Abstract
Food storing is a well-known phenomenon among birds, described in shrikes Laniidae. The preferred explanation suggests that its function is to signify male quality to females and to mark territorial borders during the mating period. The importance of food storing must vary among different species, however, because in the Red-backed Shrike Lanius collurio, this behaviour takes place during the nestling care period. The aim of this study was to evaluate the significance of food storing by male Red-backed Shrikes. We did not find any differences in the breeding parameters between pairs in which the males did or did not store food. There was, however, a significant tendency for the number of stored prey items to increase when the atmospheric pressure was falling prior to the onset of rain. The mates of males that did store food had a larger body mass than those females whose partners did not do so. This study showed that food storage have a variety of functions, including different ones compared with those described for the best-known shrike species. Differences in food storing phenology in various shrike species are probably due to the period when they start nesting: Red-backed Shrikes start their breeding season in late spring, when food is plentiful. Our study demonstrates that the abundance of food in territories and one’s own condition, as expressed by the number of prey items stored when food is readily available, is not really so relevant. The food store of a Red-backed Shrike thus functions as a larder, which the female makes use of when her energy requirements are the highest, i.e. when caring for her young. Larders are thus essential if females are to remain in good bodily condition: this could be significant if a clutch is lost and a replacement one has to be laid.
Introduction
Food storing is a well-known phenomenon among animals like mammals and birds (Kallander & Smith Citation1990; Vander Wall Citation1990), and storing prey is frequently described in the shrikes Laniidae (Cramp & Perrins Citation1993; Harris & Franklin Citation2000). Unlike other species of animals, shrikes exhibit a characteristic though rare type of behaviour in that they impale their prey on the thorns of trees and shrubs and even on barbed wire fencing used in farming (Yosef & Pinshow Citation2005). Many hypotheses have been set up relating to this way of storing food. It is commonly believed that since shrikes, unlike raptors, do not have talons, impaling their prey makes it easier for them to manipulate it, and dismembered prey is eaten by adult birds, and smaller fragments are fed to nestlings (Yosef Citation1992; Yosef & Pinshow Citation2005). In the course of evolution, storing food has taken on other functions. Keeping larders may attest to male quality, and males may use the impaled prey items to mark their territories (Antczak et al. Citation2005a). In many species of shrikes, the males arrive at the breeding grounds before the females, so appropriate marking of territory (by impaling prey) may attract the earliest arriving females, leading to better reproductive effects, i.e. a greater number of nestlings (clutch sizes decrease as the breeding season progresses), a greater frequency of re-nesting if the first clutch is lost (Yosef & Pinshow Citation1989; Antczak et al. Citation2009), and in some cases even polygyny in males having the best stocked larders (Yosef & Pinshow Citation1988). During the egg incubation period or in the first days of the life of the nestlings, Great Grey Shrike Lanius excubitor females used the larders established by their mates, which significantly shortened their absence from the nest (Yosef Citation1992). Prey items may also be stored in order to allow the toxic substances contained, e.g. in the skin of toads, to decompose, after which such food, unavailable to other species, can be consumed (Antczak et al. Citation2005b). One function of prey storing in shrikes that has been speculated upon (direct evidence is lacking) is buffering against temporary food shortage, e.g. during bad weather, when insects are less active (Tryjanowski et al. Citation2003; Nikolov et al. Citation2004); this has been demonstrated also in other animals (Korpimaki Citation1987; Kotler et al. Citation1999). Prey may also be stored in periods of increased energy expenditure, i.e. during egg laying or when large nestlings are being fed to support increased energetic costs (Carlson Citation1989).
The storing of prey is best known in the Loggerhead Shrike Lanius ludovicianus, Great Grey Shrike and Southern Grey Shrike Lanius meridionalis (Yosef & Pinshow Citation1989; Esely & Bollinger Citation2003; Antczak et al. Citation2005a; Keynan & Yosef Citation2010). The preferred explanation suggests that the function of this activity is to store food, to give females a signal of male quality and to mark territorial borders.
The Red-backed Shrike Lanius collurio is the most numerous shrike species in Europe (Harris & Franklin Citation2000). However, even though there has been much speculation regarding the purpose of food storing in this species (Kuzniak & Tryjanowski Citation2003), we still do not know the main aim of this behaviour. Hence, our objective in this study was to establish the basic reason why Red-backed Shrikes store food during the breeding season, focusing on associating this behaviour with breeding phenology and parameters. We also wish to point out that the following hypotheses are not mutually exclusive. The storage of prey items by males soon after they have returned to their breeding areas appears to be a demonstration to females of their quality or the delimitation of the boundaries of their territories (Yosef & Pinshow Citation1989; Antczak et al. Citation2005b). If that is the case, then food storing should function as a sign by which a female can recognize a male and/or his territory. On the other hand, food stored during the nestling period should simply be more quickly accessible for feeding the young birds, as this stage of the breeding period requires the greatest energy expenditure on the part of the parent birds (Esely & Bollinger Citation2003; Antczak et al. Citation2005b). Males storing food for their mates and nestlings ought to have superior breeding parameters, i.e. more eggs, nestlings and fledglings. These set-aside prey items would act in the same way as the additional food provided in experiments to assess the influence of such food on breeding parameters (e.g. Heath et al. Citation2008; Ruffino et al. Citation2014). Another aspect that we investigated is the link between storing prey items and inclement weather. Atmospheric fronts bringing cooler weather and rainfall severely restrict the activity of insects (Vicens & Bosch Citation2000; Arbeiter et al. Citation2016), which are the main constituents of the Red-backed Shrike’s diet (Tryjanowski et al. Citation2003; Golawski Citation2006a). Since birds are capable of detecting changes in atmospheric pressure (Astheimer et al. Citation1995; Breuner et al. Citation2013) and can sense approaching rains, the shrikes could accumulate sufficient food for them and their young to tie them over the period of bad weather. We also considered the possibility that food storing was dependent on the male’s condition (body mass), assuming that accumulating a store of food would require the expenditure of extra energy and that only heavier birds would be capable of doing so (Antczak et al. Citation2005b). In addition, we analysed the condition (body mass) of females, as they had frequently been found to suffer appreciable losses of body mass during the nestling period, and experiments involving the provision of extra food had reduced such losses (Nagy et al. Citation2007; Eldegard & Sonerud Citation2010). We thus anticipated that the females’ use of the larders would have much the same effect as their consumption of experimentally provided extra food. The last factor we studied, undoubtedly a key one, determining whether food would be stored or not, was the richness of shrike territories in potential food resources.
