Abstract
The genus Paramuricea is present in the Mediterranean Sea with two species, P. clavata and P. macrospina. These species have similar bathymetric distributions and can live in sympatry. P. macrospina shows morphological plasticity leading sometimes to an erroneous classification as P. clavata. Studying four ambiguous morphotypes of P. macrospina collected in different localities of the Mediterranean Sea, we provide new insights on both the taxonomy and the distribution of this species. Our analyses reveal high morphological plasticity within the sampled colonies, which in two cases were characterised by a peculiar pigmentation and a morphological pattern resembling that of P. clavata. After having confirmed the genetic identity of the samples, we tested the validity of traditional taxonomic characters, and found that the most reliable character to discriminate between P. macrospina and P. clavata is the number of rows of spindles in the collaret of the polyps. All other features are highly variable and therefore do not allow a correct identification. The bathymetric and geographic distribution of P. macrospina is here updated, showing, with the exception of the south-eastern side, broad preference in the whole basin. Moreover, further studies are needed to investigate the gene flow among and within the Mediterranean populations of P. macrospina and to assess whether the morphological plasticity is driven by adaptive genetic processes of populations exposed to different environmental, climatic and bathymetric conditions.
Introduction
The taxonomy of gorgonians is mainly based on morphological characters such as the branching pattern of the colony, the shape of the anthocodia and the type and arrangement of sclerites (Bayer Citation1961). These characters show high morphological plasticity due to the influence of environmental factors (Sánchez et al. Citation2007; Prada et al. Citation2008; Gutiérrez-Rodríguez et al. Citation2009) which, sometimes, may lead to the formation of puzzling features (West et al. Citation1993; Wirshing & Baker Citation2015). Therefore, species-level identification is not a trivial process, hiding, in some cases, distinct phylogenetic lineages (Grigg Citation1972; Brazeau & Lasker Citation1988; Prada et al. Citation2008; Gutiérrez-Rodríguez et al. Citation2009; Bilewitch et al. Citation2014; Wirshing & Baker Citation2015; Ament-Velásquez et al. Citation2016).
The genus Paramuricea (Kölliker, Citation1865) includes 25 valid species, distribute in tropical, temperate and polar areas (Cordeiro et al. Citation2018). In the Mediterranean Sea, only two species are known, Paramuricea clavata (Risso, Citation1826) and the endemic Paramuricea macrospina (Koch, Citation1882). While the interest in P. clavata as an ecosystem engineer is rapidly growing (Ponti et al. Citation2014, Citation2016, Citation2018; Valisano et al. Citation2016), especially after its severe involvement in widespread and frequent massive mortality events in shallow waters (Cerrano et al. Citation2000; Calvisi et al. Citation2003; Linares et al. Citation2005; Garrabou et al. Citation2009; Huete-Stauffer et al. Citation2011; Vezzulli et al. Citation2013), only little, scattered information, mostly in the form of records, is available for P. macrospina (Aguilar et al. Citation2015; Grinyó et al. Citation2017). The importance of gorgonians as an “animal forest” in the Mediterranean Sea has been documented (Cerrano et al. Citation2000) and is currently receiving attention (Ponti et al. Citation2016; Valisano et al. Citation2016; Gori et al. Citation2017) due to their structural and functional role. They increase the three-dimensional complexity of the habitat, modifying local environmental parameters such as water movement and sedimentation processes (Valisano et al. Citation2016), creating new ecological niches and therefore affecting the diversity of associated species (Linares et al. Citation2005; Ponti et al. Citation2014, Citation2018; Gori et al. Citation2017). Gorgonians can build wide facies, sometimes monospecific assemblages, as reported for P. macrospina in the Marmara Sea and Menorca Channel (Topçu & Öztürk Citation2015; Grinyó et al. Citation2016). Despite being assessed as “Data Deficient” due to little information and few records (Aguilar et al. Citation2015; Otero et al. Citation2017), P. macrospina was listed on the International Union for Conservation of Nature (IUCN) Red List as suspected to be in decline, owing to indiscriminate bottom trawling and trammel net fishing activities. Paramuricea macrospina inhabits hard, detritic or sandy bottoms, preferentially between 20 and 150 m in depth (Carpine & Grasshoff Citation1975; Topçu & Öztürk Citation2015; Grinyó et al. Citation2016), and except for the Aegean Sea and Marmara Sea, where it was found only between 20 and 50 m depth (Carpine & Grasshoff Citation1975; Vafidis et al. Citation1994; Bo et al. Citation2012; Topçu & Öztürk Citation2015), it usually colonises mesophotic habitats in the Mediterranean Sea below 60 m depth. Paramuricea clavata shows a bathymetric distribution ranging from 10 to 200 m in depth (Mokhtar-Jamai et al. Citation2011; Gori et al. Citation2017), and is more frequent on hard substrates with vertical sloping (Carpine & Grasshoff Citation1975). The morphological difference between the two species is an overall characterisation (Carpine & Grasshoff Citation1975). Paramuricea clavata shows usually large colonies (up to 1 m high) with thick branches, completely purple or purple to yellow in colour, polyps closely packed on the branches, thornscales of the calyx without a long point and five to eight rows of spindles in the collaret of the polyps with tubercles mainly on the convex side. In contrast, samples of P. macrospina are usually described as small colonies (up to ca. 10 cm) with rather thin branches and unpacked polyps, yellow to pinkish in colour, the thornscales in the calyces may have a very long point, and there are three to four rows of spindles in the collaret, covered all around by tubercles (Stiasny Citation1942; Carpine & Grasshoff Citation1975; Grasshoff Citation1977). Unlike P. clavata which shows more stable characters, colonies of P. macrospina with different shapes and colours have been recorded in the Mediterranean Sea (Pax & Müller Citation1962; Carpine Citation1963; Topçu & Öztürk Citation2013, Citation2015; Grinyó et al. Citation2016, Citation2017). For instance, Carpine (Citation1963) reported pinkish-grey colonies (up to 20 cm) from Corsica (France), while Pax and Müller (Citation1962) recorded bright orange, translucent white and almost colourless colonies in the Adriatic Sea. In the Marmara Sea, P. macrospina colonies are usually yellow, but sometimes creamy to orange and brownish pink variations in colour are recorded (Topçu & Öztürk Citation2013, Citation2015). Specimens collected in the Marmara Sea and in Menorca Channel showed taller colonies (up to 22 and 56 cm high, respectively) well ramified (Topçu & Öztürk Citation2013; Grinyó et al. Citation2016, Citation2017). Colonies of P. macrospina are characterised by peculiar long thornscale points in the calix, which were firstly described by Koch (Citation1882) in holotype material. This character is largely variable in terms of shape and size, representing one of the main causes of erroneous identification among and within P. clavata and P. macrospina colonies (Carpine & Grasshoff Citation1975). Indeed, thornscales have variable sizes and, few years later, the same author (Koch Citation1887) considered P. macrospina to be a variety of P. clavata (P. chamaeleon var. macrospina). Afterwards, Stiasny (Citation1942) wrote that P. macrospina might be clearly distinguishable from P. clavata, but the considered diagnostic characters are not fully accepted. For example, the long thornscale sclerites recorded in many specimens of P. macrospina (Koch Citation1882; Stiasny Citation1942; Pax & Müller Citation1962 as P. placomus) are considered too variable (Carpine & Grasshoff Citation1975). By contrast, the same authors, following Stiasny (Citation1942), claimed that the shape and number of spindles in the collaret of the polyps might be considered the main diagnostic features to differentiate the two species. Nevertheless, these features have been overlooked, and were not taken into account by all authors. Due to its wide morphological and chromatic plasticity, P. macrospina has often been confused with P. clavata (Koch Citation1887; Stiasny Citation1942; Pax & Müller Citation1962; Carpine & Grasshoff Citation1975; Aguilar et al. Citation2015) even if these two species are genetically separated (Poliseno et al. Citation2017).
