Abstract
Length–weight relationships of bivalve species collected during razor clam (Ensis minor) surveys along Italian coastal waters (Northern Adriatic and Tyrrhenian Sea) in the 3-year period 2016–2018 are reported. A total of 13,588 individuals, belonging to 12 bivalve species and 20 populations between the Adriatic and Tyrrhenian Seas, were sampled for this study. Analyzing growth parameters for each population, we found 11 allometries and nine isometries. About half of the species investigated showed different growth characteristics between the two areas.
Introduction
The razor clam Ensis minor (Chenu, 1843), a bivalve species occurring in Italian coastal waters, lives buried in the sandy bottom between 2.5 and 4 m depth (Del Piero & Dacaprile Citation1998). The development of its gonads occurs in winter and the reproductive period is concentrated in early springtime. The minimum landing size of 8 cm in length is reached in about a year (Froglia Citation1975; Del Piero & Dacaprile Citation1998). Froglia (Citation1975) found 6-cm individuals with mature gonads, demonstrating that the first reproduction event occurs within the first year of life. This species is an important fishery resource in the North Adriatic Sea and Central Tyrrhenian Sea, and it is actively exploited by hydraulic dredges. Landings for the year 2017 reported by fishermen’s logbooks were 42,831.0 kg for the Central Tyrrhenian and 7228.6 kg for the North Adriatic. Annual surveys are carried out by the National Research Council, Institute for Biological Resources and Marine Biotechnologies (CNR-IRBIM), aiming to assess the exploitation status of populations in these two areas, and to propose fishery management measures. Fishing with hydraulic dredges leads to the catch of other bivalve species occurring in the same habitat, and so allows collecting and analyzing a wide variety of bivalves of broad size ranges in different geographical areas. As information available on bivalve morphometric relationships is scarce and limited to specific areas (Gaspar et al. Citation2001, Citation2002; Charef et al. Citation2012; Vasconcelos et al. Citation2018), we here report the weight–length relationship of 12 bivalve species from the North Adriatic Sea and the Central Tyrrhenian Sea. Morphometric data could be used in fishery models and in improving the selectivity of hydraulic dredges (Gaspar et al. Citation2002), while comparison between populations from different habitats is useful to understand growth rates as a function of environmental and/or anthropogenic factors (Petrakis & Stergiou Citation1995; Gonçalves et al. Citation1997).
Materials and methods
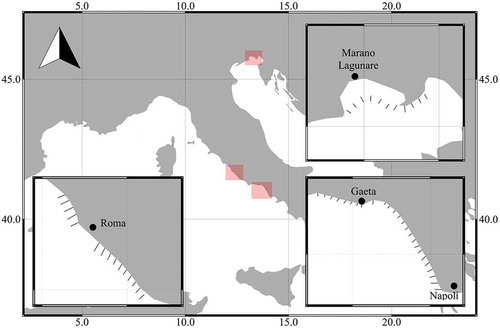
The specimens were collected during fishing surveys carried out along the Tyrrhenian and Adriatic coasts in the 3-year period 2016–2018 aboard fishing vessels. A commercial dredge 3 m wide was used to penetrate about 20 cm into the sediment and catch razor clams; the dredge was designed to catch specimens of legal size (from 8 cm upward), with a grating made of rods spaced 7 mm apart, following the Italian law (DM 22/12/Citation2000). To collect juvenile specimens, a net sampler with fine meshes (14 mm) and dimensions 40 cm × 18 cm was fixed inside the dredge. Transects perpendicular to the coastline, spaced about 2 miles apart, were chosen (11 for the Adriatic, 40 for the Tyrrhenian Sea; ). For each transect, three random sampling stations were established at depths between 1 and 4 m. Each haul was carried out by towing the hydraulic dredge in sandy bottom for 100 m at a constant speed of 0.8 knots.
Figure 1. Map of the razor clam surveys carried out in the 3-year period 2016–2018 in Italian coastal waters.
