Abstract
The taxonomic position of two species Plutella hufnagelii Zeller 1839 and Caunaca insulella Walsingham 1900, in the family Plutellidae: has been unclear. In current fauna studies, these species are usually placed in the genera Rhigognostis and Eidophasia respectively. In this paper, I address the taxonomic position of P. hufnagelli and C. insulella and describethe genitalia of both sexes. Analysis of genital structure confirms the correct placement of these species into the genus Eidophasia.
Introduction
The systematic position of the family Plutellidae within the superfamily Yponomeutoidea has been clarified relatively recently (Dugdale et al. Citation1999; Nieukerken et al. Citation2011; Sohn et al. Citation2013). The two species analyzed here, E. hufnagelii (Zeller 1838) and E. insulella (Walsingham Citation1900) were originally described in the genera Plutella and Caunaca respectively.Baraniak (Citation2007) clarified the taxonomic position of the Palearctic species of the genus Plutella. Wallengren (Citation1880) described the genus Caunaca and designated a type species of genus Plutella schmaltzella (Zetterstedt 1839). Fletcher (Citation1929) recognized this as a good genus in the familly Plutellidae: Moriuti (Citation1977) used his systematic proposal in the development of the Japanese fauna Yponomeutidae s. lato. The taxonomic position of genus Caunaca has now been changed, and it is now considered synonymous with the genus Rhigognostis (Nye & Fletcher Citation1991). Species C. insulella has been placed into the genus Eidophasia (Leraut Citation1980; Agassiz & Friese Citation1996; Baraniak Citation2010; Agassiz Citation2013).
In the last few decades, scholars have placed the species P. hufnagelii in the genus: Rhigognostis (Leraut Citation1980; Moreno Citation1992; Agassiz & Friese, Citation1996; Agassiz Citation2013). Robinson and Sattler (Citation2001) proposed a provisional list of the world genera and species of family Plutellidae, however, in recent years, several species of this family have changed their position (Baraniak Citation2007, Citation2016; Baraniak & Sohn Citation2016; Sohn & Baraniak Citation2016). E. hufnagelii is widespread in Central and southern Europe, and E. insulella is restricted to Corsica.
Material and methods
The terminology of the male and female genitalia, follows Klots (Citation1970), Razowski (Citation2008) and Ulenberg (Citation2009). The drawings of genitalia were made from fragments placed in a drop of glycerin (Landry Citation1991; Kaila Citation1999). This study is based on material from the following collections:
NHMUK Natural History Museum, London, U.K.
HNHM Hungarian Natural History Museum, Bupapest, Hungary
MNHN Museum national d’Histoire naturelle, Paris, France
MHB Museum für Naturkunde der Humboldt-Universitat, Berlin, Germany
NM Naturhistorisches Museum, Vienna, Austria
SDEI Senckenberg Deutsches Entomologisches Institut, Muncheberg, Germany
ZI Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia
ZM Zoologisk Museum, Uniwersity of Copenhagen, Copenhagen, Denmark
ZSS Zoologische Staatssammlung, Munich, Germany
EB The author’s collection, Poznań, Poland
Taxonomy
Eidophasia hufnagelii (Zeller Citation1839), .
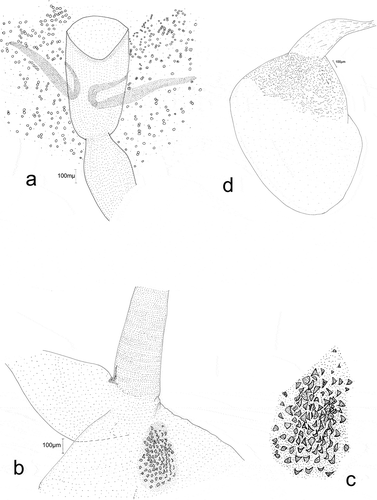
Figure 1. Eidophasia hufnagelii, adult male, Poland, Suwałki, Okrągłe, 12.06.1988, J. Buszko leg., habitus.

Figure 2. Eidophasia hufnagelii, adult male, Poland, Suwałki, Okrągłe, 12.06.1988, J. Buszko leg., labial palpus.

Plutella hufnagelii Zeller Citation1839: 188
Rhigognostis hufnagelii (1839) Agassiz & Friese Citation1996: 60.