Materials and methods
Study species
The Red-backed Shrike is a small passerine bird species widely distributed in Europe and western Asia, with population estimates ranging from 24 to 48 million breeding pairs (BirdLife International Citation2019). It is a long distance migrant, which arrives at its breeding grounds from Africa in early May (Harris & Franklin Citation2000). In eastern Poland, the majority of the population inhabits agricultural landscapes, breeding on the edge of woods, in clumps of trees, in orchards and near villages (Golawski & Meissner Citation2008). The breeding season usually starts in mid-May and extends into August. Four to seven eggs are laid and incubated for 14 days. The nestlings remain in the nest for the next 14 days, and fledglings stay around the nest for another two weeks or so (Harris & Franklin Citation2000). This species is normally single-brooded, but in case of first-brood failure, replacement clutches are laid regularly (Antczak et al. Citation2009). The Red-backed Shrike is a mainly insectivorous species, and the storing of food by males is a well-known phenomenon (Hernandez Citation1995; Tryjanowski et al. Citation2003; Golawski Citation2006a).
Study area
The research was conducted in the agricultural landscape around the town of Siedlce in eastern Poland (52.14° N, 21.93°E). The study area abounded in meadows and pastures, divided into small sectors by barbed wire fences; there were also single bushes and trees. Part of the research area was situated along a railway line with plenty of shrubs suitable as shrike nesting sites. Most of the nests were widely scattered, so it was relatively easy to assign most feeding grounds to the breeding sites (nests) of a given pair. As we had studied the breeding ecology of the Red-backed Shrike earlier, its population in this area was quite familiar to us (e.g. Golawski & Golawska Citation2008; Antczak et al. Citation2009).
Data collection
The study was carried out during seven breeding seasons (2012–2018). In each season observations started in mid-May, when the first shrikes arrived, and ended in July/August, when the last pairs departed with their broods. The research addressed the following topics.
Breeding parameters of shrikes. Information was gathered about the date of clutch initiation (helpful for estimating the hatching date) and clutch size, the date and number of hatched nestlings and the number of fledglings. We also observed the birds foraging: each pair was investigated for a minimum of 2 h per season, including the storing of prey. This enabled us to precisely assign each bird to its breeding and feeding sites, and to delimit the borders of its territory. Since a considerable proportion of broods was lost, new nests had to be searched for throughout the breeding season. The nests we found were then monitored approximately every 5 days. Male Red-backed Shrikes regularly bring food to their incubating mates (Harris & Franklin Citation2000), so it was not difficult to link a foraging area with a particular nest. Moreover, each pair was associated with a particular clump of trees and shrubs.
Species composition and phenology of impaled prey items. The fieldwork involved walking slowly along the fences and/or bushes, searching for impaled prey. Every fence, solitary shrub, and shrub on the borders of the small hedgerows found in the study area was looked at. We identified prey species and marked the impaling sites in the territory (on the barbed wire or shrub), so that during the next visit we could see whether the item in question had disappeared or was still in situ. These surveys were carried out twice a week. All doubtful situations were omitted from the subsequent analysis.
Biometrics of birds. Body size was used to calculate individual condition (Labocha & Hayes Citation2012). Shrikes are sensitive to disturbance by observers, especially in the early stages of breeding and frequently abandon their clutches as a result (Tryjanowski & Kuzniak Citation1999). The birds were trapped in mist nets during the period when their young birds were 14–17 days old. This was the most appropriate moment to trap them, as the period of heightened energy expenditure associated with nestling feeding was behind them, when their body masses had dropped to the lowest level (Leugger-Eggimann Citation1997). At the same time, it was very easy to catch them when they were aggressively defending their fledglings, which were still fluttering around near the nest (see also: Tryjanowski & Golawski Citation2004). We measured the wing length of all birds (maximum chord; Svensson Citation1992) with a ruler to the nearest 1 mm and weighed them with a Pesola spring balance to the nearest 0.5 g. In the analysis we used the Scaled Mass Index following Peig and Green (Citation2009). SMI scales individuals being compared to produce a better indicator of body condition based on relative size. The formula for SMI is: SMIi = Mi (L0/Li)bSMA, where Mi and Li are the body mass and wing length of an individual, respectively. LO is the arithmetic mean of wing length for all birds being considered in the scaling calculations, and bSMA is the slope derived from the standardized major-axis (SMA) regression between body mass and wing length, excluding outliers (Peig & Green Citation2009). To determine this index, we used the body mass and wing length data of 32 males and 25 females in two separate calculations. All capture procedures were in accordance with the relevant Polish laws and the license of the first author of this paper.
Food storing and inclement weather. Data on the phenology of the stored victims were available. Undoubtedly, most of the victims were impaled by shrikes on the day of the survey, because their legs and antennae were still moving; only a few victims could have been killed 1–3 days before the survey day. Moreover, the time-lapse digital camera recordings showed that the vast majority of victims were eaten in the evening of the day they were caught. The weather data (atmospheric pressure, rainfall, air temperature) required to test the hypothesis about food being stored before the onset of bad weather and the level of activity of potential shrike victims resulting therefrom were obtained from the Internet (http://www.tutiempo.net) for the meteorological station in Siedlce, located about 15 km from the study area. Because birds can sense a fall in atmospheric pressure up to 24 hours or so before the arrival of a front (Astheimer et al. Citation1995; Breuner et al. Citation2013), we took the fall to be the difference in pressure between the survey day and the following one. The variables describing the mean air temperature and the occurrence or non-occurrence of rain also took into account the relevant data for the survey day and the day after. Air temperature and rainfall strongly affect insect activity, so these factors were included in the analysis as well (Tryjanowski et al. Citation2003; Hušek & Adamík Citation2008).
