Abstract
Hexavalent chromium (Cr(VI)) is a harmful environmental pollutant, which is widely distributed in the marine environment. However, the mechanisms underlying Cr-induced oxidative stress and reproductive toxicity in clams remain largely unknown. The objective of this study was to explore the acute toxic effects of Cr(VI) and estimate the applicability of male clam Geloina coaxans as a bioindicator in mangrove ecosystems. In this study, G. coaxans were exposed to 4.34, 8,69, 17.38 and 34.76 mg/L Cr(VI) for 24, 48 and 72 h. The activities of catalase (CAT), glutathione S-transferase (GST) and the content of malondialdehyde (MDA) were determined. The gene expression of CAT, GST, autophagy-related gene 5 (ATG5), cyclin B3 and spermatogenesis associated protein 4 (SAP4) were measured by RT-qPCR. Furthermore, histological alteration of the gonad and sperm was observed by light microscopy and transmission electron microscopy.
The results showed that Cr(VI) exposure induced the accumulation of MDA and activities of enzymes. Cr(VI) exposure also inhibited the mRNA expression of ATG5, cyclin B3 and SAP4 in a dose-dependent manner, which might suggest that Cr(VI) caused reproductive toxicity by affecting gene expression. Significant changes in the histological structure of gonad were observed. The results demonstrate that Cr(VI) significantly affected functional and molecular parameters in Geloina coaxans, and indicate that gonad represents the target of exposure to Cr(VI).
1. Introduction
Chromium (Cr) is a highly toxic heavy metal with its wide usage in industries (Jacobs & Testa Citation2005). With the continuous development of industrialization and urbanization, a large amount of Cr was discharged into the environment. In some areas, the concentration of Cr was up to 12 mg L−1 in the aquatic environment (Jaishankar et al. Citation2014). Many countries, such as China and the United States, have listed chromium in the blacklist of priority water pollutants (China Citation2002; EPA Citation2009). Common states of Cr appear as hexavalent (VI) and trivalent (III) in the natural environment (Bregnbak et al. Citation2015), and Cr(VI) is considered to be the most toxic valence among the states of chromium. Cr (VI) is highly toxic and soluble in water, so it is easier to move in water environment than trivalent chromium (Bagchi et al. Citation1995; Huang et al. Citation2014).
Mangrove is deemed to be significant sinks for toxic compounds because of its considerable capacity for retaining various harmful substances (Shi et al. Citation2019). Due to the rapid development of industrialization and urbanization, a large number of metal pollutants were discharged into the environment. Mangrove was seriously polluted (MacFarlane & Burchett Citation2001; Wasserman et al. Citation2001; Jacobs & Testa Citation2005; Donnini et al. Citation2007; Sandilyan & Kandasamy Citation2014), and Cr(VI) was a dominate contaminant in many mangrove sites (Kumar et al. Citation2015). Studies on mangrove in south China indicated that the content of Cr(VI) in the surface sediments was up to 109 mg/kg (Vane et al. Citation2008).
Cr(VI) is considered as a model oxidative toxicant. A variety of enzymes have antioxidant and detoxicate effects such as catalase (CAT) and glutathione S-transferase (GST). Some investigations demonstrated that Cr(VI) exposure reduced the activities of antioxidant enzyme and gene expression (Papadimitriou & Loumbourdis Citation2003; Begum et al. Citation2006; Davey et al. Citation2006; Lushchak et al. Citation2008; Vasylkiv et al. Citation2010). Cr(VI) has been shown to inhibit the activities and mRNA expression of CAT and GST in Mytilus galloprovincialis under sublethal concentration exposure (Ciacci et al. Citation2011, Citation2012; Franzellitti et al. Citation2012). In liver tissue and digestive gland, Cr(VI) affected oxidative stress markers, DNA status and antioxidant enzymes (Franzellitti et al. Citation2012; Madejczyk et al. Citation2015). Studies also found that oxidative stress would also inhibit the autophagy and cyclin gene expression, which might be a potential mechanism of chromium toxicity (Muñoz et al. Citation2001; Kanzawa et al. Citation2003; Kim et al. Citation2007; Scherz-Shouval & Elazar Citation2007). In addition, Cr(VI) can also cause inhibition of sperm production, changes in gonad ultrastructure, leakage of cell junction, damage of sperm and reduction of sperm activity (Marouani et al. Citation2012). From previous studies, Cr(VI) could alter the expression of spermatogenesis-related genes (Schulz et al. Citation2009) and reproductive function of Japanese medaka (Chen et al. Citation2016).
Biomarkers are the early indicators of changes in the morphofunctional state of bivalves under the effect of pollutants, because, a toxic effect will be apparent at the subcellular level before it is noticeable at higher biological levels. In contrast to the simple measurement of contaminants accumulating in body tissues, biomarkers can offer more complete and biologically more relevant information on the potential impact of pollutants on the health of the organisms. Bivalves can accumulate heavy metals from food, water and also from the ingestion of inorganic particulate materials, hence fulfilling the criteria as good bioindicators. Bivalves are well established as bioindicators for monitoring the concentrations of heavy metals in coastal areas. Geloina coaxans is a marine bivalve widely distributed in mangrove areas, it has important ecological value (Morton Citation1976; Clemente & Ingole Citation2009), and has potential as an indicator in marine monitoring (Wang et al. Citation2020). However, there are a very few studies on the effects of Cr(VI) in G. coaxans. In this study, G. coaxans were selected as subjects to explore the toxicological effects of Cr(VI) in gonad tissue. Acute toxicity test method was carried out to investigate the alteration of MDA content, enzyme activity and gene expression (CAT, GST, autophagy-related gene 5 (ATG5), cyclin B3 and spermatogenesis associated protein 4 (SAP4)) in the spermary of male G. coaxans. Moreover, the ultrastructure of the spermary and sperms of G. coaxans was observed by tissue sectioning and transmission electron microscopy.