The present study was triggered by the finding of several puzzling colonies of Paramuricea with unusual phenotypes. However, given the lack of morphological diagnostic characters and the high phenotypic plasticity showed by the investigated samples, two partial mitochondrial genes, COI and mtMutS, have been sequenced in order to validate the species identification of the colonies. The aim of this study is to update the taxonomic and ecological knowledge on the Mediterranean endemic P. macrospina, by testing the validity of traditional taxonomic characters for congeneric discrimination and assessing its bathymetric and geographic distribution by reviewing the available literatures.
Materials and methods
Morphological characterisation
Samples from four distinct morphotypes of Paramuricea macrospina and from colonies of P. clavata were collected by scuba diving at a depth ranging from 30 to 70 m in different Mediterranean areas ().
Table I. Studied sites and codification of Paramuricea spp. sampled.
All specimens were photographed before sampling to describe the general shape of the colonies. Two branches for each colony were collected and preserved in absolute ethanol for molecular analysis, whereas further branches preserved in 75% ethanol were considered for morphological characterisation. The morphological characterisation includes the general shape of the colony, anthocodia distribution, sclerite arrangement and shape and size of sclerites. For each sample, we took digital images using a Nikon D600 camera attached to a Nikon SMZ18 stereomicroscope. Sclerites were analysed by dissolving the different parts of the colony separately, with sodium hypochlorite: the coenenchyme, the anthostele and the anthocodia. For each part, several fragments were analysed to encompass the variability of sclerites inside one colony. Non-permanent slides were prepared following Bayer (Citation1961) and observed under a Nikon Eclipse Ni-U microscope and the morphology and the dimension of the sclerites were recorded. The sclerites were also cleaned for Scanning Electron Microscopy (SEM) analysis following Fabricius and Alderslade (Citation2001). The sclerites were mounted on stubs, coated with gold–palladium in a Balzer Union evaporator and examined with a Philips XL20 SEM. The total length of the sclerites was then measured by means of ImageJ software.
Molecular analyses
DNA extraction of ethanol-preserved samples was carried out using either a modified Cetyltrimethylammonium Bromide (CTAB) phenol-choloroform protocol (Doyle & Doyle Citation1987) or the Macherey-Nagel NucleoSpin® Tissue kit (M&N, Düren, Germany). Partial fragments (< 1000 bp) of COI and mtMutS were amplified following McFadden et al. (Citation2011). PCR products were purified using a polyethylene glycol (PEG)-NaCl precipitation and were sequenced with the BigDye® Terminator v. 3.1 chemistry in an ABI PRISM® 3700 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Partial mtMutS and COI sequences of P. macrospina (SNSB-BSPG 2015 XXXII GW4713) and P. clavata (SNSB-BSPG 2015 XXXII GW4701) were extracted from the complete mitochondrial DNA sequences available in the public repositories (LT576167–LT576168). The recently published mtMutS sequence (LT576169) of P. macrospina (SNSB-BSPG 2015 XXXII GW4778) was also considered in this study. Sequences generated here were deposited at the National Center for Biotechnology Information under accession numbers MH251313–MH251317 (see Table S2). Affinity among the sequences obtained in this study and those deposited in the public repositories was assessed through the Basic Local Alignment Search Tool (BLAST) in National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi). All P. macrospina morphotypes considered in this study share identical haplotypes for both COI and mtMutS. Thus, for simplicity, the phylogenetic tree of mtMutS was performed considering a single specimen per morphotype. The mtMutS sequences generated for P. macrospina morphotypes were added to the dataset generated by Poliseno et al. (Citation2017) (DOI: http://dx.doi.org/10.5282/ubm/data.89) and were aligned using MUSCLE (Edgar Citation2004) in SeaView 4.5.3 (Gouy et al. Citation2010). For phylogenetic reconstructions, the maximum likelihood (ML) and Bayesian trees were inferred using RAxML 7.2.8 (Stamatakis Citation2006) and BEAST 2.4.4 (Bouckaert et al. Citation2014), respectively. The ML analysis was performed with a GTR+ G substitution model, using a rapid bootstrap analysis (Stamatakis et al. Citation2008) and 1000 pseudo-replicates for branch support. For Bayesian analysis the best-fit substitution model (GTR+ G) was selected using the Akaike information criterion (AIC) in jModeltest 2.1.3 (Darriba et al. Citation2012). Metropolis-coupled Markov chain Monte Carlo (MCMCMC) ran for 10 million generations, sampling every 1000, and a quarter of the sampled trees was discarded as burn-in. MCMCMC convergence was assessed with Tracer 1.6 (Rambaut et al. Citation2014).
Statistical analysis
Among the analysed specimens, we selected four different specimens of P. macrospina and one specimen of P. clavata in order to explore whether sclerite length could be considered a valid diagnostic feature. For each type of sclerite (thornscale of the anthostele, spindle of the coenenchyme, hockey-stick spindle of the point, spindle of the collaret and rod of the tentacle) 30 sclerites were measured using a Nikon Eclipse Ni-U microscope. Sclerite lengths are reported as minimum–average ± standard deviation–maximum size. Because the assumptions for parametric analyses were not met, the equality of the medians of the length of sclerites was tested using Kruskal–Wallis analyses (statistic: H). Each type of sclerite was considered. Post hoc comparison was made using Bonferroni-corrected Mann–Whitney pairwise tests.