Determination of the abundance of potential prey in territories. This was one of the most important issues relating to the possibility of prey being stored by individual males. Animals were counted once in every ten-day period, around noon, in good weather, i.e. without rain and only light wind if any, as these weather factors strongly impact on the activity of animals (Vicens & Bosch Citation2000; Arbeiter et al. Citation2016). We counted all the animals ca. > 1 cm long (the shrikes stored only these) along transects 50 m in length. All the surveys were carried out by the same person (EM), so any errors in estimating the size of victims affect all the data. Each transect was 1 m wide on either side of the path taken by the observer, so it had an overall area of 100 m2. Counting began in mid-May and ended in late July; the transects were therefore monitored 8 times in each season (8 ten-day periods). As we were familiar with the distribution of Red-backed Shrike territories from our earlier work, selecting the routes of the transects was quite easy. In our subsequent calculations we used the total number of animals found during the 8 surveys of each transect characterizing a male’s territory. We recorded the numbers of all animals, in particular insects from the orders Coleoptera and Orthoptera, as these were by far the most numerous items stored by the shrikes. This method of assessing the faunal richness of a site has frequently been used in studies of invertebrates, including the orders most commonly found in this study, i.e. coleopterans and orthopterans (Schulze et al. Citation2004; Moller & Mousseau Citation2009; Schirmel et al. Citation2010). The assumption was that the different numbers of potential prey items of the shrike found during the research is an indication of the richness of the territory’s food resources. This, in turn, should translate into the possibility of the more extensive storage of prey in more affluent territories.
Statistical procedure
Since larders are set up mainly during the nestling and fledgling periods, this analysis was based solely on successfully completed broods, that is, when at least one young bird left the nest. By doing so, we were able categorize males into those storing prey items (at least one) and those that did not establish a larder. Hence, we took 82 broods out of the 176 found in 2012–2018 into consideration. At various stages of nesting 94 clutches/broods were lost, i.e. 53.4% of all those that we found. Because red-backed shrikes in Poland have low philopatry (Tryjanowski et al. Citation2007), there was little probability of experimenting on the same individual in two years.
We applied Pearson’s Chi-square test to compare the proportion of males that did or did not store food during the various stages of nesting. The Mann-Whitney test served to compare breeding parameters like clutch initiation date, clutch size, number of hatchlings and number of fledglings between broods where males did or did not store food.
The differences in the Scaled Mass Index between the two groups of males and their mates were calculated using a General Linear Mixed Model (GLMM) with binomial errors and logit link function. Two analyses were done. In first one, the response variable was males (0 – males did not store food, 1 – males did store food). In the second analysis, the response variable was females (0 – females whose mates did not store food, 1 – females whose mates did store food). The SMI (males or females) was included as a fixed effect, and the random effect included the year of the study. In like manner, the GLMM was used to analyse the food resource richness of territories held by males that did (response variable – 1) and did not store food (response variable – 0). The fixed effects were three variables describing the food richness of the territories: the numbers of all animals, the numbers of coleopteran insects and the numbers of orthopterans. The year of the study was treated as a random effect.
A General Linear Mixed Model with Poisson distribution and logarithmic link function was used to analyse the numbers of stored prey items with respect to the following breeding stages (the numbers of prey items were skewed towards zero): 1) mating and egg laying, 2) incubation, 3) care of nestlings, 4) care of fledglings. Each stage lasted for around 14 days. We decided to combine mating and egg laying into a single category because the birds behaved in similar ways during both: females foraged on their own, only occasionally receiving food from their mates. By contrast, during the incubation period, females rarely foraged alone, and males regularly brought them food while they were incubating the eggs (Harris & Franklin Citation2000; Kuzniak & Tryjanowski Citation2003; authors’ observations). Breeding stage (the four levels described above) was introduced as a fixed effect, and pair ID was a random factor. The dependent variable was the number of prey items found during a particular stage.
The factors governing larder formation with respect to weather conditions were analysed using a General Linear Model (GLM). The dependent variable was the number of prey items divided by the number of males impaling prey that on the survey day had nestlings or fledglings (log-transformed for normality). This limitation to the nestling and fledgling stages was dictated by the large number of surveys with zero values during the earlier mating and incubation stages. During 7 years we carried out 92 such surveys fulfilling the above conditions (we carried out a total of ca 180 surveys, but half of them took place before the nestling/fledgling stages). The explanatory variables were the difference in atmospheric pressure between the survey day and the following one, as well as the mean air temperature and occurrence or non-occurrence of rain on those two days.
The GLMM analysis was carried out in SPSS 21.0 (IBM SPSS Inc. Citation2012), the other calculations in Statistica 12 (Statsoft Inc. Citation2014). Only results with a probability of α ≤ 0.05 were regarded as statistically significant.
Results
Prey storing and brood phenology
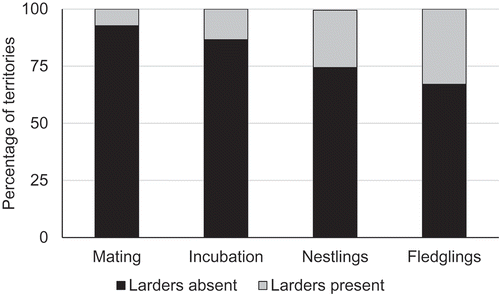
In 2012–2018 we found 82 pairs of Red-backed Shrikes in our study area that had successfully raised their broods to fledging. We discovered that food had been stored in 39 male territories (47.6%), but failed to record such behaviour in 43 males (52.4%). We found 534 prey items, 92.0% of which were insects, 5.8% amphibians, 0.9% reptiles, 0.7% mammals as well as two snails and one spider. The dominant insect orders were Coleoptera (55.4% of all prey items) and Orthoptera (22.7%), while Diptera took a distant third place with just 8.8%. The vast majority of prey (98%) had been impaled on the thorns of bushes or the spikes of barbed wire, while the remainder had been suspended from or wedged into branch forks in bushes or cracks in fence posts. The mean number of items stored by one male was 13.7 ± 3.20 SE (N = 39 males). The percentage of males storing food clearly increased as the breeding season progressed. During the mating and egg-laying stage just 7.9% of males displayed such behaviour, whereas during the fledgling care stage 32.9% of 82 males stored food (). The proportions of males that did and did not store food during the various breeding stages differed significantly (Pearson’s Chi-square test, chi2 = 20.78, df = 3, p < 0.001).