2. Materials and methods
2.1. Treatments and exposure of clams
G. coaxans were collected from Puqian Town, Wenchang City, Hainan Province in October 2018. Mature male individuals of similar size were selected for experiments. The average fresh weight of individuals was 31.73 ± 1.29 g/individual (mean ± SD) and the average shell height was 49.6 ± 2.5 mm (mean ± SD). Clams were acclimated in plastic tanks (980 × 760 × 680 mm) containing artificial seawater (1 L/clam) for 7 days. The salinity of seawater was 22‰, the temperature was maintained at 24°C and the clams were fed with dried algal powder. Before the formal experiment, we carried out the pre-acute toxicology experiment and measured the 96-h LC50 of Cr(VI) in G. coaxans (69.51 mg/L).
According to the results of pre-acute toxicity test, groups of male G. coaxans were separately exposed for 24 h, 48 h and 72 h to 4.34, 8.69, 17.38 and 34.76 mg/L of Cr(VI) (corresponding to 1/16, 1/8, 1/4 and 1/2 of the 96-h LC50, the mother liquor of 1000 mg/L was prepared first with analytically pure K2Cr2O7, and then diluted to each experimental concentration). Each group contained 12 clams and was separated into three replicates.
At 24 h, 48 h and 72 h, three individuals were taken from each replicate. The gonad was cut off and frozen in liquid nitrogen for different parameter assays. Sample processing procedures for histological analysis and sperm count determination were described in 2.4.
2.2. Determination of MDA content and enzyme activity
Samples for measurement MDA content, CAT and GST activity were homogenized in 0.1 M Tris–HCl buffer (pH 7.0) and centrifuged at 10,000 × g at 4°C for 20 min. The MDA contents and enzyme activities were determined according to the instructions of the kits (Nanjing, China); the serial numbers of MDA content kit, CAT and GST enzyme activity kits were A003-1, A007-1-1 and A004-1-1, respectively. The activity was expressed in units per gram (wet weight). One unit of CAT activity was defined as the amount of 1 μmol H2O2 decomposed per gram of tissue (wet weight) per second under 37°C. One unit of GST activity was defined as the amount of enzyme that reduced the GSH concentration in the reaction system by 1 μmol/L per min per gram (wet weight) under 37°C.
2.3. RNA isolation and RT-qPCR
Total RNA was extracted from the gonad using RNAiso Plus (Takara Corp., Dalian, China), the concentrations and purities of the isolated RNA were assessed using a NanoDrop 2000 (Thermofisher Scientific, USA). RNA was then reverse-transcribed to cDNA using PrimeScript™ RT reagent Kit (Takara, Japan) following the instructions. The amplification was implemented on a 96-well plate, consisting 5 µL 2 SYBR Premix Ex Taq, 2 µL cDNA, 1.6 µL of 10 µM specific primer pairs and 1.4 µL Milli-Q water. Thermal program consisted of 30 s at 95°C following 50 cycles (5 s at 95°C, 30 s at 54°C and 30 s at 72°C). Partial sequences of CAT, GST, ATG5, cyclin B3, SAP4 and β-actin were obtained from the transcriptome of G. coaxans, which was established by our laboratory (unpublished). All primers used in this study were designed by software Primer premier 5.0 and synthesized by Sangon Co. Ltd., Shanghai, China (). mRNA expression of selected genes was normalized according to the expression levels of β-actin. The relative expression was calculated with the 2−ΔΔCt method.
Table I. Primers used for quantitative RT-PCR analysis.
Table II. The result of pre-acute toxicity test of Cr(VI) to G. coaxans during 96-h exposure.
2.4. Histological evaluation and sperm number count
At 72 h, samples for light microscope observation were soaked in Bouin’s. After 18 h, samples were then dehydrated with ethanol, embedded in paraffin, sliced into 4 µm thick slices, stained with Ehrlich's haematoxylin and eosin (H&E) and observed with a light microscope (Olympus IX71).
For transmission electron microscopy (TEM) observation, gonad samples were cut into 1 mm3 pieces, pre-fixed in 2.5% glutaraldehyde and then fixed in 1% osmium tetraoxide, embedded in 812 epoxy resin, sliced with ultramicrotome, stained with lead citrate and observed with a transmission electron microscope (Hitachi, Japan). The number of sperm was measured according to Tian et al. (Citation2013). Gonad samples (0.1–0.2 g) were cut off and weighted with electronic balance, minced in 1 mL seawater, diluted with 19 mL seawater and filtered through a 100-mesh strainer; then, the sperm numbers were counted with hemocytometer.