Geographic and bathymetric distributions
In order to assess the bathymetric and geographic distribution of Paramuricea macrospina, we reviewed the information published so far in 30 papers, including the present one. Records of occurrence, together with the location and bathymetric range, were compiled (Table S1). Information about the substrate was also included in the analysis when available. The coordinates of the records were plotted using the open-source software QGISS 2.4.0 (http://www.qgis.org/it/site/).
Results
Morphological characterisation
The specimens of Paramuricea clavata collected from different geographic areas (Croatia, Sardinia and Ustica Island) did not show significant variation in the morphology of sclerites. Thus, a single sample (i.e. GW4701) is here considered for morphological descriptions. Regarding P. macrospina, the phylogenetic tree of the partial mitochondrial MutS gene indicates that the studied morphotypes fall into a single group (; Figure S1), despite the fact that our morphological analysis showed wide variation. Colonies of P. macrospina from Skerki Bank, Sciacca Shoal and Pantelleria Island are characterised by different, distinctive and peculiar shapes and morphological characters, while P. clavata showed a more constant morphology among the studied specimens. Below are reported the descriptions of P. macrospina morphotypes. However, colonies from Corsica Island (), identified as P. macrospina, encompass a heterogeneous set of morphological characters shared with other morphotypes (Pantelleria Island and Sciacca Shoal) and therefore are not described. In order to highlight the morphological divergence(s) among different P. macrospina morphotypes we compared them against a typical colony of P. clavata colonies collected from Ustica Island.
Paramuricea macrospina morphotype GW4781, Skerki Bank
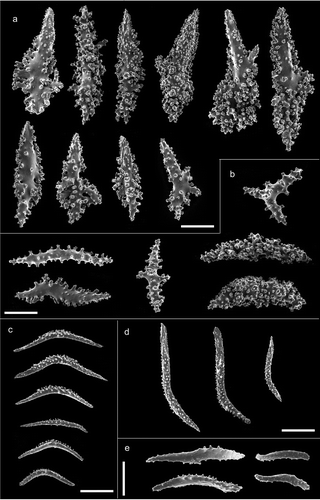
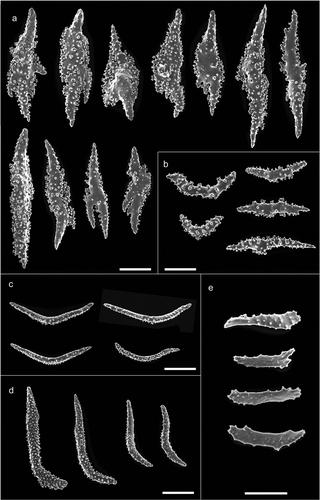
The colony is dark yellow to orange in colour, up to 33 cm high (). The arborescent, irregularly branched colony has a more or less planar shape with polyps densely packed along the branches (). The anthostele is not spiny and is formed by thornscales arranged “en chevron” ( and ). Thornscales of the anthostele are small with a short point and a well-developed base (). The coenenchyme is composed by spiny spindles, sometimes bent (). The retractile polyps have sclerites arranged in “collaret and point” (,). The collaret is formed by 3–4 rows of spindles (). These latter are arched in shape with the tubercles homogeneously scattered along the sclerite, and sometimes are more developed on the convex side (). The points are made by hockey-stick spiny spindles () arranged at the base of the tentacles. The tentacles contain flattened rods with small tubercles (). The size of sclerites is reported in .
Table II. Length of sclerites (µm), with mean and standard deviation for each morphotype analysed, of Paramuricea macrospina and the specimen of P. clavata. The distribution of the sclerites in the colony is reported in parentheses. Length is expressed in µm as minimum–mean ± standard deviation–maximum.
Figure 1. Morphological and chromatic variations of Paramuricea macrospina (a–d) and P. clavata (e). (a) Corsica Island (credit: Laurent Ballesta, Andromede Océanologie). (b) Skerki Bank. (c) Pantelleria Island. (d) Sciacca Shoal. Scale bars: a–d = 5 cm; e = 10 cm.

Figure 2. Morphology of the branches and arrangement of the polyps in Paramuricea macrospina from: (a) Skerki Bank; (b) Pantelleria Island; (c) Sciacca Shoal. (d) Branches of the colony of Paramuricea clavata. Scanning electron microscope pictures of sclerite shape and arrangement in: (e) P. macrospina from Pantelleria Island and (f) P. clavata. Scale bars: a–d, f = 1 mm; e = 0.5 mm.
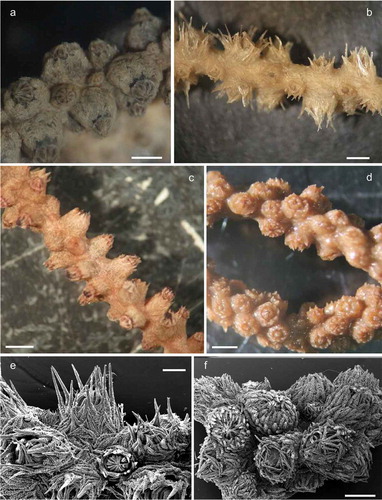
Figure 3. Arrangement of sclerites in the polyps of Paramuricea macrospina collected in (a,b) Skerki Bank; (c,d) Pantelleria Island; (e,f) Sciacca Shoal. (g,h) Arrangement of sclerites in the polyps of P. clavata. Scale bars: 0.5 mm.
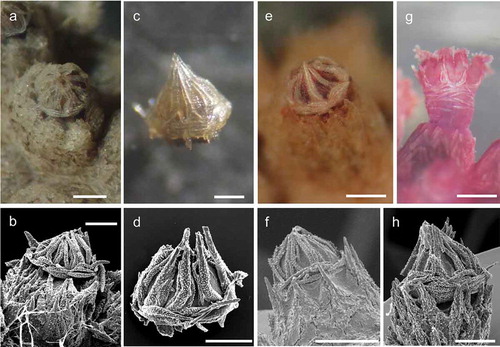
Figure 4. Scanning electron microscope pictures of sclerites of Paramuricea macrospina from Skerki Bank. (a) Thornscales from anthostele; (b) spindles of the coenenchyme; (c) spindles of the collaret; (d) hockey-stick spindles of the point; (e) rods of the tentacle. Scale bars: a–d = 200 µm; e = 100 µm.