Figure 1. Percentage of Red-backed Shrike territories where larders were present and absent, N = 82 males.
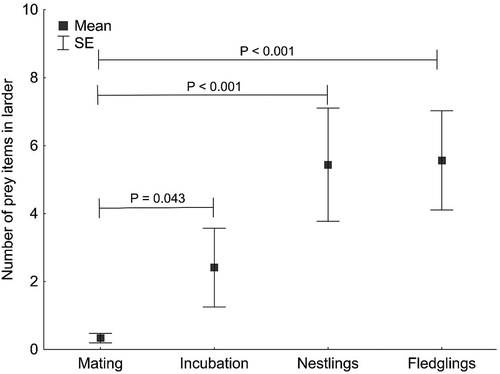
There was a distinct increase in the number of prey items stored as the breeding season advanced. During mating and egg laying, males stored on average just 0.3 prey items, but during the nestling and fledgling care stages the respective figures were 5.4 and 5.6. The number of items in larders differed between the various breeding stages (GLMM, F 3,152 = 3.21, p = 0.025). The mating and egg laying stages differed significantly from the nestling (pairwise comparisons, t152 = −3.46, p < 0.001) and fledgling care stages (t152 = −3.51, p < 0.001). These stages also differed significantly from the incubation stage (pairwise comparisons, t152 = −2.04, p = 0.043, ). In contrast, pairwise comparison of the incubation and nestling care stages revealed statistical differences close to significant (t152 = −1.76, p = 0.081), as did pairwise comparison of the incubation and fledgling care stages (t152 = −1.82, p = 0.071).
Figure 2. The number of prey items (mean ± SE) in Red-backed Shrike larders in the successive stages of breeding. The statistical differences between the stages are indicated (pairwise comparison). The incubation-nestling care and incubation-fledgling care stages were close to statistical significance (pairwise comparison: p = 0.081 and 0.071 respectively).
Breeding parameters of shrikes
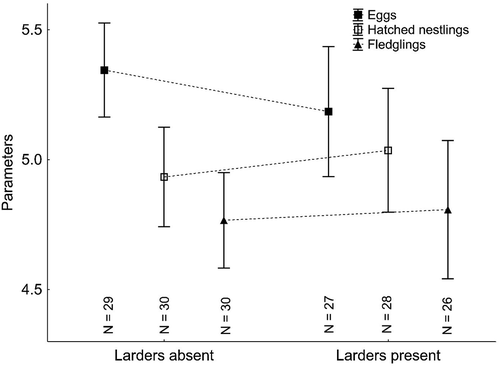
Red-backed Shrikes laid on average 5.3 ± 0.15 (SE) eggs, from which 5.0 ± 0.15 hatchlings emerged, and 4.8 ± 16 fledglings left the nest. Comparison of these breeding parameters did not reveal any significant differences between pairs that did and did not have larders (Mann-Whitney test, p > 0.469 in each case, ). In addition, the clutch initiation date (pairs stored food = 1 June; pairs did not store food = 3 June) did not differ between the two categories of pairs (Mann-Whitney test, Z = −0.03, p = 0.977, n = 82).
Biometrics of birds
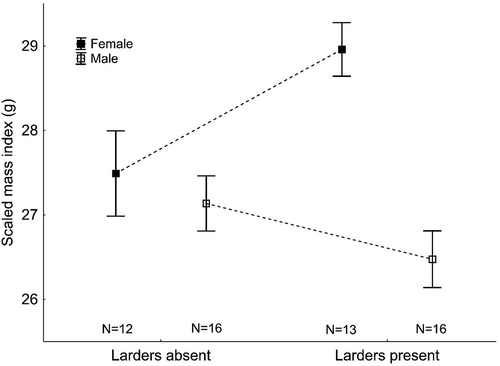
Males not storing food had a very slightly higher Scaled Mass Index (SMI) than males that did store prey items (), but the difference was not statistically significant (GLMM, F 1,30 = 2.38, p = 0.134; effect of year, p = 0.423). On the other hand, females whose mates stored food had a statistically higher SMI (difference = 1.47 g, ) than females whose mates did not set up larders (GLMM, F 1,23 = 6.30, p = 0.020; effect of year, p = 0.381).
Food storing and the weather
The measured weather factors had a slight though significant influence on the number of prey items in shrike larders on a given day (GLM, F3,88 = 3.65, p = 0.016, R2 = 8.0%). Only the difference in atmospheric pressure between the survey day and the next one significantly affected the number of prey items (). The number of prey items rose when the difference in pressure between those two days did so, i.e. the pressure on the survey day was higher than on the following day.
Table I. GLM of the number of prey items in larders of the Red-backed Shrike in relation to weather predictors.
Richness of territories in potential prey
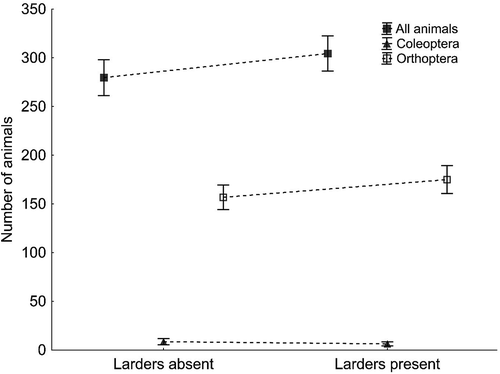
During 8 surveys of each transect in 82 shrike territories we found a total of 23 092 potential prey items, i.e. animals estimated to be more than 1 cm long. Among them, orthopterans were predominant (58.7% of all animals), while beetles made up 2.7% of the prey. The numbers of animals were roughly the same between the territories of males that did and did not store food (). We did not find that males in these two categories differed in territory richness with respect to all animals (GLMM, F 3,78 = 0.01, p = 0.970) or to the two orders of insects most frequently come across in the larders (Coleoptera, GLMM, F 3,78 = 2.92, p = 0.091; Orthoptera, GLMM, F 3,78 = 0.01, p = 0.934; effect of year, p = 0.300).