2.5. Data analysis
Results were presented as arithmetical mean ± standard deviation. Data were analyzed with one-way analysis of variance (ANOVA) and least significant difference (LSD) using SPSS software (version 20.0).
3. Results
3.1. Response of the MDA content to Cr(VI) exposure
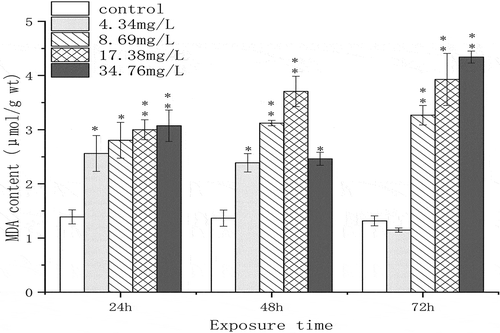
MDA, as the final decomposition product of lipid peroxidation, can indicate the degree of lipid peroxidation and oxidative damage in organisms. The effects of Cr(VI) on MDA content in the spermary of G. coaxans are shown in . MDA content in the experimental groups was significantly higher than that in the control groups. It also showed a concentration-dependent manner, the highest value was about hreefold of the control in 34.76 mg/L treatment at 72 h.
3.2. Response of the activity of CAT and GST to Cr(VI) exposure
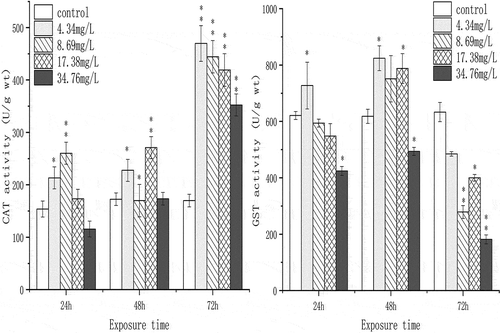
The response of CAT and GST on Cr(VI) exposure is shown in . In general, CAT showed an upward trend in a time-dependent manner. CAT activity in low concentration treatments (4.34 and 8.69 mg/L) was significantly induced at 24 h, while high concentration treatments (17.38 and 34.76 mg/L) had no significant change. CAT activity reached the highest level in 4.34 mg/L at 72 h, which was almost 3 times higher than the control. For GST, enzyme activity was significantly induced in 4.34 mg/L at 24 and 48 h, and in 34.76 mg/L, GST showed significantly inhibition trend. The lowest value was about 0.3-fold of the control.
3.3. Effect of Cr(VI) on mRNA expression of selected genes
The expressions of the selected genes (CAT, GST, ATG5, cyclin B3 and SAP4) are shown in . The mRNA expression of CAT was notably stimulated and came to the highest value (about 2.5-fold of the control) in 4.34 mg/L at 72 h. GST expression was increased at 24 h and 48 h under 4.34, 8.69 and 17.38 mg/L Cr(VI) exposure, but showed a decreased trend at 72 h. It was significantly inhibited at the highest concentration and reached the minimum value (about 0.1-fold of the control).
Figure 3. The effect of chromium (VI) on mRNA expression of CAT, GST, ATG5, cyclin B3 and SAP4 in the spermary of G. coaxans. Bars represent the mean ± SD, n = 9. Statistical significance was denoted by *P < 0.05 and **P < 0.01 versus the control group on the same day (ANOVA-LSD).
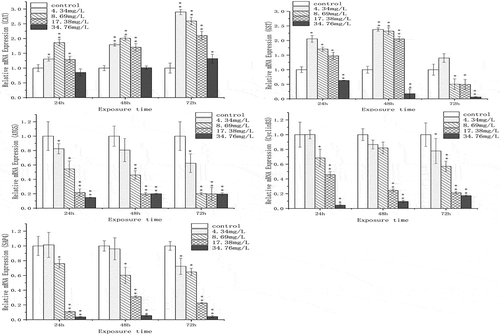
The expression of ATG5 showed a downward trend with the prolongation of exposure time and the increase of Cr(VI) concentration. The minimum value was 0.196 time of the control group. Similar performance was observed on the expression of cyclin B3 and SAP4, displaying negative time-dependent and concentration-dependent relationship.
3.4. Effect of Cr(VI) on histology and sperm number in the spermary
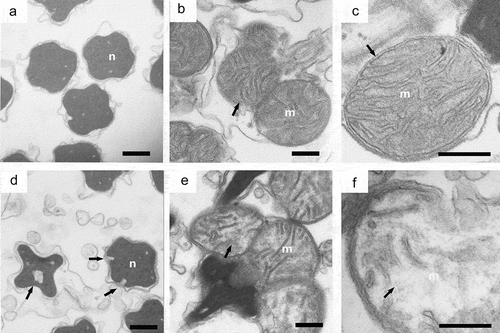
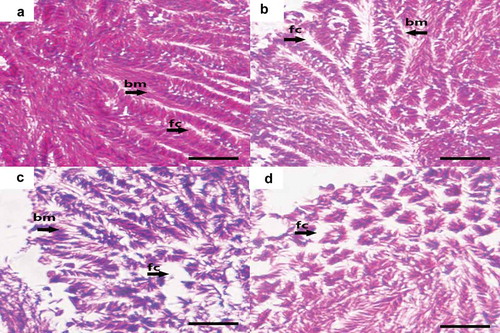
reveals the histological effects of Cr(VI) on the spermary of G. coaxans. The spermary tissue was closely arranged, the basement membrane structure was intact and the follicles were full of sperms under the lowest concentration exposure ()). After 72 h exposure to 8.69 mg/L Cr(VI), there were no significant histological changes observed except the follicles were slightly enlarged ()). However, under 17.38 mg/L and 34.76 mg/L Cr(VI) exposure, the follicles were obviously enlarged and the vacuolated structure was observed (,)). This showed inhibition of spermary development and damage of gonad tissue.
