 (Bertolino et al. Citation2014b; Ulman et al. Citation2017). Consequently, sponges may be a good proxy for evaluating possible changes in the structure of littoral hard-bottom benthic communities.
Marine caves are peculiar and vulnerable ecosystems (Sarà Citation1974, Citation1978; Harmelin et al. Citation1985), where sponges are particularly abundant, in terms of diversity, coverage and biomass (Gerovasileiou et al., Citation2015a). Today, these habitats are considered endangered in the Mediterranean Sea because of their particular sensitivity to pollution, marine litter, thermal anomalies, alien species occurrence and unregulated underwater activities (Chevaldonné & Lejeusne Citation2003; Cicogna et al. Citation2003; Martì et al. Citation2004; Di Franco et al. Citation2010; Gerovasileiou & Voultsiadou Citation2012, Citation2016; Guarnieri et al. Citation2012; Gerovasileiou et al. Citation2015b; Nepote et al. Citation2017; Montefalcone et al. Citation2018). Therefore, they are listed in the EC Habitat Directive (92/43/EEC, Habitat type 8330) as well as in the Barcelona Convention (UNEP-MAP-RAC/SPA Citation2008, Citation2015; Giakoumi et al. Citation2013) and integrated into the Action Plan for the Conservation of Coralligenous and Other Calcareous Bioconcretions in the Mediterranean Sea (UNEP-MAP-RAC/SPA Citation2008).
Semi-submerged caves are particularly suitable to verify the temporal stability of or the advent of possible changes to the benthic coastal rocky communities at a local scale, because they are easily recoverable due to their certain geographical position. Moreover, light and water movement gradients within caves cause different physical conditions along the horizontal cave axis which, in turn, promote a wide richness in small confined spaces (Dimarchopoulou et al. Citation2018). Furthermore, the lack of algal coverage facilitates the observation of zoobenthic organisms.
Recently, an ecosystem-based method for evaluating the ecological quality of Mediterranean caves (CavEBQI) was developed, opening new perspectives to the study of disturbance impacts at large geographic and temporal scales (Rastorgueff et al. Citation2015). However, these kinds of studies tend to consider and evaluate affinity, stability and changes at large systematic or functional group levels, whereas variations are often recordable at the specific level only. Bearing this in mind, the study of the sponge fauna permits us to check not only the ecological quality of littoral habitats, but also the effects of possible long-lasting environmental changes (Bertolino et al. Citation2014a, Citation2016).
Unfortunately, the lack of historical data sets and time series on local sponge biodiversity does not allow the verification of possible changes in the structure of their populations, even in the light of the global change. Fortunately, in 1961–1963, Michele Sarà (1926–2006), a pioneer of Italian sponge taxonomy, analysed the sponge assemblages present in two small semi-submerged caves in the Ligurian Eastern Riviera (Ligurian Sea) (Sarà Citation1964).
The main goals of this paper were to evaluate possible qualitative changes in the sponge biodiversity of the two semi-submerged caves after a half-century time span – a period characterised by important global and human-driven structural changes (e.g. Parravicini et al. Citation2013; Bertolino et al. Citation2016; Betti et al. Citation2017; Longobardi et al. Citation2017).
Material and methods
Site characteristics
For three summers (1961–1963), Sarà (Citation1964) surveyed and studied the sponge communities of two semi-submerged caves located in the Eastern Ligurian Riviera, called Bonassola Cave and Zoagli Cave after the names of the closest respective villages (). The distance between the two caves is about 25 km.
Figure 1. (a) Position of the two studied caves along the Ligurian coast. (b, c) Planimetry sketches of Bonassola (b) and Zoagli (c) caves.
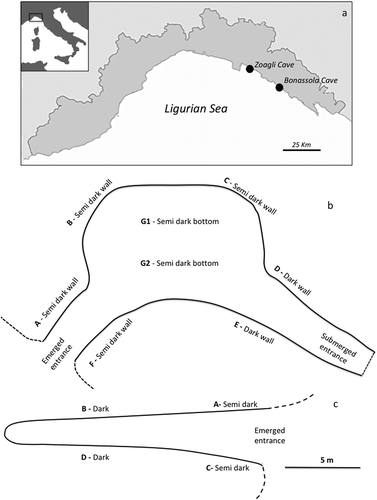
The Bonassola Cave is a semi-submerged, horizontal cave which opens on gabbro rocks about 1 km eastward of the homonymous village (). The bottom of the cave consists of irregular boulders and pebbles. The cavity is accessed through a 4–5 m long passage, 2–2.5 m wide and with a ceiling 2 m above sea level. This corridor opens into a large cavity (12 m × 7 m), with a height of about 6 m. Numerous crevices and small galleries are present on the side walls. The cave continues in a south-east direction with a passage, 12 m long and 2 m wide, connected to the exterior through a second, fully submerged opening. Inside the cave, sunlight penetrates mainly through a wide opening in the vault. Light conditions create a semi-obscure environment, while total darkness is reached only in parts of the walls and in the south-east corridor. Depending on light intensity and cave morphology, it is possible to distinguish three zones, according to Sarà (Citation1964): the semi-dark walls, the dark walls and the semi-dark bottom of the cave.