Identification of bivalve species was made to the lowest possible taxonomic level, and the nomenclature adopted was the most updated available in the World Register of Marine Species – WoRMS (http://www.marinespecies.org). Bivalve shell length (i.e. the maximum distance between anterior and posterior margins) was determined to the nearest 0.01 mm using a manual calliper; total wet weight was detected using a digital balance with a precision of 0.1 g. Damaged individuals were discarded and not considered in the analyses. The relationship W = aLb, one of the most commonly used in any analysis of fishery data, allows the estimation of weight (W) from length (L) or vice versa for individuals (Le Cren Citation1951; Petrakis & Stergiou Citation1995; Froese Citation2006). This relationship (1) is useful for comparison between populations of the same species living in different geographical areas (Gaspar et al. Citation2002); (2) is used in fishery models for stock monitoring and management (Robinson et al. Citation2010); and (3) can be used, in bivalve fishery, in improving dredge and onboard sorting sieve selectivity (Gaspar et al. Citation2002; Vasconcelos et al. Citation2018). The a parameter is the intercept, representing the initial growth coefficient. The exponent b is the slope, representing relative growth rates of the variables and so providing information on growth (Morey et al. Citation2003): when b = 3 growth is isometric, and when b is significantly different from 3 growth is allometric (positive if b > 3, negative if b < 3). The equation W = aLb and its linearized form logW = loga + b*logL were determined for eight species in both sampling areas, and for four species only in one sampling area (one for the Adriatic Sea and three for the Tyrrhenian Sea). A t-test (ts = (b − 3)/sb) with a confidence level of 95% was applied to determine the significance of morphometric relationships using the coefficient b (= , < or > 3) and its standard error Sb (Huxley & Teissier Citation1936; Sokal & Rohlf Citation1987). An analysis of variance (ANOVA) was carried out to assess differences in growth between areas. All the analyses were performed with the free R-package FSA (Ogle et al. Citation2018, version 0.8.22).
Results
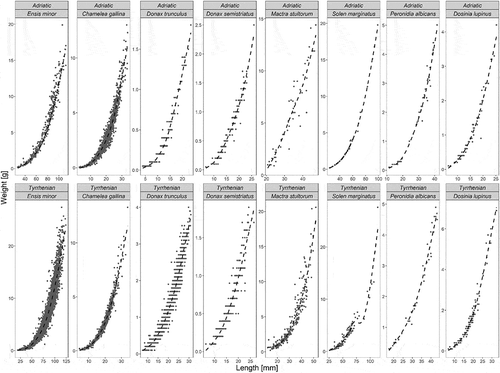
A total of 13,588 individuals, belonging to 12 bivalve species and 20 populations between the Adriatic and Tyrrhenian Seas, were sampled for this study, and a summary of the statistics referring to their length–weight relationship is listed in . It is important to specify that the gear used for sampling is not designed for all species of bivalves or all sizes within species. Nevertheless, the presence of the fine mesh sampler allowed us to sample individuals of these bivalve communities belonging to a wide range of size classes. The length–weight relationships estimated for each population were significant (p < 0.001) in some cases (). In total, 11 allometries and nine isometries were found; for the North Adriatic Sea, allometry was observed in E. minor, Chamelea gallina (Linnaeus, 1758), Donax trunculus (Linnaeus, 1758), Solen marginatus (Pulteney, 1799), Peronidia albicans (Gmelin, 1791) and Loripes orbiculatus (Poli, 1795); for the Central Tyrrhenian Sea, allometry was observed in E. minor, C. gallina, D. trunculus, Donax semistriatus (Poli, 1795) and Macomopsis cumana (O.G. Costa, 1830).
Table I. Length–weight relationship and growth parameters of the bivalve species investigated.
For eight species out of 12 we obtained growth curves from both areas (Adriatic and Tyrrhenian Seas), which are summarized in . Results from ANOVA between the two sampling areas gave significant differences in growth for E. minor (F1, 63 = 9.41, p < 0.002), D. trunculus (F1, 26 = 65.35, p < 0.001), D. semistriatus (F1, 48 = 9.64, p < 0.002) and C. gallina (F1, 32 = 8.66, p < 0.003). No statistical significance was observed for Mactra stultorum (Linnaeus, 1758) (F1,31 = 3.01, p = 0.08), Dosinia lupines (Linnaeus, 1758) (F1,16 = 0.31, p = 0.58), S. marginatus (F1,19 = 1.39, p = 0.24) or P. albicans (F1, 72 = 0.63, p = 0.43).