Figure 4. The effect of chromium (VI) in the spermary of G. coaxans. (a) exposed to 4.34 mg/L Cr(VI) for 72 h (×400); (b) exposed to 8.69 mg/L Cr(VI) for 72 h (×400); (c) exposed to 17.38 mg/L Cr(VI) for 72 h (×400); (d) exposed to 34.76 mg/L Cr(VI) for 72 h (×400). (bm: Basement membrane; fc: follicle. Paraffin sections, 4 µm-thick, hematoxylin-eosin staining). Bar = 10 μm.
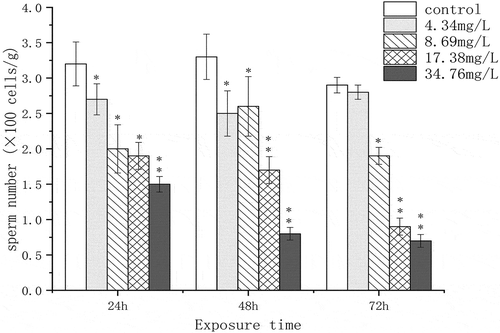
In addition, we observed the ultrastructure of the spermary under a high concentration of Cr(VI) exposure (34.76 mg/L Cr(VI), 72 h) using transmission electron microscopy (). In the control group, the nucleus of sperm showed a regular shape, with a clear and complete plasma membrane, and the mitochondria structure was complete (). After Cr(VI) treatment, the plasma membrane of the nucleus was broken and the vesicle structure was observed. The cristae in the mitochondria were broken and the adventitia was partially dissolved (shown by a black arrow in ). The number of sperms in spermary under Cr(VI) exposure was measured by using a hemocytometer. Exposure to the higher Cr(VI) concentrations resulted in a significant decrease in sperm numbers ().
4. Discussion
Cr(VI) is a toxicant that primarily targets the antioxidant system in organisms, if the toxicant exposure leads to excessive oxidation, which exceeds the ability of cells to recovery, it will cause cell damage (Madejczyk et al. Citation2015). Cr(VI) also has reproductive toxicity, which can cause damage to the reproductive system. Transcription of various genes was also affected by Cr(VI) exposure (Gül et al. Citation2004; Emmanouil et al. Citation2007; Yu et al. Citation2018). However, little research has been done to investigate the effects of Cr(VI) on spermary of marine bivalves.
MDA, a product of lipid peroxidation (LPO), is generated by free radicals attacking the plasma membrane of cells. It has been commonly used to indicate oxidative stress (Chen et al. Citation2017). shows that the lowest concentration of Cr(VI) induced significant upregulation of MDA in 24 h and 48 h, but no significant changes in MDA content were observed in the spermary exposed to 72 h. These results demonstrate that the antioxidant system worked at low concentration, and the organism was not greatly affected. However, under high concentration exposure (8.69 mg/L, 17.38 mg/L and 34.76 mg/L), the content of MDA was significantly higher than the control group, which suggested the imbalance between lipid peroxidation and antioxidant defenses. Tissue damage was also observed by histological observation (,)).
Antioxidant enzyme system plays an important role in maintaining the balance of oxygen-free radical metabolism. CAT and GST are important components of antioxidant enzymes in organisms. Under normal physiological conditions, antioxidant system can effectively remove reactive oxygen species (ROS) and protect cells from injury of lipid peroxidation (Zhu et al. Citation2008; Ni et al. Citation2019). The toxicity of heavy metals to organisms is mainly reflected in the activity of antioxidant enzymes, and Cr(VI) exposure can inhibit the enzyme activity by causing LPO or affecting gene expression (Kumar et al. Citation2012; Bolognesi & Cirillo Citation2014; Kim & Kang Citation2016).
The activity of CAT showed significant inhibition only in low concentration (4.34 mg/L and 8.69 mg/L). When Cr(VI) treatments were prolonged to 72 h, CAT activities in the spermary were significantly increased. The expression of CAT also remained at a higher level at under 34.76 mg/L Cr(VI). After exposure to Cr(VI), the activity of GST showed an increasing trend in the early stage of exposure, but with the extension of time and increase of Cr(VI) concentration, Cr(VI) exposure induced significant downregulation of GST activity. The pre-acute toxicity test demonstrated that G. coaxans had a high tolerance to Cr(VI) exposure (96-h LC50 of Cr(VI) was 69.51 mg/L). The gradual increase of CAT activities suggested it played a key role in post-antioxidant capacity.