The Zoagli Cave is a small blind-end (cul-de-sac) semi-submerged cave, which opens in a marly cliff about 1 km west of the Zoagli village (). It is about 16 m long and 4 m wide at the entrance, with a height of the vault decreasing from about 3 m at the entrance to about 1 m at the end of the cave. The pebble floor steadily rises from a depth of 2 m to the end of the cave where it forms a small beach. The walls are mostly smooth. The opening is oriented to the east. According to Sarà’s (Citation1964) observations, four different zones with different light intensity can be recognised: A-zone (exposed towards the south), external and semi-dark; B-zone (towards the south), internal and dark; C-zone (towards the north), external and semi-dark; and D-zone (towards the north), internal and dark.
During summer 2016, the sponge assemblages of these caves were studied again; samples were collected for taxonomic determinations and photographs were taken on standard surfaces of 400 cm2 in the different sectors defined by Sarà (Citation1964), according to the light intensity. In the Bonassola Cave, 10 replicates were conducted in each of eight sectors: four on the cave walls (A, B, C, F) characterised by semi-dark conditions; two on the bottom in semi-darkness (G1, G2) and two on the dark walls (D, E), for a total of 80 images (). In the Zoagli Cave, 10 replicates were conducted in each of the four sectors, two (A, C) characterised by semi-dark conditions and two (B, D) by dark conditions, for a total of 40 images (). For each cave data were elaborated by grouping the different sectors according to the light intensity conditions (Sarà Citation1964). For each light condition the abundance of each species was evaluated as percent presence in the studied standard surfaces. According to the growth habit, the recorded sponges were divided into encrusting (En) and massive species (Ms).
To compare the recent data with those of Sarà (Citation1964), who used a semi-quantitative analysis, we have transformed our data (presented in and ) as follows: +, present (< 5% of the studied images); ++ abundant (5–24% of the studied images); +++, very abundant (> 24% of the studied images).
Table I. Sponge diversity and abundance in the Bonassola Cave in both considered periods. En, encrusting sponges; Ms, massive sponges.
Table II. Sponge diversity and abundance in the Zoagli Cave in both considered periods. En, encrusting sponges; Ms, massive sponges.
Results
During the 2016 survey, a total of 30 species were found in both of the studied semi-submerged caves; seven of them were shared by the two caves.
The Bonassola Cave, characterised by a complex morphology, hosted 21 species (; ). Sarcotragus spinulosus Schmidt, 1862 was the most abundant species in each examined sector. Other frequent species were Erylus discophorus (Schmidt, 1862), Aplysina sp., Ircinia variabilis (Schmidt, 1862), Spongia (Spongia) officinalis Linnaeus, 1759, and Rhabderemia topsenti van Soest and Hooper, Citation1993 recorded with different frequencies in the three sectors (). The richest sector was the dark wall, followed by the semi-dark wall and by the semi-dark bottom (). In the complex the number of encrusting species was double that of the massive ones (). The sponge assemblages present in the three zones of the cave were widely different: 24% of the recorded species were shared by all zones, while 57% were recorded in a single zone.
Figure 2. Number of sponge species recorded in the studied caves during both periods. Grey bars represent the species in common between the two periods.
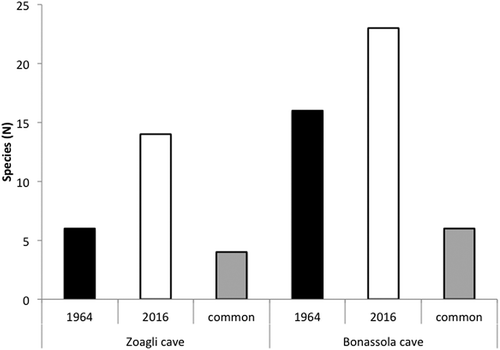
Figure 3. Frequencies of the most abundant sponge species in both caves under different light conditions and positions. The frequency is the percentage presence of a species in the studied standard surface.
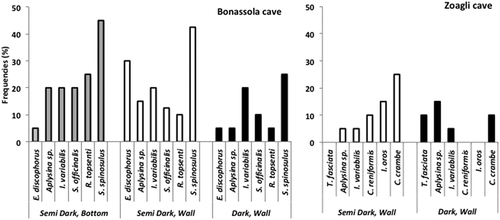
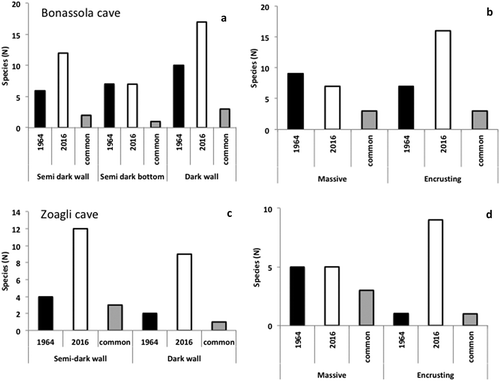
Figure 4. (a, c) Number of sponge species recorded in the different zones and in both sampling periods within the two studied caves. (b, d) Number of sponge species in both caves according to different growth patterns and sampling periods.