Paramuricea macrospina morphotype GW4778, Pantelleria Island
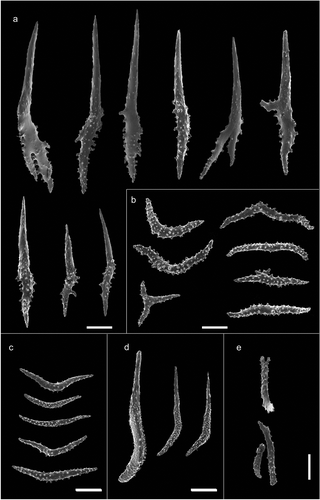
The colony is violet with a pale-coloured base and up to 10 cm high (). The colony is slight with few dichotomously ramifications arranged in one plane. The polyps are unpacked on the branches and the anthostele has very long thornscales, which give a spiny aspect to the colony (,). The thornscales have long points and their base has very few digitiform processes (). The coenenchyme contains spindles that are often bent and branched, completely covered by tubercles (). The retractile polyps have sclerites arranged in “collaret and point” (,). The collarets of the polyps are formed by long bent spindles completely covered by tubercles (), and are organised on the polyps in 2–3 rows (,). The points are made by hockey-stick spindles with the tubercles mainly in the basal part (). The tentacles have rods with flattened points, with few tubercles (). The size of the sclerites is reported in .
Figure 5. Scanning electron microscope pictures of sclerites of Paramuricea macrospina from Pantelleria Island. (a) Thornscales from anthostele; (b) spindles of the coenenchyme; (c) spindles of the collaret; (d) hockey-stick spindles of the point; (e) rods of the tentacle. Scale bars: a–d = 200 µm; e = 100 µm.
Paramuricea macrospina morphotype GW4556, Sciacca Shoal
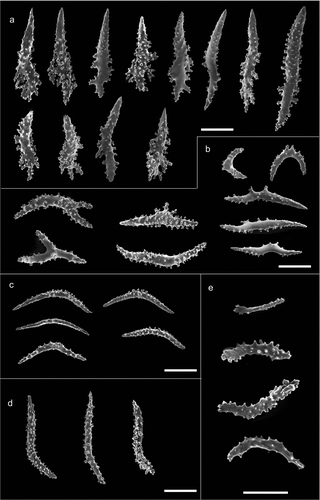
The colonies are dark yellow to pale orange in colour, up to 30 cm high (). They are robust, with numerous branches, which often give the colonies a bushy aspect (). Polyps are crowded along the branches (). The anthostele is a little spiny with sclerites arranged “en chevron” (). The thornscales are more or less fusiform, with short digitiform processes at the base (). Sometimes they show a spindle-like shape with two well-defined points. The coenenchyme is formed by slightly bent or branched spindles (). The retractile polyps have sclerites arranged in “collaret and point” (,). The spindles of the collaret are curved and tubercled, but sometimes the tubercles are more developed on the convex side of the sclerite (). The collaret is formed by 2–3 rows of sclerites (,). The hockey-stick spindles of the point have many tubercles along the entire sclerite (). The tentacle has flattened spiny rods (). The size of the sclerites is reported in .
Figure 6. Scanning electron microscope pictures of sclerites of Paramuricea macrospina from Sciacca Shoal. (a) Thornscales from anthostele; (b) spindles of the coenenchyme; (c) spindles of the collaret; (d) hockey-stick spindles of the point; (e) rods of the tentacle. Scale bars: a–d = 200 µm; e = 100 µm.
Paramuricea clavata GW4701, Ustica Island
The colonies are usually planar in shape, but sometimes can be bushy, and may show different colour patterns, varying from purple to yellow. The colonies are up to 70 cm high (). The branched colonies are usually planar in shape, but sometimes can be bushy. Polyps are closely packed along the branches (,). The anthostele is not spiny () and is composed by thornscales with short tubercled points and numerous short digitiform processes at the base (). The spindles of the coenenchyme are usually highly tubercled but also tiny and branched (). The retractile polyps have sclerites arranged in “collaret and point” (,). The collaret of the polyps is composed by spindles with the tubercles mainly on the convex side (). The collaret is composed by 5–8 rows of sclerites (,). The points are made by hockey-stick spindles, homogeneously covered with tubercles (). The rods of the tentacles are flattened, with few tubercles (). The size of the sclerites is reported in .
Statistical analyses
Kruskal–Wallis analysis evidences significant differences in relation to the sclerite length of the studied colonies, except for the spindle of the collaret. In particular, Bonferroni-corrected pairwise Mann–Whitney shows significant differences according to the type of sclerite investigated. Significant differences are reported between Pantelleria Island and Sciacca Shoal for the spindles of coenenchyme and hockey-stick spindles of the point (p < 0.01 and p < 0.05, respectively). The sizes of thornscales, hockey-stick spindles and rods were significantly different between Sciacca Shoal and Skerki Bank (p < 0.01). The size of spindles of the coenenchyme was significantly different between Pantelleria Island–Skerki Bank (p < 0.01) and Ustica Island–Skerki Bank (p < 0.05). Rods of the tentacle of Ustica Island were significantly different from those of Sciacca Shoal and Corsica Island (p < 0.01 and p < 0.05, respectively).
Geographic and bathymetric distributions
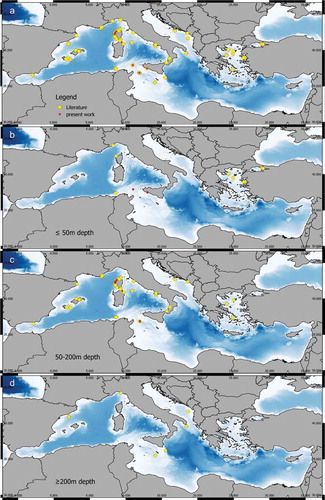
Among the 30 reviewed papers naming Paramuricea macrospina, 78 records were extracted (Table S1). This species was mainly recorded in the western Mediterranean Sea (). Only a few records from the continental shelves of the eastern Mediterranean Sea Basin are reported (). These records are mostly from the Adriatic Sea and Aegean Sea. Paramuricea macrospina has been recorded between 30–1002 m in depth, although the deepest record is yet to be confirmed, as stated by Cartes et al. (Citation2009) (Table S1). The shallower records (≤ 50 m deep) are rare and are reported in the Marmara Sea, Aegean Sea, Gallinara Island, Corsica, Sardinia and Tunisian coasts (). To date, the majority of the records are distributed between 50 and 200 m in depth () while few are ≥ 200 m depth and are limited to central and western Mediterranean (). In 10.3% of the records reviewed (eight out of 78), the substrate features where P. macrospina lived were not specified (Table S1). 56.4% of the records (44 out of 78) were found on hard substrate, in particular 25.4% (20 out of 78) identified as coralligenous habitat. In 30.8% (24 out of 78) the bottom was detritic, for example maerl bed, fragments of corals, mollusc shells, and coarse gravel and sand (Table S1). In only two records, P. macrospina was found on a muddy sand bottom.