The alteration of enzyme activities caused by poisons at low concentration was a stimulus response without causing toxicity (Stebbing Citation1982; Oller & Bates Citation2004; Berry & Lopez-Martinez Citation2020). In the present experiment, activities of CAT and GST both showed significant changes in low Cr(VI) concentration, which suggested that they could be effective indicators in the early stage of exposure. In short, our findings showed that in G. coaxans, the antioxidant capacity of CAT and GST worked at different stages, it revealed that GST was more susceptible to Cr(VI) exposure while CAT mainly played a role at late stage of exposure. And the antioxidant capacity may be inhibited by excess ROS induced by Cr(VI).
Autophagy is a cellular physiological mechanism that maintains homeostasis and plays an important role in cell growth and stress response (Chai et al. Citation2016). Autophagy can eliminate the damage caused by oxidation through the degradation process (Li et al. Citation2015a, Citation2015b), and Atg5-dependent autophagy serves to protect cells from cytotoxicity (Takanezawa et al. Citation2016). Although the mechanism of autophagy is not clear at present, many compounds and biological processes, such as lipid peroxidation and damage of mitochondria can influence the process of autophagy (Kanzawa et al. Citation2003; Kim et al. Citation2007; Scherz-Shouval & Elazar Citation2007). In this study, we investigated the relative expression of ATG5 in spermary tissue, which was significantly lower than the control group during the experiment. In addition, the rapid decrease of ATG5 expression was accompanied by an increase in MDA content. Yang et al. (Citation2018) indicated that autophagy could enhance the viability of cells under heavy metal stress, the inhibition of autophagy could further aggravate the stress. And autophagy may provide a defense against Cr (VI)-induced oxidative stress (Ge et al. Citation2018). However, the inhibition of ATG expression can lead to cell damage. Our study suggested that autophagy did play a role in Cr exposure. Cr(VI) could lead to spermary damage by inhibiting the expression of ATG5 in clams. The decrease of ATG5 in exposure revealed that organism could not maintain the cell homeostasis and protect cells from oxidative damage. This might be a possible mechanism of reproductive toxicity induced by Cr(VI).
Cyclin B is a member of the cyclin family. It is also the first cell cycle regulator found in sea urchins. It plays a key role in achieving gamete development and was most dominantly expressed during spermatogenesis (Nguyen et al. Citation2002; Kajiura-Kobayashi et al. Citation2004). It has been reported that oxidative stress could inhibit the cyclin gene expression (Muñoz et al. Citation2001). In the present study, with the increase of exposure time, the expression of cyclin B was significantly decreased, showing a negative concentration-dependent relationship (). At the same time, the delay of gonad development and reduction of sperm numbers was also observed (,) and ). The results indicate that Cr(VI) could inhibit the expression of cyclin B3 in the gonad, thereby inducing delay of gonad development by mechanisms including spermatogonial proliferation which targeted cyclin B pathway.
Spermatogenesis is a complex process by which spermatogonial stem cells develop into functional sperm, it is regulated by a variety of factors (Ozaki et al. Citation2011). SAP4 is a highly conservative gene in many organisms, it was used as biomarkers for spermatogenesis disruption and had great effects in spermatogenesis and gonadal development (Liu et al. Citation2005a, Citation2005b, Citation2017). In this study, the expression of SAP4 significantly decreased under Cr(VI) exposure (48 and 72 h). The results of microscopic examination also showed that after 72 h of chromium (VI) exposure, sperm development was delayed and the nucleus and mitochondria were damaged (,) and ,,)). The numbers of sperms were also significantly inhibited (). Therefore, we suggested that SAP4 gene might be a potential regulatory gene related to spermatogenesis of the clams, and Cr(VI) could cause reproductive toxicity by interfering SAP4 gene expression.
Histological observation showed that after exposure to Cr(VI), the spermary had histological changes, vacuoles and deformation. This shows that the spermary is damaged. Cr(VI) also changed the ultrastructure of sperms. There was a visible disruption in the nucleus and mitochondria. Such alterations in the spermary and sperms indicated that Cr(VI) may result in the reproductive toxicity.
5. Conclusion
The results demonstrate that exposure to concentrations of Cr(VI) in the low mg/L range resulted in MDA accumulation in the spermary of mussels (from +0 to +400% with respect to controls) and induced changes in a number of antioxidation biomarkers, as well as in gene transcription, in spermary tissue. These results indicated that Cr(VI) could produce oxidative damage by affecting enzyme activity and gene expression. Observed histological abnormalities also suggest that excess Cr(VI) caused development delay and spermatogenesis disruption by damaging the spermary structure. The present work is the first attempt to assess the effects of Cr(VI) on G. coaxans, the data presented here provided information for further investigations to assess the toxic effects of hexavalent chromium on marine invertebrates and indicated the possible usage of G. coaxans as a mangrove ecosystem indicator.
Geolocation information
This study was developed in Puqian Town, Hainan Province (20°01′ E, 110°59′ N).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bagchi D, Hassoun EA, Bagchi M, Muldoon D, Stohs S. 1995. Oxidative stress induced by chronic administration of sodium dichromate [Cr(VI)] to rats. Comparative Biochemistry and Physiology. Part C, Pharmacology, Toxicology & Endocrinology 110:281–287. DOI: 10.1016/0742-8413(94)00103-H.
- Begum G, Rao J, Srikanth K. 2006. Oxidative stress and changes in locomotor behavior and gill morphology of Gambusia affinis exposed to chromium. Environmental Toxicology and Chemistry 88:355–365. DOI: 10.1080/02772240600635985.