Discussion
About half of the species investigated in the two areas could present a significantly different growth. Populations of the same species living in different areas are in fact conditioned by different variables, which can be abiotic (environmental) or biotic (physiological) (Gaspar et al. Citation2002), although in the present study these variables were not taken into account. Growth patterns also vary considering different life stages (Gaspar et al. Citation2002) and seem to vary even within the year, as demonstrated by different b values in monthly length–weight relationships (Tirado & Salas Citation1998; Ngor et al. Citation2018). Between the North Adriatic and the Central Tyrrhenian Sea, a possible hypothesis explaining this difference may be related to latitude (Beukema & Meehan Citation1985), depth, type of bottom (Claxton et al. Citation1998) and/or type of sediment (Newell & Hidu Citation1982). Our data included a wide size range for each species and area, so we can exclude differences due to sampling.
Interestingly, only the commercial species showed significantly different growth parameters between the two areas. In particular, for E. minor, C. gallina and D. trunculus we found that b values were significantly lower in the North Adriatic Sea than in the Central Tyrrhenian Sea; in contrast, D. semistriatus showed a b value higher in the Adriatic Sea than in the Tyrrhenian Sea.
Growth parameters observed in the literature review showed clear differences among the present study and previous observations made in the Mediterranean Sea and on the European Atlantic coasts for some of the species (). A qualitative comparison between our findings on C. gallina and D. trunculus and those available in the literature revealed a common negative allometry, indicating that individuals of both species grow more in length than in weight. Other species such as D. semistriatus, S. marginatus, D. lupinus and P. albicans displayed different types of growth depending on the area where they were collected.
Table II. Morphometric growth patterns available in the literature.
Our results improve the existing knowledge of growth parameters of some bivalve species that are very common along the Italian coasts, and point to the extreme variability of the length–weight relationship within the species in different areas. Further studies are required to better comprehend morphometric patterns especially of commercial species, in order to identify direct and/or indirect anthropogenic stressors to better manage these economic resources.
Acknowledgements
The research was conducted under the European Data Collection Framework and financially supported by the Italian Ministry for Agricultural, Food, Forestry and Tourism Policies (MIPAAFT). The authors are grateful to Pierluigi Strafella and Vera Salvalaggio for their contributions to the identification of bivalve species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ansell AD, Lagardčre F. 1980. Observations on the biology of Donax trunculus and Donax vittatus at Ile d’Oleron (French Atlantic Coast). Marine Biology 57:287–300. DOI: 10.1007/BF00387572.
- Beukema JJ, Meehan BW. 1985. Latitudinal variation in linear growth and other shell characteristics of Macoma balthica. Marine Biology 90:27–33. DOI: 10.1007/BF00428211.
- Charef A, Langar NZ, Gharsallah IH. 2012. Stock size assessment and spatial distribution of bivalve species in the Gulf of Tunis. Journal of the Marine Biological Association of the United Kingdom 92:179–186. DOI: 10.1017/S0025315411000403.
- Claxton WT, Wilson AB, Mackie GL, Boulding EG. 1998. A genetic and morphological comparison of shallow-and deep-water populations of the introduced dreissenid bivalve Dreissena bugensis. Canadian Journal of Zoology 76:1269–1276. DOI: 10.1139/z98-064.
- Çolakoğlu S. 2014. Population structure, growth and production of the wedge clam Donax trunculus (Bivalvia, Donacidae) in the West Marmara Sea, Turkey. Turkish Journal of Fisheries and Aquatic Sciences 14:221–230. DOI: 10.4194/1303-2712-v14_1_24.
- Da Costa F, Martínez-Patiño D. 2009. Culture potential of the razor clam Solen marginatus (Pennánt, 1777). Aquaculture 288:57–64. DOI: 10.1016/j.aquaculture.2008.11.001.
- Decreto Ministeriale Ministero delle Risorse Agricole, Alimentari, e Forestali. 2000. Disciplina della pesca dei molluschi bivalvi. Modifiche al DM 21/7/1998. 22/12/200.
- Del Piero D, Dacaprile R. 1998. The alternating recruitment pattern in Ensis minor, an exploited bivalve in the Gulf of Trieste, Italy. Hydrobiologia 375:67–72. DOI: 10.1023/A:1017017224280.
- Froese R. 2006. Cube law, condition factor and weight–length relationships: History, meta‐analysis and recommendations. Journal of Applied Ichthyology 22:241–253. DOI: 10.1111/jai.2006.22.issue-4.
- Froglia C. 1975. Osservazioni sull’accrescimento di Chamelea gallina (L.) ed Ensis minor (Chenu) nel medio Adriatico. Quaderni del Laboratorio di Tecnologia della Pesca 2:37–48.