The Zoagli Cave assemblage included 13 demosponges and the calcarean Clathrina coriacea (Montagu, 1814) (; ). The different sectors of the cave are quite different in terms of species composition: Crambe crambe (Schmidt, 1862) and Ircinia oros (Schmidt, 1864) were the most abundant species on semi-dark walls while in the dark area the most frequent sponges were C. crambe, Aplysina sp. and Timea fasciata Topsent, 1934 (). The number of species was higher in the semi-dark zone while, in accordance with the growth patterns, the encrusting species represented the higher number (). In this cave, half of the observed species were recorded only in one zone.
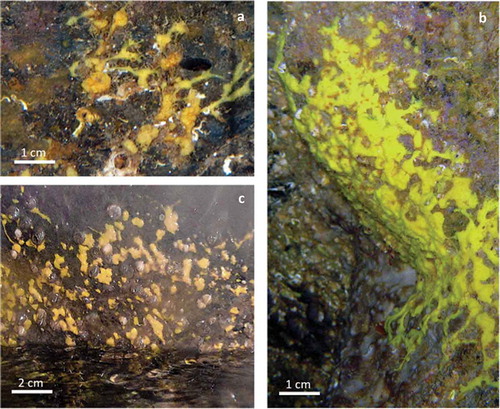
Particularly interesting was the finding of a species of Aplysina () characterised by only one kind of pithed fibres devoid of foreign bodies, forming a regular network of large polygonal meshes as usual in the genus. Nevertheless, the growth habit of our specimens differs from that shown by the two Mediterranean species of the genus: A. aerophoba (Nardo, 1833) and A. cavernicola (Vacelet, 1959). In fact, the specimens recorded in the caves are small, yellow, cushion-shaped sponges (2–5 mm in diameter). They form clusters composed of 3–10 specimens joined together by thin, sometimes branching processes (). Sometimes several small specimens fuse together, forming large encrusting plates (). The colour and the conulose surface of the specimens are the same as in the other two Mediterranean Aplysina. The species is widely distributed in the semi-dark and dark portions of both caves. Several specimens recorded in the tidal zone of the Zoagli Cave remain completely emerged during low tides ().
Figure 5. Aplysina sp. (a) Cushion-shaped specimens joined together by thin, sometimes branching processes. (b) Large encrusting specimens formed by the coalescence of several small sponges. (c) Emerged specimens during a low tide.
Three other species, Clathria (Clathria) depressa Sarà and Melone, 1966, Stelletta hispida (Buccich, 1886) and Latrunculia (Biannulata) citharistae Vacelet, 1969, were recorded for the first time in the Ligurian Sea.
The comparative analysis of our data and those reported by Sarà (Citation1964), who visited these semi-submerged caves several times between 1961 and 1963, was surprising. Firstly, the number of species recorded by Sarà in both the caves was about half that found in our survey ( and II; ). Secondly, the species composition appeared widely changed ( and II).
In the Bonassola Cave, only six species (Erylus discophorus, Spongia (Spongia) officinalis, Scalarispongia scalaris (Schmidt, 1862), Crambe crambe, Ircinia variabilis and Rhabderemia topsenti van Soest and Hooper Citation1993) are still present () while 10 species have disappeared. Among them, five species (Aplysilla sulfurea Schulze, 1878, Haliclona (Haliclona) varia (Sarà, Citation1958), Mycale (Aegogropila) tunicata (Schmidt, 1862), Phorbas fictitius and Spongia (Spongia) virgultosa) were considered very abundant by Sarà (Citation1964).
In the Zoagli Cave, only four species (Clathrina coriacea, Crambe crambe, Chondrosia reniformis and Ircinia oros) were recorded again, while two species, Dysidea fragilis and Haliclona (Reniera) cinerea, disappeared.
Despite changes in the specific composition, the sponge distribution in the different zones of the caves remained quite similar: in general, the species richness increased in each zone of the caves ( and II; ).
As for growth patterns, the number of massive species decreased or remained constant in both caves, whereas the encrusting ones strongly increased ( and ; ).
Discussion
In the absence of long-term series of historical data that could provide a reliable picture of the environmental changes within marine communities (Boero et al. Citation2015), the comparative analysis of richness and abundance of a peculiar taxon such as Porifera in two different temporal periods remains a good tool for highlighting the changes that have occurred. Sponges have been recently suggested as a surrogate taxon for the structural and functional study of sessile benthic diversity in Mediterranean marine caves (Gerovasileiou et al. Citation2017). They appear therefore a good proxy, being the most specific taxon inside Mediterranean hard-bottom benthic communities and in particular within marine caves, where they were widely studied (Sarà Citation1958, Citation1959, Citation1961a,b, 1962, Citation1968; Labate Citation1965; Pansini et al. Citation1977; Bibiloni et al. Citation1989; Corriero et al. Citation1997, Citation2000, Citation2003; Harmelin et al. Citation2003; Bussotti et al. Citation2006; Gerovasileiou & Voultsiadou Citation2012).