- Berry R, Lopez-Martinez G. 2020. A dose of experimental hormesis: When mild stress protects and improves animal performance. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 242:110658. DOI: 10.1016/j.cbpa.2020.110658.
- Bolognesi C, Cirillo S. 2014. Genotoxicity biomarkers in aquatic bioindicators. Current Zoology 60:273–284. DOI: 10.1093/czoolo/60.2.273.
- Bregnbak D, Johansen J, Jellesen M, Zachariae C, Menné T, Thyssen J. 2015. Chromium allergy and dermatitis: Prevalence and main findings. Contact Dermatitis 73:261–280. DOI: 10.1111/cod.12436.
- Chai P, Ni H, Zhang H, Fan X. 2016. The evolving functions of autophagy in ocular health: A double-edged sword. International Journal of Biological Sciences 12:1332–1340. DOI: 10.7150/ijbs.16245.
- Chen H, Cao J, Li LX, Wu X, Bi R, Klerks P, Xie L. 2016. Maternal transfer and reproductive effects of Cr(VI) in Japanese medaka (Oryzias latipes) under acute and chronic exposures. Aquatic Toxicology (Amsterdam, Netherlands) 171:59–68. doi:10.1016/j.aquatox.2015.12.011.
- Chen QL, Sun YL, Liu ZH, Li YW. 2017. Sex-dependent effects of subacute mercuric chloride exposure on histology, antioxidant status and immune-related gene expression in the liver of adult zebrafish (Danio rerio). Chemosphere 188:1–9. DOI: 10.1016/j.chemosphere.2017.08.148.
- China. 2002. Ministry of Environmental Protection of China. Standard of seawater quality. Available: http://kjs.mee.gov.cn/hjbhbz/bzwb/shjbh/shjzlbz/199807/W020061027511546974673.pdf. Accessed Oct 2019 26.
- Ciacci C, Barmo C, Fabbri R, Canonico B, Gallo G, Canesi L. 2011. Immunomodulation in Mytilus galloprovincialis by non-toxic doses of hexavalent Chromium. Fish & Shellfish Immunology 31:1026–1033. DOI: 10.1016/j.fsi.2011.09.002.
- Ciacci C, Barmo C, Gallo G, Maisano M, Cappello T, D’Agata A, Leonzio C, Mauceri A, Fasulo S, Canesi L. 2012. Effects of sublethal, environmentally relevant concentrations of hexavalent chromium in the gills of Mytilus galloprovincialis. Aquatic Toxicology (Amsterdam, Netherlands) 120-121:109–118. DOI: 10.1016/j.aquatox.2012.04.015.
- Clemente S, Ingole B. 2009. Gametogenic development and spawning of the mud clam, Polymesoda erosa (Solander, 1876) at Chorao Island, Goa. Marine Biology Research 5:109–121. DOI: 10.1080/17451000802317709.
- Davey M, Stals E, Panis B, Keulemans J, Swennen R. 2006. High-throughput determination of malondialdehyde in plant tissues. Analytical Biochemistry 347:201–207. DOI: 10.1016/j.ab.2005.09.041.
- Donnini F, Dinelli E, Sangiorgi F, Fabbri E. 2007. A biological and geochemical integrated approach to assess the environmental quality of a coastal lagoon (Ravenna, Italy). Environment International 33:919–928. DOI: 10.1016/j.envint.2007.05.002.
- Emmanouil C, Sheehan T, Chipman J. 2007. Macromolecule oxidation and DNA repair in mussel (Mytilus edulis L.) gill following exposure to Cd and Cr(VI). Aquatic Toxicology (Amsterdam, Netherlands) 82:27–35. DOI: 10.1016/j.aquatox.2007.01.009.
- EPA. 2009. National primary drinking water regulations. Available: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations.
- Franzellitti S, Viarengo A, Dinelli E, Fabbri E. 2012. Molecular and cellular effects induced by hexavalent chromium in Mediterranean mussels. Aquatic Toxicology (Amsterdam, Netherlands) 124-125:125–132. DOI: 10.1016/j.aquatox.2012.07.011.
- Gagné F, Blaise C, Pellerin J, Pelletier E, Strand J. 2006. Health status of Mya arenaria bivalves collected from contaminated sites in Canada (Saguenay Fjord) and Denmark (Odense Fjord) during their reproductive period. Ecotoxicology and Environmental Safety 64:348–361. doi:10.1016/j.ecoenv.2005.04.007.
- Ge H, Li Z, Jiang L, Li Q, Geng C, Yao X, Shi X, Liu Y, Cao J. 2018. Cr (VI) induces crosstalk between apoptosis and autophagy through endoplasmic reticulum stress in A549 cells. Chemico-Biological Interactions 298. DOI: 10.1016/j.cbi.2018.10.024.
- Gül Ş, Belge-Kurutaş E, Yildiz E, Şahan A, Doran F. 2004. Pollution correlated modifications of liver antioxidant systems and histopathology of fish (Cyprinidae) living in Seyhan Dam Lake, Turkey. Environment International 30:605–609. DOI: 10.1016/S0160-4120(03)00059-X.