Discussion
The results of this research show clearly the dominant part played by larders during the periods of care for nestlings and fledglings compared with the mating, egg laying and incubation stages, as well as their various functions in different shrike species. Compared with the larders of the Red-backed Shrike, those of the Great Grey Shrike serve quite a different purpose, since the largest numbers of them were found at the start of their breeding period (Yosef & Pinshow Citation1989; Antczak et al. Citation2005a). The differences between these two species with regard to their prey storing periods may arise because the Great Grey Shrike starts breeding earlier, when potential prey is less readily available. By storing a large number of prey items, some never consumed, along the borders of their territories, male Great Grey Shrikes demonstrated to potential mates that they were in good condition and had a territory with abundant food, and to other males that this site was occupied. In contrast, when male Red-backed Shrikes started nesting some two months later, prey items were now abundant, so there was probably no need to convince females that they were good hunters or to mark territorial borders. That this supposition is correct is confirmed by studies of the Southern Grey Shrike in Israel (Yosef Citation1992; Keynan & Yosef Citation2010). The largest number of impaled victims (the researchers supplied food) was found during the periods preceding the actual breeding season, providing information about the males’ territories. In contrast, the fewest prey items were found during the nestling care period, which the authors associated with the very poor foraging offered by the habitat (Keynan & Yosef Citation2010). Our results are no exception, as the peaking of food storing activities by Red-backed Shrikes during the fledgling care period has been recorded in other areas (Carlson Citation1989; Hernandez Citation1995; Morelli et al. Citation2015).
The significance of food caches during lean periods has been shown in many birds (Vander Wall Citation1990), especially as a buffer against temporary food shortage in the breeding season (Balgooyen Citation1976; Korpimaki Citation1987; Hakkarainen et al. Citation1997). Among the factors affecting this behaviour are the periodically reduced availability of food and the possibility of rapidly replenishing energy reserves at dusk and dawn (Mikusek Citation2019). In mammals, squirrels Sciurus vulgaris, food storage allowed animals to survive in better condition until the breeding season and achieve better reproductive success (Wauters et al. Citation1995; Lee Citation2002). Since food storing by shrikes clearly intensifies during the nestling and fledgling care periods, it would be reasonable to expect an association between the presence of a larder and breeding parameters. However, data in support of this hypothesis have been published in just two papers and, moreover, are based on rather small samples (Yosef & Pinshow Citation1989; Esely Citation1998). In Israel, female Southern Grey Shrike, mated with males having plentiful larders, laid more eggs and reared more young in comparison with males storing fewer prey items (Yosef & Pinshow Citation1989). However, these superior nesting parameters were due to the larger number of broods (3 per season) rather than to the size of just a single brood. A study of the Loggerhead Shrike in the USA showed a weak positive correlation between the number of young fledged from successful nests and the abundance of impaled prey (Esely Citation1998). In eastern Poland we did not find any differences whatsoever in clutch initiation date, clutch sizes, numbers of hatchlings or numbers of fledglings between broods whose males that did and did not store food; this was an entirely unexpected result. It should be added that the shrikes vary slightly in their breeding parameters, which can make it difficult to detect any differences.
During periods of inclement weather individuals are more likely to rely on cached food because they are unable to hunt (Vander Wall Citation1990). This has been well described in owls (Korpimaki Citation1987; Korpimaki & Hakkarainen Citation1991; Halonen et al. Citation2007), passerine birds such as tits (McNamara et al. Citation1990) and mammals (Thompson & Thompson Citation1980; Persson Citation2005). Food storing should alleviate the effects of short-term deficiencies in food caused by inclement weather in shrikes as well (Tryjanowski et al. Citation2003; Yosef & Pinshow Citation2005). The low level of breeding success in the Lesser Grey Shrike Lanius minor as a consequence of bad weather, reported by Lovászi et al. (Citation2000), indicates that since this shrike rarely stores food, it must be more dependent on good weather. Our results show that Red-backed Shrikes did not really intensify their efforts to store food with the approach of bad weather. The only significant factor turned out to be the difference in atmospheric pressure between the survey day and the following day: the greater the pressure difference, the more prey items that were accumulated. This accords with earlier conjectures that birds can sense changes in atmospheric pressure (Breuner et al. Citation2013) and anticipate approaching rains. This weak reaction of Red-backed Shrikes to changes in the weather could be due to the position of eastern Poland, where the climate zone has continental features and the weather in summer is generally sunny, with infrequent and usually short-lived periods of rain; hence, weather-limited access to food is not really a factor of any importance. In the same study area, fairly weak relationships were found between weather conditions and partial losses of nestlings, egg dimensions and clutch sizes in the Red-backed Shrike (Golawski Citation2006b, Citation2008). In Spain and Italy, where the weather is usually sunny, food storing in anticipation of rainy days was not found to occur; indeed, any manifestations of such behaviour would be regarded as exceptional (Hernandez Citation1995; Morelli et al. Citation2013).