Discussion
According to the descriptions reported in the literature (Koch Citation1882; Stiasny Citation1942; Pax & Müller Citation1962), Paramuricea macrospina shows a higher morphological plasticity than its Mediterranean congener. This variability often caused misidentification of some specimens that were erroneously identified as P. clavata or P. placomus (Koch Citation1887; Kükenthal Citation1924; Pax & Müller Citation1962). Although some authors (Stiasny Citation1942; Carpine & Grasshoff Citation1975) recognised in the shape and the number of the spindles in the collaret of the polyps, two distinct diagnostic characters useful for species discrimination among Mediterranean Paramuricea until now, no one had tested the validity of these morphological characters with the support of genetic analyses.
In the present paper, genetic analyses were performed as an integrative approach with the aim to validate the species identification of the colonies based on morphology. Our morphological analyses revealed high morphological plasticity within P. macrospina colonies sampled from different geographic sites. In particular, the colonies of P. macrospina collected from Skerki Bank and Sciacca Shoal have a general shape, polyp distribution and sclerite morphology more closely resembling P. clavata colonies than the typical P. macrospina reported in the literature. However, the genetic analysis confirms the species identification of the colonies as P. macrospina.
The two morphotypes show large colonies, up to 33 cm, with a bright orange to yellowish colour. The size of these specimens is unusually big and to date only Grinyó et al. (Citation2016) described colonies of P. macrospina up to 55 cm high from Menorca. Regarding the colour, only Pax and Müller (Citation1962) and Topçu & Öztürk (Citation2013, Citation2015) reported yellowish-orange colouration of the specimens. In fact, colonies of P. macrospina were described in the past as whitish/yellowish to pinkish-grey, or sometimes translucent white in colour (Stiasny Citation1942; Carpine Citation1963; Carpine & Grasshoff Citation1975). By contrast, P. clavata was usually described as purple, purple/yellow or completely dark yellow, similarly to P. macrospina from Sciacca Shoal (Carpine & Grasshoff Citation1975). In agreement with Carpine and Grasshoff (Citation1975), we showed that the only taxonomic character actually reliable for species discrimination in Mediterranean Paramuricea is the number of rows in the collaret (5–8 rows in P. clavata vs 2–4 rows in P. macrospina). Moreover, the spindles of the collaret in the Mediterranean Paramuricea species may be different: in P. macrospina the spindles in general have tubercles more or less dispersed, while in P. clavata the tubercles are mainly located on the convex side of the spindles. This character was considered by Carpine and Grasshoff (Citation1975) to be diagnostic in discriminating P. clavata from P. macrospina, but we showed that it is too variable and therefore not suitable for species-level identification. In this respect, colonies of P. macrospina from Skerki Bank and Sciacca Shoal have many spindles of the collaret with tubercles mainly located on the convex side, as reported in P. clavata.
The typical, but not diagnostic, long thornscales of the anthostele were recorded only in the colony from Pantelleria. Thornscales and spindles of the coenenchyme in P. macrospina from Skerki Bank and Sciacca Shoal are, in terms of shape, close to those of P. clavata. These sclerites, in fact, are described as too variable in shape and length to be considered a valid diagnostic taxonomic character (Carpine & Grasshoff Citation1975).
Even though more colonies should be studied, our preliminary statistical analyses of the size of sclerites showed that in P. macrospina this character is variable enough that it cannot be used to discriminate between the two Mediterranean species. Sclerites show a high variability in shape and size, and constitute an important trait in octocoral taxonomy (e.g. Bayer Citation1961; Fabricius & Alderslade Citation2001; Tentori & Ofwegen Citation2011). Moreover, abiotic and biotic factors such as light intensity, water movement, depth and predation rate can affect their shape (Velimirov Citation1976; West et al. Citation1993; West Citation1998; Kim et al. Citation2004; Clavico et al. Citation2007; Prada et al. Citation2008), but clear links still need to be demonstrated. The sharing of partial morphological features between red gorgonians and some P. macrospina morphotypes highlights the role of the environment in species adaptation and plasticity. Environmental conditions may influence the branching pattern of the colonies and the size of sclerites of Paramuricea macrospina, as already reported for other gorgonian species (Sánchez et al. Citation2007; Prada et al. Citation2008; Gutiérrez-Rodríguez et al. Citation2009), leading to the formation of these morphotypes in shallow water. The “thin morph” of P. macrospina is likely more common where water movement is low, while the “thick morph” has been found in more exposed shallow areas.
The Mediterranean Paramuricea species represent two highly divergent lineages resulting from two independent vicariance events related to the Messinian and Gelasian crises, as documented by Poliseno et al. (Citation2017). This diversity has been considered to reflect also the ecology of the endemic Paramuricea species, with P. clavata occurring at shallower depths and P. macrospina being restricted to deeper habitats. The present data suggest a higher morphological and ecological plasticity of P. macrospina that, in shallow habitats, has similar characters of P. clavata.
This study updates and summarises the distribution of P. macrospina from the literature data considering the whole Mediterranean Basin (Table S1). Our results show that this species is widely distributed, with the exception of the eastern side (Marmara Sea). Within the Mediterranean Sea there is an overall lack of knowledge regarding the area between the Northern and Southern sides and the Western and Eastern parts of the basin. In particular, only a few studies concerning the distribution of gorgonians from the eastern Mediterranean are available (Di Camillo et al. Citation2018), so the absence of P. macrospina in the region could mirror this gap. Over the last two decades, new data on the distribution of several Mediterranean benthic species have been provided through the exploration of mesophotic and deep habitats (from 60 to 300 m depth; i.e. Aguilar et al. Citation2009; Bo et al. Citation2011, Citation2012; Giusti et al. Citation2015; Grinyó et al. Citation2016). Data from remotely operated vehicle (ROV) studies may explain in part why the majority of the records from the recent literature are reported from 50–200 m depth, confirming the preferential bathymetric distribution of P. macrospina (Carpine & Grasshoff Citation1975). Colonies of P. macrospina were also recorded in deeper waters; however, the deepest record, reported from 970–1002 m depth in the Catalan Basin, is considered doubtful due to the uncertainty of the species identification (Cartes et al. Citation2009). Further deep-sea records were reported also for the Apulian coast at 538–826 m depth (Mastrototaro et al. Citation2010) and for the Montenegrin slope at 430–490 m depth (Angeletti et al. Citation2014), confirming the scattered presence of this species in the Mediterranean deep-sea waters.