- Huang Z, Kuang X, Chen Z, Fang Z, Wang S, Shi P. 2014. Comparative studies of tri- and hexavalent chromium cytotoxicity and their effects on oxidative state of Saccharomyces cerevisiae cells. Current Microbiology 68:448–456. DOI: 10.1007/s00284-013-0496-1.
- Jacobs JA, Testa SM. 2005. Overview of chromium(VI) in the environment: Background and history. Chromium(VI) Handbook 1–21. DOI: 10.1017/S1047951104006225.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. 2014. Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology 7(2):60–72. DOI:10.2478/intox-2014-0009.
- Kajiura-Kobayashi H, Kobayashi T, Nagahama Y. 2004. The cloning of cyclin B3 and its gene expression during hormonally induced spermatogenesis in the teleost, Anguilla japonica. Biochemical and Biophysical Research Communications 323:288–292. DOI: 10.1016/j.bbrc.2004.08.097.
- Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. 2003. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Research 63:2103–2108.
- Kim EH, Sohn S, Kwon H, Kim S, Kim M, Lee S, Choi KS. 2007. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Research 67(13):6314–6324. DOI:10.1158/0008-5472.CAN-06-4217.
- Kim J, Kang J. 2016. Oxidative stress, neurotoxicity, and metallothionein (MT) gene expression in juvenile rock fish Sebastes schlegelii under the different levels of dietary chromium (Cr6+) exposure. Ecotoxicology and Environmental Safety 125:78–84. DOI: 10.1016/j.ecoenv.2015.12.001.
- Kumar DP, Kumar R, Nagpure N, Nautiyal P, Dabas A, Kushwaha B, Lakra W. 2012. Genotoxic and mutagenic assessment of hexavalent chromium in fish following in vivo chronic exposure. Human and Ecological Risk Assessment: HERA 18:855–870. DOI: 10.1080/10807039.2012.688713.
- Kumar G, Kumar M, Ramanathan A. 2015. Assessment of heavy metal contamination in the surface sediments in the mangrove ecosystem of Gulf of Kachchh, West Coast of India. Environmental Earth Sciences 74:545–556. DOI: 10.1007/s12665-015-4062-y.
- Li J, Yang Y, Liu Q, Zhou Z, Pan S, He Z, Zhang X, Yang T, Pan S, Duan W, He S, Chen X, Qiu J, Zhou S. 2015a. The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells. Drug Design, Development and Therapy 9:1027–1062. DOI: 10.2147/DDDT.S74412.
- Li L, Tan J, Miao Y, Lei P, Zhang Q. 2015b. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cellular and Molecular Neurobiology 35:615–621. DOI: 10.1007/s10571-015-0166-x.
- Liu P, Miao J, Song Y, Pan L, Yin P. 2017. Effects of 2,2ʹ,4,4ʹ-tetrabromodipheny ether (BDE-47) on gonadogenesis of the manila clam Ruditapes philippinarum. Aquatic Toxicology 193:178–186. DOI: 10.1016/j.aquatox.2017.10.022.
- Liu S, Ai C, Ge Z, Liu H, Liu B, He S, Wang Z. 2005a. Molecular cloning and bioinformatic analysis of SPATA4 gene. BMB Reports 38:739–747. DOI: 10.5483/BMBRep.2005.38.6.739.
- Liu S, Liu B, He S, Zhao Y, Wang Z. 2005b. Cloning and characterization of zebra fish SPATA4 gene and analysis of its gonad specific expression. Biochemistry 70:638–644. DOI: 10.1007/s10541-005-0163-7.
- Lushchak O, Kubrak O, Nykorak M, Storey K, Lushchak V. 2008. The effect of potassium dichromate on free radical processes in goldfish: Possible protective role of glutathione. Aquatic Toxicology (Amsterdam, Netherlands) 87:108–114. DOI: 10.1016/j.aquatox.2008.01.007.
- MacFarlane G, Burchett M. 2001. Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Marine Pollution Bulletin 42:233–240. DOI: 10.1016/S0025-326X(00)00147-8.
- Madejczyk M, Baer C, Dennis W, Minarchick V, Leonard S, Jackson D, Stallings J, Lewis J. 2015. Temporal changes in rat liver gene expression after acute cadmium and chromium exposure. PloS One 10:e127327. DOI: 10.1371/journal.pone.0127327.
- Marouani N, Tebourbi O, Mahjoub S, Yacoubi M, Sakly M, Benkhalifa M, Rhouma K. 2012. Effects of hexavalent chromium on reproductive functions of male adult rats. Reproductive Biology 12:119–133. DOI: 10.1016/S1642-431X(12)60081-3.
- Morton B. 1976. The biology and functional morphology of the Southeast Asian mangrove bivalve, Polymesoda (Geloina) erosa (Solander, 1786) (Bivalvia: Corbiculidae). Canadian Journal of Zoology 54:482–500. DOI: 10.1139/z76-055.
- Muñoz C, Post J, Verkleij AJ, Verrips T, Boonstra J. 2001. The effect of hydrogen peroxide on the cyclin D expression in fibroblasts. Cellular and Molecular Life Sciences: CMLS 58:990–996. DOI: 10.1007/PL00013204.