In our opinion the most interesting discovery to emerge from this research was the difference between the masses of females whose mates did or did not store food. That females consume food stored by their mates is indicated in the literature (e.g. Yosef Citation1992), but also by the films taken by the time-lapse digital cameras we deployed. These films showed that during the nestling care stage, it was mainly the female that consumed the stored prey items on the spot. Since the stored prey items, impaled on thorns or on barbed wire, consisted only of larger animals no less than 1 cm in length, they could not have been fed to the nestlings, which at least in the first days of life require small and delicate food (Favini et al. Citation1998). The fact that females remain in better condition thanks to food stored in larders may enable them, if necessary, to rear a replacement brood; in eastern Poland, the proportions of clutch/brood losses were roughly the same during the incubation and nestling care stages (Golawski & Golawska Citation2019). The breeding success of the Red-backed Shrike was ca 50%, so a substantial number of pairs would have had to repeat their breeding attempts, which is energetically costly (Antczak et al. Citation2009). If a clutch/brood is lost, females in better condition should therefore have a better chance of rearing a replacement one (and also to lay a larger number of larger eggs). Applegate (Citation1977) long ago suggested that the establishment of a larder by the male was a means by which the incubating female could save energy: she could devote all her energy to incubating her eggs, while leaving hunting duties to her mate. In the intervals between incubation bouts, females of both the Loggerhead Shrike and the Great Grey Shrike visited larders to consume the food left there by their mates (Applegate Citation1977; Yosef Citation1992). Also, female Eurasian Kestrels Falco tinnunculus responded to food supplements by decreasing their hunting effort and were heavier than those at the control nests, whereas the body mass of males was not affected by feeding (Wiehn & Korpimaki Citation1997), so exactly as in our research. Females of Canada Jays Perisoreus canadensis rely primarily on cached food to support this weight gain and with increasing final weight, females tended to lay larger clutches and hatched more nestlings (Sechley et al. Citation2014). As we stated earlier, the intensity of storing prey items by the different shrike species peaks in different stages of brood advancement; quite clearly, the critical period for the Red-backed Shrike is when the birds are caring for their nestlings and fledglings. Females avail themselves of the food stored by males also because this entails only a short absence from the nest: were they off the nest for longer, the nestlings would quickly lose heat and also be exposed to predators (Yosef & Pinshow Citation2005). In any case, shrikes are aggressive birds and are capable of driving some predators away from the nest (Tryjanowski & Golawski Citation2004). On the other hand, the most important effect of storing food really ought to be better breeding parameters achieved by the males that do so, but the present study failed to confirm this. Red-backed Shrikes came to the larders for food mainly in the morning and evening (Hernandez Citation1995, films from our time-lapse digital cameras), when it is much cooler than in the hours around noon, when prey is being brought to the larders. The activity of the shrikes’ potential victims, i.e. insects, is lower early and late in the day, so making use of the larders at those times of the day should enable the birds to feed their young for longer during the interval between. The extensively managed meadows and pastureland in eastern Poland are probably abundant in food (Golawski & Golawska Citation2008; Golawski & Meissner Citation2008), so the establishment and later utilization of larders are not so important as to affect breeding parameters, especially the number of young birds successfully reared. The pastures and regularly mown meadows are evidently a highly appropriate habitat for Red-backed Shrikes, since they achieved the highest breeding parameters and densities there, thanks to the great abundance and detectability of potential prey (Golawski & Meissner Citation2008; Brambilla et al. Citation2009). It is probably because of the fairly uniform habitat that we did not find any differences in the food richness of Red-backed Shrike territories.
Conclusion
Our study has shown that Red-backed Shrikes store prey mainly during the nestling and fledgling care periods. We failed to find any differences in breeding parameters between pairs in which the males did or did not store food. There was a significant tendency for the number of stored prey items to rise with falling atmospheric pressure prior to rainfall. Moreover, we found that the mates of males that did store food had higher body masses compared with females whose mates did not do so.
This research also showed that storing food could fulfil a variety of functions, different to those described in the Great Grey Shrike and Loggerhead Shrike (Yosef & Pinshow Citation2005; Antczak et al. Citation2005a). We consider that these differences are due to the Red-backed Shrike’s breeding phenology: the onset of breeding in this species takes place later, when access to food is incomparably better than in early spring, when the former two species start to breed. When food is readily available, demonstrations of a site’s food-richness of and one’s own condition as expressed by the number of prey items in the larder is not so important in the Red-backed Shrike. The prey stored by males of this species thus function as a classical larder, used mainly by the females when their energy requirements are the greatest, i.e. while they are caring for their young. Our study also suggests that females whose mates set up larders may stay in better condition than their counterparts without access to additional food. The better condition of a female may be significant if she loses a clutch/brood and has to lay a replacement clutch; clutch/brood losses in the Red-backed Shrike, in eastern Poland as elsewhere, can reach 50% (Tryjanowski et al. Citation2000; Polak Citation2016; Golawski & Golawska Citation2019).
Acknowledgements
We would like to thank Włodzimierz Meissner and Cezary Mitrus for their constructive comments on the earlier draft of the manuscript, Peter Senn for the English language translation and editing, and the two anonymous reviewers for their comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Antczak M, Golawski A, Kuzniak S, Tryjanowski P. 2009. Costly replacement – How do different stages of nest failure affect clutch replacement in the red-backed shrikes Lanius collurio? Ethology, Ecology & Evolution 21(2):127–136. DOI: 10.1080/08927014.2009.9522501.
- Antczak M, Hromada M, Tryjanowski P. 2005a. Spatio-temporal changes in Great Grey Shrike Lanius excubitor impaling behaviour: From food catching to communication sings. Ardea 93:101–107.
- Antczak M, Hromada M, Tryjanowski P. 2005b. Frogs and toads in the food of the Great Grey Shrike (Lanius excubitor): Larders and skinning as two ways to consume dangerous prey. Animal Biology 55(3):227–233. DOI: 10.1163/1570756054472836.
- Applegate RD. 1977. Possible ecological role of food caches of Loggerhead Shrikes. Auk 94:192–199. DOI: 10.1093/auk/94.2.391.
- Arbeiter S, Schulze M, Tamm P, Hahn S. 2016. Strong cascading effect of weather conditions on prey availability and annual breeding performance in European bee-eaters Merops apiaster. Journal of Ornithology 157(1):155–163. DOI: 10.1007/s10336-015-1262-x.
- Astheimer LB, Buttemer WA, Wingfield JC. 1995. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Hormones and Behavior 29(4):442–457. DOI: 10.1006/hbeh.1995.1276.
- Balgooyen TG. 1976. Behaviour and ecology of the American kestrel (Falco sparverius L.) in the Sierra Nevada of California. University of California Publications in Zoology 103:1–83.
- BirdLife International. 2019. Species factsheet: Lanius collurio. Available: http://www.birdlife.org. Accessed Apr 2019 05.
- Brambilla M, Casale F, Bergero V, Crovetto GM, Falco R, Negri I, Siccardi P, Bogliani G. 2009. GIS-models work well, but are not enough: Habitat preferences of Lanius collurio at multiple levels and conservation implications. Biological Conservation 142(10):2033–2042. DOI: 10.1016/j.biocon.2009.03.033.
- Breuner CW, Sprague RS, Patterson SH, Woods HA. 2013. Environment, behavior and physiology. Do Birds Use Barometric Pressure to Predict Storms? Journal of Experimental Biology 216(Pt 11):1982–1990. DOI: 10.1242/jeb.081067.
- Carlson A. 1989. Courtship Feeding and Clutch Size in Red backed Shrikes (Lanius collurio). American Naturalist 133(3):454–457. DOI: 10.1086/284928.