Species composition, abundance and distribution of sponges within a cave are driven not only by its dimensions, but also by the differences of light and water-movement gradients related to exposition and topography of the cave. In particular, semi-submerged, tunnel-shaped caves generally show richer communities due to the more intense water movement usually recorded in these spots (Riedl Citation1966; Balduzzi et al. Citation1989; Martì et al. Citation2004; Gerovasileiou & Voultsiadou Citation2016). Moreover, caves are particularly selective environments and each seems to host its own sponge assemblage (Corriero et al. Citation2003). Only a small number of species are, in fact, widespread within Mediterranean marine caves: Petrosia (Petrosia) ficiformis, Ircinia variabilis, Agelas oroides and Spirastrella cunctatrix were found in one-third of the explored Mediterranean caves (Gerovasileiou & Voultsiadou Citation2012).
However, attention must be paid to the fact that 67% of the species recorded in total were found in less than five caves and 34.5% were found only in one (Gerovasileiou & Voultsiadou Citation2012). This is particularly true for the semi-submerged caves characterised by the presence of sciaphilous species, also able to live outside. The most frequent species, present in almost 70% of the 10 semi-submerged caves until now analysed along the Italian coast (Cinelli et al. Citation1977; Pansini et al. Citation1977; Corriero Citation1989; Corriero et al. Citation1997, Citation2000), were Clathrina coriacea, Crambe crambe, Spirastrella cunctatrix, Chondrosia reniformis and Ircinia variabilis. The above-cited species were recorded also during the study of the Sarà caves in the Ligurian Sea.
Moreover, in this type of caves massive species tend to show an encrusting growth habit, becoming flat, as a consequence of the intense water-movement (Corriero et al. Citation2003).
Long-term studies to evaluate possible changes in the sponge composition and structure within Mediterranean caves are yet to come. Thanks to the observations conducted by Sarà (Citation1964) about 55 years ago, a first comparative analysis of the sponge assemblages present within two semi-submerged caves in the Ligurian Sea was possible. During this time span, the sponge assemblages of the considered caves experienced an increase in terms of specific richness and a significant change in the structural aspects. The three-dimensional growth forms decreased or remained quite similar, while numerous new two-dimensional ones were recorded in both caves, regardless of differences in cave morphologies, sunlight gradients, and water-movement and lithology variables which determine the spatial distribution of each species. Similar results were obtained by Montefalcone et al. (Citation2018) studying the benthic community settled inside the Bergeggi Cave (Western Ligurian Riviera).
A reduction in massive demosponges in the Mediterranean was also observed in other littoral communities (Bertolino et al. Citation2016; Betti et al. Citation2017). In particular, species of the genus Spongia have suffered intense mass mortalities since the last two decades of the past century (Cerrano et al. Citation2000; Garrabou et al. Citation2009). Our data are in agreement with this tendency. On the other hand, the Mediterranean rocky communities have experienced a widespread increase of encrusting species in terms of both diversity and abundance (Cerrano et al. Citation2000; Bianchi et al. Citation2014a,b; Bertolino et al. Citation2016; Betti et al. Citation2017). Also, palaeontological data, obtained for the coralligenous community and extended to the entire Holocene, strongly suggest that sponge diversity increased in warmer periods (Bertolino et al. Citation2017).
Over the past few decades, the coasts onto which the caves open were not particularly disturbed by anthropogenic activities. We may therefore hypothesise that the recorded changes could be exclusively attributed to climatic events, which favoured some species and disadvantaged others. In conclusion, the two sponge communities of the semi-submerged caves appear poorly resilient and the massive sponges might have suffered from the positive thermal anomalies which occurred in the Ligurian Sea in the last few decades, and were replaced by the encrusting ones. The process of cave recolonisation and recovery after major disturbances appears slow and studying it is necessary for the management and conservation of these habitats (Parravicini et al. Citation2010).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Airoldi L, Beck M. 2007. Loss, status and trends for coastal marine habitats in Europe. Oceanography and Marine Biology: an Annual Review 45:345–405.
- Balduzzi A, Bianchi CN, Boero F, Cattaneo-Vietti R, Pansini M, Sarà M. 1989. The suspension-feeder communities of a Mediterranean Sea cave. Scientia Marina 53:387–395.
- Bavestrello G, Bertone S, Cattaneo-Vietti R, Cerrano C, Gaino E, Zanzi D. 1994. Mass mortality of Paramuricea clavata (Anthozoa, Cnidaria) on Portofino Promontory cliffs, Ligurian Sea, Mediterranean Sea. Marine Life 4:15–19.
- Bertolino M, Betti F, Bo M, Cattaneo-Vietti R, Pansini M, Romero J, Bavestrello G. 2016. Changes and stability of a Mediterranean hard bottom benthic community over 25 years. Journal of the Marine Biological Association of the United Kingdom 96:341–350. DOI:10.1017/S0025315415001186.
- Bertolino M, Calcinai B, Cattaneo-Vietti R, Cerrano C, Lafratta A, Pansini M, Pica D, Bavestrello G. 2014a. Stability of the sponge assemblage of the Mediterranean coralligenous along a millennial span of time. Marine Ecology 35:149–158. DOI:10.1111/maec.12063.
- Bertolino M, Costa G, Carella M, Cattaneo-Vietti R, Cerrano C, Pansini M, Quarta G, Calcagnile L, Bavestrello G. 2017. The dynamics of a Mediterranean coralligenous sponge assemblage at decennial and millennial temporal scales. PLoS ONE 12:e0177945. DOI:10.1371/journal.pone.0177945.