- Gaspar MB, Santos MN, Vasconcelos P. 2001. Weight–length relationships of 25 bivalve species (Mollusca: Bivalvia) from the Algarve coast (southern Portugal). Journal of the Marine Biological Association of the United Kingdom 81:805–807. DOI: 10.1017/S0025315401004623.
- Gaspar MB, Santos MN, Vasconcelos P, Monteiro CC. 2002. Shell morphometric relationships of the most common bivalve species (Mollusca: Bivalvia) of the Algarve coast (southern Portugal). Hydrobiologia 477:73–80. DOI: 10.1023/A:1021009031717.
- Gonçalves JMS, Bentes L, Lino PG, Ribeiro J, Canario AV, Erzini K. 1997. Weight-length relationships for selected fish species of the small-scale demersal fisheries of the south and south-west coast of Portugal. Fisheries Research 30:253–256. DOI: 10.1016/S0165-7836(96)00569-3.
- Huxley JS, Teissier G. 1936. Terminology of relative growth. Nature 137:780–781. DOI: 10.1038/137780b0.
- Kasapoglu N, Duzgunes E. 2013. Length-weight relationships of marine species caught by five gears from the Black Sea. Mediterranean Marine Science 15:95–100. DOI: 10.12681/mms.463.
- Le Cren ED. 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). The Journal of Animal Ecology 20:201–219. DOI: 10.2307/1540.
- Morey G, Moranta J, Massutı E, Grau A, Linde M, Riera F, Morales-Nin B. 2003. Weight–Length relationships of littoral to lower slope fishes from the western Mediterranean. Fisheries Research 62:89–96. DOI: 10.1016/S0165-7836(02)00250-3.
- Newell CR, Hidu H. 1982. The effects of sediment type on growth rate and shell allometry in the soft shelled clam Mya arenaria L. Journal of Experimental Marine Biology and Ecology 65:285–295. DOI: 10.1016/0022-0981(82)90060-0.
- Ngor PB, Sor R, Prak LH, So N, Hogan ZS, Lek S. 2018. Mollusc fisheries and length–Weight relationship in Tonle Sap flood pulse system, Cambodia. Annales De Limnologie-International Journal of Limnology 54:34. EDP Sciences.
- Ogle DH, Wheeler P, Dinno A. 2018. FSA: Fisheries stock analysis. R package version 0.8.22. Available: https://github.com/droglenc/FSA
- Petrakis G, Stergiou KI. 1995. Weight-length relationships for 33 fish species in Greek waters. Fisheries Research 21:465–469.
- Ramón M. 1993. Estudio de las poblaciones de Chamelea gallina (Linnaeus, 1758) y Donax trunculus Linnaeus, 1758 (Mollusca: Bivalvia) en el Golfo de Valencia (Mediterráneo occidental). PhD Thesis. Universidad de Barcelona.
- Remacha-Triviño AI, Anadon N. 2006. Reproductive cycle of the razor clam Solen marginatus (Pulteney 1799) in Spain: A comparative study in three different locations. Journal of Shellfish Research 25:869–876.
- Robinson LA, Greenstreet SPR, Reiss H, Callaway R, Craeymeersch J, De Boois I, Kröncke I. 2010. Length–weight relationships of 216 North Sea benthic invertebrates and fish. Journal of the Marine Biological Association of the United Kingdom 90:95–104.
- Rufino MM, Gaspar MB, Pereira AM, Vasconcelos P. 2006. Use of shape to distinguish Chamelea gallina and Chamelea striatula (Bivalvia: Veneridae): Linear and geometric morphometric methods. Journal of Morphology 267:1433–1440.
- Sokal RR, Rohlf FJ. 1987. Introduction to Biostatistics. 2nd ed. New York: Freeman.
- Tirado C, Salas C. 1998. Reproduction and fecundity of Donax trunculus L., 1758 (Bivalvia: Donacidae) in the littoral of Málaga (southern Spain). Journal of Shellfish Research 17:169–176.
- Tunçer S, Erdemir C. 2002. A preliminary study on some properties for Chamelea gallina (L.) (Bivalvia: Veneridae from Karabiga-Canakkale). Turkish Journal of Fisheries and Aquatic Sciences 2:117–120.
- Vasconcelos P, Moura P, Pereira F, Pereira A, Gaspar M. 2018. Morphometric relationships and relative growth of 20 uncommon bivalve species from the Algarve coast (southern Portugal). Journal of the Marine Biological Association of the United Kingdom 98:463–474.