- Cramp S, Perrins CM. 1993. Red-backed Shrike, Lanius collurio. Handbook of the Birds of Europe the Middle East and North Africa. Vol. 7. Oxford: Oxford University Press.
- Eldegard K, Sonerud GA. 2010. Experimental increase in food supply influences the outcome of within-family conflicts in Tengmalm’s owl. Behavioral Ecology 64(5):815–826. DOI: 10.1007/s00265-009-0898-z.
- Esely JD. 1998. Habitat selection, reproductive success, and impaling patterns of a migratory population of Loggerhead Shrikes. Masters Theses, Eastern Illinois University.
- Esely JD, Bollinger EK. 2003. Patterns of impaling in a migratory population of the loggerhead shrike. Prairie National 35:1–8.
- Favini G, Fornasari L, Bottoni L, Massa R. 1998. A video-taping approach to study nestling diet and parental care of Red-backed Shrike (Lanius collurio). IBCE Technical Publications 7:76–78.
- Golawski A. 2006a. Comparison of methods for diet analysis and prey preference: A case study on the Red-backed Shrike Lanius collurio. Ornis Fennica 83:108–116.
- Golawski A. 2006b. Impact of weather on partial loss of nestlings in the Red-backed Shrike Lanius collurio in eastern Poland. Acta Ornithologica 41(1):15–20. DOI: 10.3161/000164506777834705.
- Golawski A. 2008. No evidence of weather effect found on the clutch size, eggs sizes and their hatchability in the red-backed shrike Lanius collurio in eastern Poland. Annales Zoologici Fennici 45(6):513–520. DOI: 10.5735/086.045.0606.
- Golawski A, Golawska S. 2008. Habitat preference in territories of the Red-backed Shrike Lanius collurio and their food richness in an extensive agriculture landscape. Acta Zoologica Academiae Scientiarum Hungarica 54:89–97.
- Golawski A, Golawska S. 2019. Weather and predation pressure: The case of the Red-backed Shrike (Lanius collurio). Acta Zoologica Academiae Scientiarum Hungarica 65(4):371–379. DOI: 10.17109/AZH.65.4.371.2019.
- Golawski A, Meissner W. 2008. The influence of territory characteristics and food supply on the breeding performance of the Red-backed Shrike (Lanius collurio) in an extensively farmed region of eastern Poland. Ecological Research 23(2):347–353. DOI: 10.1007/s11284-007-0383-y.
- Hakkarainen H, Koivunen V, Korpimäki E. 1997. Reproductive success and parental effort of Tengmalm’s owls: Effects of spatial and temporal variation in habitat quality. Ecoscience 4(1):35–42. DOI: 10.1080/11956860.1997.11682374.
- Halonen M, Mappes T, Meri T, Suhonen J. 2007. Influence of snow cover on food hoarding in Pygmy Owls Glaucidium passerinum. Ornis Fennica 84:105–111.
- Harris T, Franklin K. 2000. Shrikes and Bush-Shrikes. London: Christoper Helm.
- Heath SR, Kershner EL, Cooper DM, Lynn S, Turner JM, Warnock N, Farabaugh S, Brock K, Garcelon DK. 2008. Rodent control and food supplementation increase productivity of endangered San Clemente Loggerhead Shrikes (Lanius ludovicianus mearnsi). Biological Conservation 141(10):2506–2515. DOI: 10.1016/j.biocon.2008.07.011.
- Hernandez A. 1995. Temporal-spatial patterns of food caching in two sympatric Shrike species. Condor 97:1002–1010. DOI: 10.2307/1369539.
- Hušek J, Adamík P. 2008. Long-term trends in the timing of breeding and brood size in the Red-Backed Shrike Lanius collurio in the Czech Republic, 1964–2004. Journal of Ornithology 149(1):97–103. DOI: 10.1007/s10336-007-0222-5.
- IBM SPSS Statistics for Windows. 2012. Version 21.0. Amrock, NY: IBM Corporation.
- Kallander H, Smith HG. 1990. Food storing in birds. An evolutionary perspective. Current Ornithology 7:147–207.
- Keynan O, Yosef R. 2010. Temporal changes and sexual differences of impaling behaviour in Southern Grey Shrike (Lanius meridionalis). Behavioural Processes 85(1):47–51. DOI: 10.1016/j.beproc.2010.06.005.
- Korpimaki E. 1987. Prey caching of breeding Tengmalm’s owls Aegolius funereus as a buffer against temporary food shortage. Ibis 129:499–510. DOI: 10.1111/j.1474-919X.1987.tb08237.x.
- Korpimaki E, Hakkarainen H. 1991. Fluctuating food supply affects the clutch size of Tengmalm’s owl independent of laying date. Oecologia 85(4):543–552. DOI: 10.1007/BF00323767.
- Kotler BP, Brown JS, Hickey M. 1999. Food storability and the foraging behavior of fox squirrels (Sciurus niger). American Midland Naturalist 142(1):77–86. DOI: 10.1674/0003-0031(1999)142[0077:FSATFB]2.0.CO;2.
- Kuzniak S, Tryjanowski P. 2003. Red-backed Shrike. Świebodzin: Wydawnictwo Klubu Przyrodników.
- Labocha MK, Hayes JP. 2012. Morphometric indices of body condition in birds: A review. Journal of Ornithology 153(1):1–22. DOI: 10.1007/s10336-011-0706-1.
- Lee TH. 2002. Feeding and hoarding behaviour of the Eurasian red squirrelSciurus vulgaris during autumn in Hokkaido, Japan. Acta Theriologica 47(4):459–470. DOI: 10.1007/BF03192470.
- Leugger-Eggimann U. 1997. Parental expenditure of red-backed shrikes Lanius collurio in habitats of varying farming intensity. PhD thesis, University of Basel.
- Lovászi P, Bártol I, Moskát C. 2000. Nest-site selection and breeding success of the Lesser Grey Shrike (Lanius minor) in Hungary. Ring 22:157–164.
- McNamara JM, Houston AI, Krebs JR. 1990. Why hoard? The economics of food storing in tits, Parus spp. Behavioral Ecology 1(1):12–23. DOI: 10.1093/beheco/1.1.12.