Type locality
: southern Europe: (Zeller Citation1839),
Holotype
: 1 male, Zeller’s holotype preserved in coll. Walsingham (NHMUK), information from DJL. Agassiz
Material examined
: 45 males, 20 females
Albania: 1 male, Albania Exp.1918, 23–31.07. Korab., 8 male, 3 female, [Wien], Prater, [without data], Mann leg., ZSS;
Austria: 4 males, 1 female, Guttenstein [bei Schneeberg] 20.07. coll. Krone, Austria, Mödling, 1 male, 2.06. coll. Krone, Austria, Eichkogel, 1 male, 30.06., 1 female, 9.06. 1905, coll. Krone, Austria, Gumpodsk[irschen], 1 female, 24.06., HNHM; Wien, without data Mann leg, 6 males, 3 females, MHB; 10 males, 9 females, Austria, Superior, Wegscheid, 21.06.1940, J. Klimesch leg., 1 female, Austria, Superior, Umgebung Linz, Traun, 20.08.1938, J. Klimesch leg., NM; 1 female, Wien, 1873, Mann leg., 1 male, 1 female, Guttenstein bei Schneeberg, 24.08., SDEI; 1 male, Austria inf. Glaslauterriegel sudl. Gumpoldskirchen, 20.09.1982, E. Kasy leg., MNHN; 3 males, Vienna [without data], ZMUC; 8 males, 3 females, [Wien], Prater, [without data], Mann leg., ZSM; 31 specimens from different localities in Austria preserved in NHM;
Croatia: 1 male, 4 females, Oester. Küstenland, Fužine, 24.06.1906, M. Hilf leg., Leonhard coll., SDEI;
France: 3 specimens, S[outh] France, Basy Albes, 01.07.1913, Walsingham leg., NHM;
Italy: 2 females, Sicilia [without data], MNHN;
Poland: 1 male, Wielkopolski Park Narodowy, 14.07.1981, Niwka, E. Baraniak leg., 2 males, 2 females, Suwałki, Okrągłe, 12.06.1988, J. Buszko leg., 1 male, Kattowitz (currently Katowice), 28.08.1942, Gr. v. Toll leg., coll. EB; 3 males; Poland, Suwałki, Okrągłe, 12.06.1988, O. Karsholt leg., ZMUC.
Russia: Janwarcewo, 1 male, 1 specimen without abdomen, 21.06.1950, Kuznetzow leg., ZI
Slovenia: Nanos, 2 males, 05.1854, Mann leg., coll. Wocke, ZI;
The forewing span is 10–12 mm (). The head is white or white-gray, with scattered white scales. The labial palp is 3-segmented. The first and second segments are light brown the second bicolored, with a characteristic long tuft of scales. The outer surface is light brown, with a small proportion of white scales; on the inner surface, white-gray scales are more frequent. The third segment pointed apically and white-gray. The maxillary palp is small, inconspicuous, and dark gray (). The antennae are long, the scape and pedicel are white-gray, and flagellomeres are white with tiny gray bands.
The forewings are dark brown, with a conspicuous pure white narrow patch along costa from the base to one-third of the wing length. A white patch from the base narrow along the dorsum and widening to the middle of the wing and then extending obliquely toward costaturing toward the dorsum again, forms an inverted letter V. The apex is lighter because of a white triangular patch. White elements, which form the wing pattern, always contain some brown scales. The cilia are bicolored, white and brown.
The hindwing lanceolate are mostly light or dark gray, or sometimes clear brown.
Thethorax is white.
The abdominal segments are white, with a small proportion of gray scales.
Description of genitalia
Male genitalia (prep. gen. no. YPO 16/2016, )).
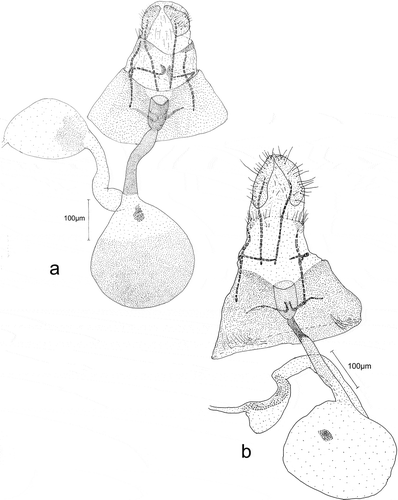
The anal tube is wide, short, and membranous. Soccii are clearly wider in apical part and covered with fine, scanty spinules. The valva is rectangular, with its apex gently rounded ()). Its outer surface (ventral margin), is slightly concave near the apex, its distal part is straight, and its margin is membranous. At the valva apex, on ventral side, there is a small membranous sacculus with scanty minute spinules ()). In its central part, there are conspicuous groups of single spinules. Near the dorsal margin of valva there is a group of numerous fine spinules. The processus basalis is broad, short, and curved, with its apex pointed. The costal margin of the valva is not sclerotized. The vinculum is narrow. The saccus is short and not wide, noticeably narrowing, with a rounded apex. The aedeagus is membranous, only slightly longer than the valva (), (b,c)), of even width and slightly widened near at the base. It is straight to three-quarters of its length, but the remaining part is slightly curved. The vesica has no cornuti (spines).The bulbus ejaculatorius is membranous, small, and circular, and the ductus ejaculatorius is thin and membranous.