- Bertolino M, Longo C, Marra MV, Corriero G, Pansini M. 2014b. Paraleucilla magna Klautau et al., 2004 (Porifera, Calcarea), an alien species extending its range in the Mediterranean Sea. Biologia Marina Mediterranea 21:109–110.
- Betti F, Bavestrello G, Bo M, Asnaghi V, Chiantore M, Bava S, Cattaneo-Vietti R. 2017. Over 10 years of variation in Mediterranean reef benthic communities. Marine Ecology 38:e12439. DOI:10.1111/maec.12439.
- Bianchi CN, Caroli F, Guidetti P, Morri C. 2017. Seawater warming at the northern reach for southern species: Gulf of Genoa, NW Mediterranean. Journal of the Marine Biological Association of the United Kingdom 98:1–12. DOI:10.1017/S0025315417000819.
- Bianchi CN, Corsini-Foka M, Morri C, Zenetos A. 2014a. Thirty years after-dramatic change in the coastal marine habitats of Kos Island (Greece), 1981-2013. Mediterranean Marine Science 15:482–497. DOI:10.12681/mms.678.
- Bianchi CN, Morri C. 2004. Climate change and biological response in Mediterranean Sea ecosystems: A need for broad-scale and long-term research. Ocean Challenge 13:32–36.
- Bianchi CN, Morri C, Pronzato R. 2014b. The other side of rarity: Recent habitat expansion and increased abundance of the horny sponge Ircinia retidermata (Demospongiae: Dictyoceratida) in the southeast Aegean. Italian Journal of Zoology 81:564–570. DOI:10.1080/11250003.2014.920929.
- Bianchi CN, Peirano A, Salvati E, Morri C. 2001. Assessing interannual and decadal changes in marine epibenthic assemblages through UW photography: An example from Punta Mesco, Ligurian Sea. Archive of Oceanography and Limnology 22:83–86.
- Bibiloni MA, Uriz MJ, Gili JM. 1989. Sponge communities in three submarine caves of the Balearic Islands (Western Mediterranean): Adaptations and faunistic composition. PSZNI: Marine Ecology I 10:317–334. DOI:10.1111/j.1439-0485.1989.tb00076.x.
- Boero F, Kraberg AC, Krause G, Wiltshire KH. 2015. Time is an affliction: Why ecology cannot be as predictive as physics and why it needs time series. Journal of Sea Research 101:12–18. DOI:10.1016/j.seares.2014.07.008.
- Bussotti S, Terlizzi A, Fraschetti S, Belmonte G, Boero F. 2006. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Marine Ecology Progress Series 325:109–119. DOI:10.3354/meps325109.
- Cattaneo-Vietti R. 2018. Structural changes in Mediterranean marine communities: Lessons from the Ligurian Sea. Rendiconti Lincei. Scienze Fisiche e Naturali 29:515–524. DOI:10.1007/s12210-018-0670-2.
- Cattaneo-Vietti R, Albertelli G, Aliani S, Bava S, Bavestrello G, Benedetti Cecchi L, Bianchi CN, Bozzo E, Capello M, Castellano M, Cerrano C, Chiantore M, Corradi N, Cocito S, Cutroneo L, Diviacco G, Fabiano M, Faimali M, Ferrari M, Gasparini GP, Locritani M, Mangialajo L, Marin V, Moreno M, Morri C, Orsi Relini L, Pane L, Paoli C, Petrillo M, Povero P, Pronzato R, Relini G, Santangelo G, Tucci S, Tunesi L, Vacchi M, Vassallo P, Vezzulli L, Wurtz M. 2010. The Ligurian Sea: State of the art, problems, and perspectives. Chemistry and Ecology 26:319–340. DOI:10.1080/02757541003689845.
- Cerrano C, Arillo A, Azzini F, Calcinai B, Castellano L, Muti C, Valisano L, Zega G, Bavestrello G. 2005. Gorgonian population recovery after a mass mortality event. Aquatic Conservation Marine and Freshwater Ecosystems 15:147–157. DOI:10.1002/aqc.661.
- Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F. 2000. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecology Letters 3:284–293. DOI:10.1046/j.1461-0248.2000.00152.x.
- Cerrano C, Totti C, Sponga F, Bavestrello G. 2006. Summer disease in Parazoanthus axinellae (Schmidt, 1862) (Cnidaria, Zoanthidea). Italian Journal of Zoology 73:355–361. DOI:10.1080/11250000600911675.
- Chevaldonné P, Lejeusne C. 2003. Regional warming‐induced species shift in north‐west Mediterranean marine caves. Ecology Letters 6:371–379. DOI:10.1046/j.1461-0248.2003.00439.x.
- Cicogna F, Bianchi CN, Ferrari G, Forti P, editors. 2003. Grotte Marine. Cinquant’anni di ricerca in Italia. Ministero dell’Ambiente e Tutela del Territorio. Rapallo: Officine Grafiche Canessa. pp. 505.