- Mikusek R. 2019. The role of caches in the Eurasian Pygmy Owl Glaucidium passerinum during the breeding season. Ornis Polonica 60:1–15.
- Moller AP, Mousseau TA. 2009. Reduced abundance of insect and spiders linked to radiation at Chernobyl 20 years after the accident. Biology Letters 5(3):356–359. DOI: 10.1098/rsbl.2008.0778.
- Morelli F, Bussiere R, Golawski A, Tryjanowski P, Yosef R. 2015. Saving the best for last: Differential usage of impaled prey by red-backed shrike (Lanius collurio) during the breeding season. Behavioural Processes 119:6–13. DOI: 10.1016/j.beproc.2015.07.006.
- Morelli F, Saltarelli M, Pruscini F, Benedetti Y. 2013. First description of red-backed shrike Lanius collurio food caching in Central Italy: Prey’s type and spatial position into the larders. Avocetta 37:27–34.
- Nagy LR, Stanculescu D, Holmes RT. 2007. Mass loss by breeding female songbirds: Food supplementation supports energetic stress hypothesis in Black-throated Blue Warblers. Condor 109(2):304–311. DOI: 10.1093/condor/109.2.304.
- Nikolov BP, Kodzhabashev ND, Popov VV. 2004. Diet composition and spatial patterns of food caching in wintering Great Grey Shrike (Lanius excubitor) in Bulgaria. Biological Letters 41:119–133.
- Peig J, Green AJ. 2009. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 118(12):1883–1891. DOI: 10.1111/j.1600-0706.2009.17643.x.
- Persson J. 2005. Female wolverine (Gulo gulo) reproduction: Reproductive costs and winter food availability. Canadian Journal of Zoology 83(11):1453–1459. DOI: 10.1139/z05-143.
- Polak M. 2016. Comparative breeding ecology, nest survival, and agonistic behaviour between the Barred Warbler and the Red-backed Shrike. Journal of Ornithology 157(3):747–758. DOI: 10.1007/s10336-016-1336-4.
- Ruffino L, Salo P, Koivisto E, Banks PB, Korpimäki E. 2014. Reproductive responses of birds to experimental food supplementation: A meta-analysis. Frontiers in Zoology 11(1):80–93. DOI: 10.1186/s12983-014-0080-y.
- Schirmel J, Buchholz S, Fartmann T. 2010. Is pitfall trapping a valuable sampling method for grassland Orthoptera? Journal of Insect Conservation 14(3):289–296. DOI: 10.1007/s10841-009-9258-6.
- Schulze CH, Waltert M, Kessler PJA, Pitopang R, Veddeler D, Mühlenberg M, Gradstein SR, Leuschner C, Steffan-Dewenter I, Tscharntke T. 2004. Biodiversity indicator groups of tropical land-use systems: Comparing plants, birds, and insects. Ecological Applications 14(5):1321–1333. DOI: 10.1890/02-5409.
- Sechley TH, Strickland D, Norris DR. 2014. Causes and consequences of pre-laying weight gain in a food-caching bird that breeds in late winter. Journal of Avian Biology 45(1):85–93. DOI: 10.1111/j.1600-048X.2013.00296.x.
- StatSoft, Inc. 2014. STATISTICA (data analysis software system), version 12. Available: www.statsoft.com.
- Svensson L. 1992. Identification Guide to European Passerines. 4th ed. Stockholm: Svensson.
- Thompson DC, Thompson PS. 1980. Food habits and caching behavior of urban grey squirrels. Canadian Journal of Zoology 58(5):701–710. DOI: 10.1139/z80-101.
- Tryjanowski P, Golawski A. 2004. Sex differences in nest defence by the red-backed shrike Lanius collurio: Effects of offspring age, brood size, and stage of breeding season. Journal of Ethology 22(1):13–16. DOI: 10.1007/s10164-003-0096-9.
- Tryjanowski P, Golawski A, Kuzniak S, Mokwa T, Antczak M. 2007. Disperse or stay? Exceptionally high breeding-site infidelity in the Red-backed Shrike Lanius collurio. Ardea 95(2):316–320. DOI: 10.5253/078.095.0214.
- Tryjanowski P, Karg MK, Karg J. 2003. Diet composition and prey choice by the red-backed shrike Lanius collurio in western Poland. Belgian Journal of Zoology 133:157–162.
- Tryjanowski P, Kuzniak S. 1999. Effect of research activity on the success of Red-backed Shrike Lanius collurio nests. Ornis Fennica 76:41–43.
- Tryjanowski P, Kuzniak S, Diehl B. 2000. Does breeding performance of Red-backed Shrike Lanius collurio depend on nest site selection? Ornis Fennica 77:137–141.
- Vander Wall SB. 1990. Food hoaring in animals. Chicago: University of Chicago Press.
- Vicens N, Bosch J. 2000. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environmental Entomology 29(3):413–420. DOI: 10.1603/0046-225X-29.3.413.
- Wauters LA, Suhonen J, Dhondt AA. 1995. Fitness consequences of hoarding behaviour in the Eurasian red squirrel. Proceedings of the Royal Society B 262:277–281. DOI: 10.1098/rspb.1995.0206.
- Wiehn J, Korpimaki E. 1997. Food limitation on brood size: Experimental evidence in the Eurasian Kestrel. Ecology 78(7):2043–2050. DOI: 10.1890/0012-9658(1997)078[2043:FLOBSE]2.0.CO;2.
- Yosef R. 1992. Behavior of polygynous and monogamous Loggerhead Shrikes and a comparison with Northern Shrikes. Wilson Bulletin 104:747–749.
- Yosef R, Pinshow B. 1988. Polygyny in the Northern Shrike (Lanius excubitor) in Israel. Auk 105(3):581–582. DOI: 10.1093/auk/105.3.581.
- Yosef R, Pinshow B. 1989. Cache size influences reproductive success in the Northern Shrike, Lanius excubitor. Auk 106:418–421.
- Yosef R, Pinshow B. 2005. Impaling in true shrikes (Laniidae): A behavioral and ontogenic perspective. Behavioral Processes 69(3):363–367. DOI: 10.1016/j.beproc.2005.02.023.