In the present work, we report two new records of P. macrospina from the Skerki Bank and Sciacca Shoal, widening the distribution of its upper limit. Although observations from recreational divers, including technical divers, can contribute to increase our knowledge on the actual distribution of several species in shallow waters (Cerrano et al. Citation2016), currently few records of P. macrospina have been reported (Carpine & Grasshoff Citation1975; Balduzzi et al. Citation1994; Vafidis et al. Citation1994; Topçu & Öztürk Citation2013, Citation2015, Citation2016). Given the difficulties of species discrimination among P. clavata and the puzzling shallow-water colonies of P. macrospina, we suppose that this species may have a wider bathymetric distribution. Further ecological surveys together with valid specimen identification should be carried out to confirm the real distribution of P. macrospina in the Mediterranean Sea.
P. macrospina colonises a wide range of sea bottoms such as rocks, coralligenous habitat, maerl, detritic and sandy-mud bottoms (Carpine & Grasshoff Citation1975; Bo et al. Citation2012; Topçu & Öztürk Citation2015). The literature reviewed in this study shows that this species is usually found in the coralligenous outcrops together with other gorgonians, such as P. clavata, and antipatharians (Gori et al. Citation2017). Differently from P. clavata, P. macrospina is able to form dominant assemblages on more unstable bottoms, such as coastal detritic and maerl (Topçu & Öztürk Citation2015; Grinyó et al. Citation2016). The ability to colonise this habitat is probably related to its greater adaptability in deep waters and its fast growth rate with respect to P. clavata (Bo et al. Citation2012; Topçu & Öztürk Citation2015).
More specimens should be collected to investigate in depth the intraspecific variation of P. macrospina species in the Mediterranean Basin. Population genetics and connectivity studies could be useful to better understand the gene flow among and within the Mediterranean populations of P. macrospina. Furthermore, the use of new molecular technologies such as next-generation sequencing (NGS) could be also exploited to assess whether the morphological plasticity is driven by adaptive genetic processes of populations exposed to different environmental, climatic and bathymetric conditions. We need to increase the number of samples of these peculiar colonies of P. macrospina, and a tailored citizen science project for volunteer divers (Cerrano et al. Citation2016) could be launched in order to increase the chance of finding new colonies.
Pica_et_al_Fig_S1.pdf
Download PDF (171.8 KB)Pica_et_al_TabS1_TabS2_REV.docx
Download MS Word (32 KB)Acknowledgements
Gert Wörheide is thanked for providing access to the laboratory facilities of the Department of Earth & Environmental Sciences, Paleontology and Geobiology. The authors wish to thank Santo Tirnetta for the collection of samples and related images at Sciacca Shoal. We thank the editor and reviewers for their revisions, which improved earlier versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Aguilar R, Goffredo S, Garcia S, Bavestrello G, Linares CL, Ozalp B, Forero A 2015. Paramuricea macrospina. The IUCN Red List of Threatened Species 2015: e.T50012470A50609268. Available: http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T50012470A50609268.en. Accessed Apr 2018 24.
- Aguilar R, Pastor X, Torriente A, Garcia S 2009. Deep-sea Coralligenous and red algae concretions’ communities observed in the western Mediterranean by using ROV. First Mediterranean Symposium on the Conservation of Coralligenous and other calcareus bioconcretions. UNEP/MAP/RAC/SPA, 15-16 January 2009. Tunis. pp. 148–150.
- Ament-Velásquez S, Breedy O, Cortés J, Guzman HM, Wörheide G, Vargas S. 2016. Homoplasious colony morphology and mito-nuclear phylogenetic discordance among Eastern Pacific octocorals. Molecular Phylogenetics and Evolution 98:373–381. DOI: 10.1016/j.ympev.2016.02.023.
- Angeletti L, Taviani M, Canese S, Foglini F, Mastrototaro F, Argnani A, Trincardi F, Bakran-Petricioli T, Ceregato A, Chimienti G, Mačić V, Poliseno A. 2014. New deep-water cnidarian sites in the southern Adriatic Sea. Mediterranean Marine Science 15:263–273. DOI: 10.12681/mms.558.
- Balduzzi A, Bianchi CN, Cattaneo-Vietti R, Cerrano C, Cocito S, Cotta S, Degl’Innocenti F, Diviacco G, Morgigni M, Morri C, Pansini M, Salvatori L, Senes L, Sgorbini S, Tunesi L 1994. Primi lineamenti di bionomia bentica dell’Isola Gallinaria (Mar Ligure). Atti del 10 Congresso della Associazione italiana di Oceanologia e Limnologia, AIOL, 4-6 November 1992. Italy. pp. 603–617.
- Bayer FM. 1961. The shallow-water Octocorallia of the West Indian Region. Netherlands: The Hague. 373 pp.
- Bilewitch JP, Ekins M, Hooper J, Degnan SM. 2014. Molecular and morphological systematics of the Ellisellidae (Coelenterata: Octocorallia): Parallel evolution in a globally distributed family of octocorals. Molecular Phylogenetics and Evolution 73:106–118. DOI: 10.1016/j.ympev.2014.01.023.
- Bo M, Bertolino M, Borghini M, Castellano M, Covazzi Harriague A, Di Camillo CG, Gasparini G, Misic C, Povero P, Pusceddu A, Schroeder K, Bavestrello G. 2011. Characteristics of the mesophotic megabenthic assemblages of the Vercelli Seamount (North Tyrrhenian Sea). PLoS ONE 6:e16357. DOI: 10.1371/journal.pone.0016357.
- Bo M, Canese S, Spaggiari C, Pusceddu A, Bertolino M, Angiolillo M, Giusti M, Loreto MF, Salvati E, Greco S, Bavestrello G. 2012. Deep coral oases in the South Tyrrhenian Sea. PLoS ONE 7:e49870. DOI: 10.1371/journal.pone.0049870.
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ. 2014. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10:e1003537. DOI: 10.1371/journal.pcbi.1003537.
- Brazeau DA, Lasker HR. 1988. Inter- and intraspecific variation in gorgonian colony morphology: Quantifying branching patterns in arborescent animals. Coral Reefs 7:139–143. DOI: 10.1007/BF00300973.
- Calvisi G, Trainito E, Pais M, Franci G, Schiaparelli S. 2003. Prima segnalazione di un episodio di mortalità di gorgonacei lungo la costa dell’Isola di Tavolara (Sardegna Settentrionale). Biologia Marina Mediterranea 10:506–508.
- Carpine C. 1963. Contribution à la connaissance des Gorgones Holaxonia de la Méditerranée occidentale. Bulletin de l’Institut Océanographique 60:1–52.