- Cinelli F, Fresi E, Mazzella L, Pronzato M, Pansini M, Svoboda A. 1977. Distribution of benthic phyto- and zoocoenoses along a light gradient in a superficial marine cave. In: Keegan BF, O’Céidigh PO, Boaden PJSE, editors. Biology of benthic organisms. Oxford: Pergamon Press. pp. 173–183.
- Claudet J, Fraschetti S. 2010. Human-driven impacts on marine habitats: A regional meta-analysis in the Mediterranean Sea. Biological Conservation 143:2195–2206. DOI:10.1016/j.biocon.2010.06.004.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, Estrada M, Froglia C, Galil BS, Gasol JM, Gertwagen R, Gil J, Guilhaumon F, Kesner-Reyes K, Kitsos MS, Koukouras A, Lampadariou N, Laxamana E, López-Fé de la Cuadra CL, Lotze HK, Martin D, Mouillot D, Oro RS, Rius-Barile J, Saiz-Salinas JI, San Vicente C, Somot S, Templado J, Turon X, Vafidis D, Villanueva R, Voultsiadou E. 2010. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 5:e11842.
- Coma R, Pola E, Ribes M, Zabala M. 2004. Long-term assessment of the patterns of mortality of a temperate octocoral in protected and unprotected areas: A contribution to conservation and management needs. Ecological Applications 14:1466–1478. DOI:10.1890/03-5176.
- Corriero G. 1989. Primi dati sul popolamento di Poriferi delle grotte superficiali dell’Isola di Ustica. Nova Thalassia 10:585–588.
- Corriero G, Pansini M, Pronzato R. 2003. Porifera. In: Cicogna F, Bianchi CN, Ferrari G, Forti P, editors. Grotte Marine. Cinquant’anni di ricerca in Italia. Ministero dell’Ambiente e Tutela del Territorio. Rapallo: Officine Grafiche Canessa 147–155.
- Corriero G, Scalera Liaci L, Gristina M, Riggio S, Mercurio M. 1997. Composizione tassonomica e distribuzione della fauna a poriferi e briozoi in una grotta semisommersa della Riserva Naturale Marina “Isola di Ustica”. Biologia Marina Mediterranea 4:34–43.
- Corriero G, Scalera Liaci L, Ruggiero D, Pansini M. 2000. The sponge community of a semi-submerged Mediterranean cave. PSZNI: Marine Ecology 21:85–96. DOI:10.1046/j.1439-0485.2000.00655.x.
- Di Franco A, Ferruzza G, Baiata P, Chemello R, Milazzo M. 2010. Can recreational scuba divers alter natural gross sedimentation rate? A case study from a Mediterranean deep cave. Journal Marine Science 67:871–874.
- Dimarchopoulou D, Gerovasileiou V, Voultsiadou E. 2018. Spatial variability of sessile benthos in a semi-submerged marine cave of a remote Aegean Island (eastern Mediterranean Sea). Regional Studies in Marine Science 17:102–111. DOI:10.1016/j.rsma.2017.11.015.
- Fraschetti S, Guarnieri G, Bevilacqua S, Terlizzi A, Claudet J, Russo GF, Boero F. 2011. Conservation of Mediterranean habitats and biodiversity countdowns: What information do we really need? Aquatic Conservation: Marine and Freshwater Ecosystems 21:299–306. DOI:10.1002/aqc.v21.3.
- Garrabou J, Coma R, Benssousan N, Bally M, Chevaldonne P, Cigliano M, Diaz D, Harmelin J-G, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido N, Torrents O, Zabala M, Zuberer F, Cerrano C. 2009. Mass mortality in NW Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Global Change Biology 15:1090–1103. DOI:10.1111/j.1365-2486.2008.01823.x.
- Gatti G, Bianchi CN, Parravicini V, Rovere A, Peirano A, Montefalcone M, Massa F, Morri C. 2015. Ecological change, sliding baselines and the importance of historical data: Lessons from combing observational and quantitative data on a temperate reef over 70 years. PLoS ONE 10:e0118581. DOI:10.1371/journal.pone.0118581.
- Gerovasileiou V, Chintiroglou C, Vafidis D, Koutsoubas D, Sini M, Dailianis T, Issaris Y, Akritopoulou E, Dimarchopoulou D, Voultsiadou E. 2015a. Census of biodiversity in marine caves of the Eastern Mediterranean Sea. Mediterranean Marine Science 16:245–265. DOI:10.12681/mms.1069.
- Gerovasileiou V, Dimitriadis C, Arvanitidis C, Voultsiadou E. 2017. Taxonomic and functional surrogates of sessile benthic diversity in Mediterranean marine caves. PLoS ONE 12:e0183707. DOI:10.1371/journal.pone.0183707.
- Gerovasileiou V, Voultsiadou E. 2012. Marine caves of the Mediterranean Sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 7:e39873. DOI:10.1371/journal.pone.0039873.
- Gerovasileiou V, Voultsiadou E. 2016. Sponge diversity gradients in marine caves of the eastern Mediterranean. Journal of the Marine Biological Association of the United Kingdom 96:407–416. DOI:10.1017/S0025315415000697.
- Gerovasileiou V, Voultsiadou E, Issaris Y, Zenetos A. 2015b. Alien biodiversity in Mediterranean marine caves. Marine Ecology 37:239–256. DOI:10.1111/maec.12268.