- Nguyen T, Manova K, Capodieci P, Lindon C, Bottega S, Wang X, Refik-Rogers J, Pines J, Wolgemuth D, Koff A. 2002. Characterization and expression of mammalian cyclin B3, a prepachytene meiotic cyclin. The Journal of Biological Chemistry 277:41960–41969. DOI: 10.1074/jbc.M203951200.
- Ni H, Peng L, Gao X, Ji H, Ma J, Li Y, Jiang S. 2019. Effects of maduramicin on adult zebrafish (Danio rerio): Acute toxicity, tissue damage and oxidative stress. Ecotoxicology and Environmental Safety 168:249–259. DOI: 10.1016/j.ecoenv.2018.10.040.
- Oller A, Bates H. 2004. Metals and hormesis. Journal of Environmental Monitoring 6:14–19.
- Ozaki Y, Saito K, Shinya M, Kawasaki T, Sakai N. 2011. Evaluation of Sycp3, Plzf and Cyclin B3 expression and suitability as spermatogonia and spermatocyte markers in zebrafish. Gene Expression Patterns: GEP 11:309–315. DOI: 10.1016/j.gep.2011.03.002.
- Papadimitriou E, Loumbourdis N. 2003. Exposure of the Frog Rana ridibunda to copper: Impact on two biomarkers, lipid peroxidation, and glutathione. Bulletin of Environmental Contamination and Toxicology 69:885–891. DOI: 10.1007/s00128-002-0142-2.
- Sandilyan S, Kandasamy K. 2014. Decline of mangroves – A threat of heavy metal poisoning in Asia. Ocean & Coastal Management 102:161–168. DOI: 10.1016/j.ocecoaman.2014.09.025.
- Scherz-Shouval R, Elazar Z. 2007. ROS, mitochondria and the regulation of autophagy. Trends in Cell Biology 17:422–427. DOI: 10.1016/j.tcb.2007.07.009.
- Schulz R, França L, Lareyre J, Le Gac F, Legac F, Chiarini-Garcia H, Nóbrega R, Miura T. 2009. Spermatogenesis in fish. General and Comparative Endocrinology 165:390–411. DOI: 10.1016/j.ygcen.2009.02.013.
- Shi C, Ding H, Zan Q, Li R. 2019. Spatial variation and ecological risk assessment of heavy metals in mangrove sediments across China. Marine Pollution Bulletin 143:115–124. DOI: 10.1016/j.marpolbul.2019.04.043.
- Stebbing ARD. 1982. Hormesis — The stimulation of growth by low levels of inhibitors. The Science of the Total Environment 22(3):213–234. DOI:10.1016/0048-9697(82)90066-3.
- Takanezawa Y, Nakamura R, Sone Y, Uraguchi S, Kiyono M. 2016. Atg5-dependent autophagy plays a protective role against methylmercury-induced cytotoxicity. Toxicology Letters 262:135–141. DOI: 10.1016/j.toxlet.2016.09.007.
- Tian S, Pan L, Sun X. 2013. An investigation of endocrine disrupting effects and toxic mechanisms modulated by benzo[a]pyrene in female scallop Chlamys farreri. Aquatic Toxicology (Amsterdam, Netherlands) 144-145C:162–171. DOI: 10.1016/j.aquatox.2013.09.031.
- Vane C, Harrison I, Kim AW, Moss-Hayes V, Vickers B, Hong K. 2008. Organic and metal contamination in surface mangrove sediments of South China. Marine Pollution Bulletin 58:134–144. DOI: 10.1016/j.marpolbul.2008.09.024.
- Vasylkiv O, Kubrak O, Storey K, Lushchak V. 2010. Cytotoxicity of chromium ions may be connected with induction of oxidative stress. Chemosphere 80:1044–1049. DOI: 10.1016/j.chemosphere.2010.05.023.
- Wang G, Zhang C, Huang B. 2020. Transcriptome analysis and histopathological observations of Geloina erosa gills upon Cr(VI) exposure. Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology 231. DOI: 10.1016/j.cbpc.2020.108706.
- Wasserman J, Figueiredo A, Pellegatti F, Silva-Filho E. 2001. Elemental composition of sediment cores from a mangrove environment using neutron activation analysis. Journal of Geochemical Exploration 72:129–146. DOI: 10.1016/S0375-6742(01)00158-3.
- Yang F, Liao J, Pei R, Yu W, Han Q, Li Y, Guo J, Hu L, Pan J, Tang Z. 2018. Autophagy attenuates copper-induced mitochondrial dysfunction by regulating oxidative stress in chicken hepatocytes. Chemosphere 204:36–43. DOI: 10.1016/j.chemosphere.2018.03.192.
- Yu X, Yu R, Gui D, Zhang X, Zhan F, Sun W, Wu Y. 2018. Hexavalent chromium induces oxidative stress and mitochondria-mediated apoptosis in isolated skin fibroblasts of Indo-Pacific humpback dolphin. Aquatic Toxicology (Amsterdam, Netherlands) 203:179–186. DOI: 10.1016/j.aquatox.2018.08.012.
- Zhu X, Zhu L, Lang Y, Chen Y. 2008. Oxidative stress and growth inhibition in the freshwater fish Carassius auratus induced by chronic exposure to sublethal fullerene aggregates. Environmental Toxicology and Chemistry 27:1979–1985. DOI: 10.1897/07-573.1.