- Carpine C, Grasshoff M. 1975. Les gorgonaires de la Méditerranée. Bulletin de l’Institut Océanographique Monaco 71:1–140.
- Cartes JE, Maynou F, Fanelli E, Papiol V, Lloris D. 2009. Long-term changes in the composition and diversity of deep-slope megabenthos and trophic webs off Catalonia (western Mediterranean): Are trends related to climatic oscillations? Progress in Oceanography 82:32–46. DOI: 10.1016/j.pocean.2009.03.003.
- Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F. 2000. A catastrophic mass‐mortality episode of gorgonians and other organisms in the Ligurian Sea (North‐western Mediterranean), summer 1999. Ecology Letters 3:284–293. DOI: 10.1046/j.1461-0248.2000.00152.x.
- Cerrano C, Milanese M, Ponti M. 2016. Diving for science‐science for diving: Volunteer scuba divers support science and conservation in the Mediterranean Sea. Aquatic Conservation: Marine and Freshwater Ecosystems 27:303–323. DOI: 10.1002/aqc.2663.
- Clavico EE, De Souza AT, Da Gama BA, Pereira RC. 2007. Antipredator defense and phenotypic plasticity of sclerites from Renilla muelleri, a tropical sea pansy. The Biological Bulletin 213:135–140. DOI: 10.2307/25066647.
- Cordeiro R, van Ofwegen L, Williams G 2018. World list of Octocorallia. Paramuricea Kölliker, 1865. World Register of Marine Species. Available: http://www.marinespecies.org/aphia.php?p=taxdetails&id=125311. Accessed Mar 2018 31.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9:772. DOI: 10.1038/nmeth.2109.
- Di Camillo CG, Ponti M, Bavestrello G, Krzelj M, Cerrano C. 2018. Building a baseline for habitat-forming corals by a multi-source approach, including Web Ecological Knowledge. Biodiversity and Conservation 27:1257–1276. DOI: 10.1007/s10531-017-1492-8.
- Doyle JJ, Doyle JLA. 1987. Rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15.
- Edgar RC. 2004. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32:1792–1797. DOI: 10.1093/nar/gkh073.
- Fabricius K, Alderslade P. 2001. Soft corals and sea fans-A comprehensive guide to the tropical shallow-water genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Townsville: Australian Institute of Marine Science. 264 pp.
- Garrabou J, Coma R, Bensoussan N, Bally M, Chevaldonné P, Cigliano M, Diaz D, Harmelin JG, Gambi MC, Kersting DK, Ledoux JB, Lejeusne C, Linares C, Marschal C, Pérez T, Ribes M, Romano JC, Serrano E, Teixido N, Torrents O, Zabala M, Zuberer F, Cerrano C. 2009. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Global Change Biology 15:1090–1103. DOI: 10.1111/gcb.2009.15.issue-5.
- Giusti M, Cerrano C, Angiolillo M, Tunesi L, Canese S. 2015. An updated overview of the geographic and bathymetric distribution of Savalia savaglia. Mediterranean Marine Science 16:128–135. DOI: 10.12681/mms.890.
- Gori A, Bavestrello G, Grinyó J, Dominguez-Carrió C, Ambroso S, Bo M. 2017. Animal forests in deep coastal bottoms and Continental Shelf of the Mediterranean Sea. In: Rossi S, Bramanti L, Gori A, Orejas C, editors. Marine animal forests: The ecology of benthic biodiversity hotspots. Switzerland: Springer International Publishing. pp. 1–27.
- Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27:221–224. DOI: 10.1093/molbev/msp259.
- Grasshoff M. 1977. Die Gorgonariendes ÓstlichenNordatlantik und des Mittelmeeres.III. Die Familie Paramuriceidae (Cnidaria, Anthozoa). Meteor-Forschungsergebnisse. Reihe D. Biologie 27:5–76.
- Grigg RW. 1972. Orientation and growth form of sea fans. Limnology and Oceanography 17:185–192. DOI: 10.4319/lo.1972.17.2.0185.
- Grinyó J, Gori A, Ambroso S, Purroy A, Calatayud C, Dominguez-Carrió C, Coppari M, Lo Iacono C, López-González PJ, Gili JM. 2016. Diversity, distribution and population size structure of deep Mediterranean gorgonian assemblages (Menorca Channel, Western Mediterranean Sea). Progress in Oceanography 145:42–56. DOI: 10.1016/j.pocean.2016.05.001.
- Grinyó J, Gori A, López-González PJ, Santín A, Baena P, Gili JM. 2017. Morphological features of the gorgonian Paramuricea macrospina on the continental shelf and shelf edge (Menorca Channel, Western Mediterranean Sea). Marine Biology Research. DOI: 10.1080/17451000.2017.1375118.
- Gutiérrez-Rodríguez C, Barbeitos MS, Sánchez JA, Lasker HR. 2009. Phylogeography and morphological variation of the branching octocoral Pseudopterogorgia elisabethae. Molecular Phylogenetics and Evolution 50:1–15. DOI: 10.1016/j.ympev.2008.09.019.
- Huete-Stauffer C, Vielmini I, Palma M, Navone A, Panzalis P, Vezzulli L, Misic C, Cerrano C. 2011. Paramuricea clavata (Anthozoa, Octocorallia) loss in the Marine Protected Area of Tavolara (Sardinia, Italy) due to a mass mortality event. Marine Ecology 32:107–116. DOI: 10.1111/mae.2011.32.issue-s1.
- Kim E, Lasker HR, Coffrot MA, Kim K. 2004. Morphological and genetic variation across reef habitats in a broadcast spawning octocoral. Hydrobiologia 530:423–432.
- Koch G. 1882. Vorlaüfige Mittheilungen über die Gorgonien (Alcyonaria axifera) von Neapel und über die Entwicklung der Gorgonia verrucosa. Mittheilungen aus der Zoologischen Station zu Neapel 3:537–550.
- Koch G. 1887. Die Gorgoniden des Golfes von Neapel und der angrenzenden Meeresabschnitte. Fauna und Flora des Golfes von Neapel 15:1–99.
- Kölliker AV. 1865. Von der erhärteten Bindesubstanz oder den Skeletbildungen der Coelenteraten. Icones histiologicae, oder Atlas der vergleichenden Gewebelehre. pp. 117–171.
- Kükenthal W. 1924. Gorgonaria. Das Tierreich 47:1–459.
- Linares C, Coma R, Diaz D, Zabala M, Hereu B, Dantart L. 2005. Immediate and delayed effects of a mass mortality event on gorgonian population dynamics and benthic community structure in the NW Mediterranean Sea. Marine Ecology Progress Series 305:127–137. DOI: 10.3354/meps305127.