- Giakoumi S, Sini M, Gerovasileiou V, Mazor T, Beher J, Possingham HP, Abdulla A, Çinar ME, Dendrinos P, Gucu AC, Karamanlidis A, Rodic P, Panayotidis P, Taskin E, Jaklin A, Voultsiadou E, Webster C, Zenetos A, Katsanevakis S, Thrush S. 2013. Ecoregion-based conservation planning in the Mediterranean: Dealing with large-scale heterogeneity. PLoS ONE 8:e76449. DOI:10.1371/journal.pone.0076449.
- Guarnieri G, Terlizzi A, Bevilacqua S, Fraschetti S. 2012. Increasing heterogeneity of sensitive assemblages as a consequence of human impact in submarine caves. Marine Biology 159:1155–1164. DOI:10.1007/s00227-012-1895-8.
- Harmelin JG, Boury-Esnault N, Fichez R, Vacelet J, Zibrowius H. 2003. Peuplements de la grotte sous-marine de l’île de Bagaud (Parc National de Port-Cros, France, Méditerranée). Scientific Reports of Port-Cros National Park 19:117–134.
- Harmelin JG, Vacelet J, Vasseur P. 1985. Les grottes sous-marines obscures: Un milieu extrême et un remarquable biotope refuge. Tethys 11:214–229.
- Labate M. 1965. Ecologia dei Poriferi della Grotta della Regina (Adriatico meridionale). Bollettino di Zoologia 32:541–553. DOI:10.1080/11250006509440709.
- Linares C, Coma R, Diaz D, Zabala M, Hereu B, Dantart L. 2005. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Marine Ecology Progress Series 305:127–137. DOI:10.3354/meps305127.
- Longobardi L, Bavestrello G, Betti F, Cattaneo-Vietti R. 2017. Long-term changes in a Ligurian infralittoral community (Mediterranean Sea): a warning signal? Regional Studies in Marine Science 14:15–26. DOI:10.1016/j.rsma.2017.03.011.
- Martì R, Uriz MJ, Ballesteros E, Turon X. 2004. Benthic assemblages in two Mediterranean caves: Species diversity and coverage as a function of abiotic parameters and geographic distance. Journal of the Marine Biological Association of the United Kingdom 84:557–572. DOI:10.1017/S0025315404009567h.
- Montefalcone M, De Falco G, Nepote E, Canessa M, Bertolino M, Bavestrello G, Morri C, Bianchi CN. 2018. Thirty year ecosystem trajectories in a submerged marine cave under changing pressure regime. Marine Environmental Research 137:98–110. DOI:10.1016/j.marenvres.2018.02.022.
- Morri C, Bianchi CN. 2001. Recent changes in biodiversity in the Ligurian Sea (NW Mediterranean): Is there a climatic forcing? In: Faranda FM, Guglielmo L, Spezie G, editors. Mediterranean Ecosystems: structure and processes. Italia: Springer Verlag. pp. 375–384.
- Nepote E, Bianchi CN, Morri C, Ferrari M, Montefalcone M. 2017. Impact of a harbour construction on the benthic community of two shallow marine caves. Marine Pollution Bulletin 114:35–45. DOI:10.1016/j.marpolbul.2016.08.006.
- Pansini M, Pronzato R, Fresi E, Cinelli F, Mazzella L, Ponticelli MP. 1977. Evoluzione delle biocenosi bentoniche di substrato duro lungo un gradiente di luce in una grotta marina superficiale: Poriferi. In: Atti IX Congresso della S.I.B.M. Lacco Ameno d’Ischia. pp. 315–330.
- Parravicini V, Guidetti P, Morri C, Montefalcone M, Donato M, Bianchi CN. 2010. Consequences of sea water temperature anomalies on a Mediterranean submarine cave ecosystem. Estuarine, Coastal and Shelf Science 86:276–282. DOI:10.1016/j.ecss.2009.11.004.
- Parravicini V, Mangialajo L, Mousseau L, Peirano A, Morri C, Francour P, Kulbicki M, Bianchi CN. 2015. Climate change and warm-water species at the north-western boundary of the Mediterranean Sea. Marine Ecology 36:897–909. DOI:10.1111/maec.12277.
- Parravicini V, Micheli F, Montefalcone M, Morri C, Villa E, Castellano M, Povero P, Bianchi CN. 2013. Conserving biodiversity in a human-dominated world: Degradation of marine sessile communities within a Protected Area with conflicting human uses. PLoS ONE 8:e75767. DOI:10.1371/journal.pone.0075767.
- Pérez T, Garrabou J, Sartoretto S, Harmelin J-G, Francour P, Vacelet J. 2000. Mortalité massive d’invertébrés marins: Un evénement sans préceédent en Méditerranée nord-occidentale. Life Sciences 323:853–865.
- Puce S, Bavestrello G, Di Camillo CG, Boero F. 2009. Long term changes in hydroid (Cnidaria Hydrozoa) Assemblages: Effect of Mediterranean Warming? Marine Ecology 30:313–326.