- Mastrototaro F, D’onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A. 2010. Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep Sea Research Part II: Topical Studies in Oceanography 57:412–430. DOI: 10.1016/j.dsr2.2009.08.021.
- McFadden CS, Benayahu Y, Pante E, Thoma JN, Nevarez PA, France SC. 2011. Limitations of mitochondrial gene barcoding in the cnidarian sub-class Octocorallia. Molecular Ecology Resources 11:19–31. DOI: 10.1111/j.1755-0998.2010.02924.x.
- Mokhtar-Jamai K, Pascual M, Ledoux JB, Coma R, Féral JP, Garrabou J, Aurelle D. 2011. From global to local genetic structuring in the red gorgonian Paramuricea clavata: The interplay between oceanographic conditions and limited larval dispersal. Molecular Ecology 20:3291–3305. DOI: 10.1111/j.1365-294X.2011.05176.x.
- Otero MM, Numa C, Bo M, Orejas C, Garrabou J, Cerrano C, Kružic´ P, Antoniadou C, Aguilar R, Kipson S, Linares C, Terrón-Sigler A, Brossard J, Kersting D, Casado-Amezúa P, García S, Goffredo S, Ocaña O, Caroselli E, Maldonado M, Bavestrello G, Cattaneo-Vietti R, Özalp B. 2017. Overview of the conservation status of Mediterranean anthozoans. Malaga, Spain: IUCN. 73 pp.
- Pax F, Müller I. 1962. Die anthozoenfauna der Adria. Split: Institut für Ozeanographie und Fischerei. 376 pp.
- Poliseno A, Altuna A, Cerrano C, Wörheide G, Vargas S. 2017. Historical biogeography and mitogenomics of two endemic Mediterranean gorgonians (Holaxonia, Plexauridae). Diversity & Evolution 17:365–373. DOI: 10.1007/s13127-017-0322-x.
- Ponti M, Grech D, Mori M, Perlini RA, Ventra V, Panzalis PA, Cerrano C. 2016. The role of gorgonians on the diversity of vagile benthic fauna in Mediterranean rocky habitats. Marine Biology 163:120. DOI: 10.1007/s00227-016-2897-8.
- Ponti M, Perlini RA, Ventra V, Grech D, Abbiati M, Cerrano C, Ferse SCA. 2014. Ecological shifts in Mediterranean coralligenous assemblages related to gorgonian forest loss. PloS One 9:e102782. DOI: 10.1371/journal.pone.0102782.
- Ponti M, Turicchia E, Ferro F, Cerrano C, Abbiati M. 2018. The understorey of gorgonian forests in mesophotic temperate reefs. Aquatic Conservation in Press. DOI: 10.1002/aqc.2928.
- Prada C, Schizas N, Yoshioka P. 2008. Phenotypic plasticity or speciation? A case from a clonal marine organism. BMC Evolutionary Biology 8:47. DOI: 10.1186/1471-2148-8-47.
- Rambaut A, Suchard MA, Xie D, Drummond AJ 2014. Tracer v1.6. Available: http://beast.bio.ed.ac.uk/Tracer. Accessed Apr 2018 24.
- Risso A. 1826. Histoire naturelle des principales productions de l’Europe Méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes. Paris: Levrault 5:400.
- Sànchez JA, Aguilar C, Dorado D, Manrique N. 2007. Phenotypic plasticity and morphological integration in a marine modular invertebrate. BMC Evolution Biology 7:122. DOI: 10.1186/1471-2148-7-122.
- Stamatakis A. 2006. RAxML-VI-HPC: Maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. DOI: 10.1093/bioinformatics/btl446.
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57:758–771. DOI: 10.1080/10635150701856671.
- Stiasny G. 1942. Alcyonaria und Gorgonaria aus dem Gulf von Neapel. Pubblicazioni della Stazione Zoologica di Napoli 19:1–47.
- Tentori E, van Ofwegen LP. 2011. Patterns of distribution of calcite crystals in soft corals sclerites. Journal of Morphology 272:614–628. DOI: 10.1002/jmor.v272.5.
- Topçu EN, Öztürk B. 2013. Octocoral diversity of Balıkçı Island, the Marmara Sea. Journal of Black Sea/Mediterranean Environment 19:46–57.
- Topçu EN, Öztürk B. 2015. Composition and abundance of octocorals in the Sea of Marmara, where the Mediterranean meets the Black Sea. Scientia Marina 79:125–135. DOI: 10.3989/scimar.2015.79n1.
- Topçu EN, Öztürk B. 2016. First insights into the demography of the rare gorgonian Spinimuricea klavereni in the Mediterranean Sea. Marine Ecology 37:1154–1160. DOI: 10.1111/maec.12352.
- Vafidis D, Koukouras A, Voultsiadou-Koukoura E. 1994. Octocoral fauna of the Aegean Sea with a checklist of the Mediterranean species: New information, faunal comparisons. Annales de l’Institut Oceanographique 70:217–230.
- Valisano L, Notari F, Mori M, Cerrano C. 2016. Temporal variability of sedimentation rates and mobile fauna inside and outside a gorgonian garden. Marine Ecology 37:1303–1314. DOI: 10.1111/maec.2016.37.issue-6.
- Velimirov B. 1976. Variations in growth forms of Eunicella cavolinii Koch (Octocorallia) related to intensity of water movement. Journal of Experimental Marine Biology and Ecology 21:109–117. DOI: 10.1016/0022-0981(76)90032-0.
- Vezzulli L, Pezzati E, Huete-Stauffer C, Pruzzo C, Cerrano C. 2013. 16SrDNA pyrosequencing of the Mediterranean gorgonian Paramuricea clavata reveals a link among alterations in bacterial holobiont members, anthropogenic influence and disease outbreaks. PloS One 8:e67745. DOI: 10.1371/journal.pone.0067745.
- West JM. 1998. The dual role of sclerites in a gorgonian coral: Conflicting functions of support and defence. Evolutionary Ecology 12:803–821. DOI: 10.1023/A:1006542515553.
- West JM, Harvell CD, Walls AM. 1993. Morphological plasticity in a gorgonian coral (Briareum asbestinum) over a depth cline. Marine Ecology Progress Series 94:61–69. DOI: 10.3354/meps094061.
- Wirshing HH, Baker AC. 2015. Molecular and morphological species boundaries in the gorgonian octocoral genus Pterogorgia (Octocorallia: Gorgoniidae). PLoS ONE 10:e0133517. DOI: 10.1371/journal.pone.0133517.