- Rastorgueff PA, Bellan-Santini D, Bianchi CN, Bussotti S, Chevaldonné P, Guidetti P, Harmelin J-G, Montefalcone M, Morri C, Pérez T, Ruitton S, Vacelet J, Personnic S. 2015. An ecosystem-based approach to evaluate the ecological quality of Mediterranean undersea caves. Ecological Indicators 54:137–152. DOI:10.1016/j.ecolind.2015.02.014.
- Riedl R. 1966. Biologie der Meereshöhlen. Hamburg: Paul Parey. pp. 1–602.
- Roghi F, Parravicini V, Montefalcone M, Rovere A, Morri C, Peirano A, Firpo M, Bianchi CN, Salvati E. 2010. Decadal evolution of a coralligenous ecosystem under the influence of human impacts and climate change. Biologia Marina Mediterranea 17:59–62.
- Rossi S. 2013. The destruction of the ‘animal forests’ in the oceans: Towards an over simplification of the benthic ecosystems. Ocean Coastal Management 84:77–85. DOI:10.1016/j.ocecoaman.2013.07.004.
- Sala E, Ballesteros E, Panagiotis D, Di Franco A, Ferretti F, Foley D, Fraschetti S, Friedlander A, Garrabou J, Guclusoy H, Guidetti P, Halpern BS, Hereu B, Karamanlidis AA, Kizilkaya Z, Macpherson E, Mangialajo L, Mariani S, Micheli F, Pais A, Riser K, Rosenberg AA, Sales M, Selkoe KA, Starr R, Tomas F, Zabala M. 2012. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7:e32742. DOI:10.1371/journal.pone.0032742.
- Sarà M. 1958. Studio sui Poriferi di una grotta di marea del Golfo di Napoli. Archivio Zoologico Italiano 43:203–280.
- Sarà M. 1959. Considerazioni sulla distribuzione ed ecologia dei Poriferi nelle grotte. Annuario dell’Istituto e Museo di Zoologia dell’Università di Napoli 11:1–7.
- Sarà M. 1961a. La fauna di Poriferi delle grotte delle isole Tremiti. Studio ecologico e sistematico. Archivio Zoologico Italiano 46:1–59.
- Sarà M. 1961b. Zonazione dei poriferi nella grotta della Gaiola. Annuali dell’Istituto del Museo di Zoologia dell’Università di Napoli 13:1–32.
- Sarà M. 1962. Zonazione dei Poriferi in biotopi litorali. Pubblicazioni della Stazione Zoologica di Napoli 32(suppl.):44–57.
- Sarà M. 1964. Distribuzione ed ecologia dei Poriferi in acque superficiali della Riviera ligure di Levante. Archivio Zoologico Italiano 49:181–248.
- Sarà M. 1968. Stratification des peuplements d’éponges à recouvrement total dans certaines grottes du niveau superficiel. Rapports et Procés-Verbaux des Reunions. Commission International Pour l’Exploration Scientifique de la Mer Méditérranée Monaco 19:83–85.
- Sarà M. 1974. Il popolamento delle grotte marine e sua protezione. In: Cacucci Bari, editor. Atti IV Simposio Nazionale sulla Conservazione della Natura. Vol. 1. pp. 51–59.
- Sarà M. 1978. Il popolamento delle grotte marine: Interesse di una salvaguardia. Pubblicazioni della Stazione Zoologica di Napoli 40:502–505.
- Schiaparelli S, Castellano M, Povero P, Sartoni G, Cattaneo-Vietti R. 2007. A benthic mucilage event in North-Western Mediterranean Sea and its possible relationships with the summer 2003 European heat-wave: Short term effects on littoral rocky assemblages. Marine Ecology 28:1–13. DOI:10.1111/j.1439-0485.2007.00155.x.
- Thibaut T, Pinedo S, Torras X, Ballesteros E. 2005. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes coast (France, North-western Mediterranean). Marine Pollution Bulletin 50:1472–1489. DOI:10.1016/j.marpolbul.2005.06.014.
- Ulman A, Ferrario J, Occhipinti-Ambrogi A, Arvanitidis C, Bandi A, Bertolino M, Bogi C, Chatzigeorgiou G, Çiçek BA, Deidun A, Ramos-Esplá A, Koçak C, Lorenti M, Martinez-Laiz G, Merlo G, Princisgh E, Scribano G, Marchini A. 2017. A massive update of non-indigenous species records in Mediterranean marinas. PeerJ 5:e3954. DOI:10.7717/peerj.3954.
- UNEP-MAP-RAC/SPA. 2008. Action plan for the conservation of the coralligenous and other calcareous bio-concretions in the Mediterranean Sea. Tunis: UNEPMAP RAC-SPA publ.
- UNEP-MAP-RAC/SPA. 2015. Action plan for the conservation of habitats and species associated with seamounts, underwater caves and canyons, aphotic hard beds and chemo-synthetic phenomena in the Mediterranean Sea. Tunis: Dark Habitats Action Plan. UNEP MAP RAC-SPA publ.
- Van Soest RWM, Hooper JNA. 1993. Taxonomy, phylogeny and biogeography of the marine sponge genus Rhabderemia Topsent, 1890 (Demospongiae, Poecilosclerida). Scientia Marina 57:319–351.