Female genitalia (prep. gen. no. YPO 17/2016, ))
In female genitalia, the labii are short, triangular, and covered with very small, scanty, hair-like bristles. The apophyses posteriores and anteriores are short and thin. The antrum resembles a strongly sclerotized elongated cup ()). The ductus bursae are evenly sclerotized, except for a very short membranous section immediately before the corpus bursae, where the ductus bullae emerge ()). The bursa copulatrix is small, oval, and membranous, but its posterior part has conspicuous, scattered, very small sclerites on its walls. Signum a single dentate sclerite situated near the entrance of ductus bursae ()). The ductus bullae are broad and membranous. The bulla seminalis ()) is oval and membranous, and it is strengthened with very small sclerites only near the ductus bullae. The ductus seminalis is narrow and membranous.
Biology
: Caterpillars in May, on Arabis hirsuta L. (Schȕtze Citation1931)
Distributional area
: Known from central and eastern Europe (Agassiz & Friese Citation1996; Agassiz Citation2013), and European part of Russia (Sinev Citation2008).
Eidophasia insulella (Walsingham, Citation1900), .
Figure 3. Eidophasia insulella, adult male, Vizzavona, Corsica, 12.06.1899, coll. Walsingham, 84023, habitus.

Figure 4. Eidophasia insulella, adult male, Vizzavona, Corsica, 12.06.1899, coll. Walsingham, 84023, labial palpus.

Caunaca insulella Walsingham Citation1900: 153.
Eidophasia insulella (1900) Agassiz & Friese Citation1996: 60.
Type locality
: Corsica, Vizzavona (Walsingham Citation1900)
Holotype
examined: 1 male, Vizzavona, Corsica, 11.06.1899, preserved in coll. Walsingham and 6 males with the same data as paratypes (NHMUK) information from DJL. Agassiz
Locality
: Vizzavona is a small town with an operating railway station, at an altitude of 920 m, in the central part of Corsica.
Material examined: 23males, 8 females and 2 specimens without abdomen
France, Corsica: 2 males, Korsika, 13.07.1913, 1 male, 27.07. [19]13, P. Nagel leg., 2 females, Corsika 15.07.[19]13, P. Nagel leg. HNHM;
1 male, Korsika, 13.07.1913, P. Nagel leg. MNHN;
Vizzavona, 1 specimen without abdomen, 12.06.1899, Wlsm. [Walsingham], 1 male, 2 females, Korsika, coll. di Vizzavona, 13.07.[19]13, P. Nagel leg., 1 male, 13.07.[19]13, Korsika, col. di Vizzavona, ex. coll. E. Linack-P. Nagel, NM;
1 male, 1 female, Corsica, Vizzavona, 07.07.1899, A. Petry leg., 1 female, ditto, 18.07.1899, A. Petry leg., 1 male, Corsica, 07.07.1905, Leonhard coll., 2 males, 1 ex. without abdomen, ditto, 17.07. SDEI;
3 males, Korsika,15.07.[19]13, P. Nagel leg., ZI;
1 male, Corsica, Vizzavona, 13.07.[19]13. P. Nagel leg., 1 specimen (without abdomen), Corse [Corsika] 1300 m coll. de Vizzavona, 07. 1928, Schawerda leg. ZSS;
2 males, 3 females, Korsika, Vizzavona, 13.07.[19]13, P. Nagel leg., EB.
Redescription
The forewing span is 12–15 mm (). The head and thorax are white and brown. The labial palpus is 3-segmented. The first segment is very short and white. The second segment has a characteristic tuft of long scales, and its outer surface is covered by light brown scales with a very few white scales. The uppermost scales form a purely white tuft. The third t segment is curved, pointed, and covered with white scales, there are only a few dark scales.The maxillary palp is small, inconspicuous, and dark gray (). The antenna are long, and the scape and pedicel flagellomeres are white. There are two dark flagellomeres at half the length of the flagellum and three black flagellomeres at two-thirds of the lenght. The two terminal flagellomeres are dark.
The forewings are light brown with a conspicuous narrow white patch along one-third of the costa, starting from the base. A narrow white patch from base to middle of the dorsum, becomes wider and extends obliquely, extending the mid costal. In the apical area, from the costal margin, two white patches extend halfway to the tornus. One of them slightly bends toward the termen, and the other is Y-shaped. The apex is lighter, because there are more numerous white scales. On the dorsum, an irregular white patch extends from two-thirds to the tornus. White elements forming the wing pattern do not contain any brown scales. The cilia are bicolored white with scattered brown scales.
The hindwing lanceolate are light gray, with similarly colored long cilia.
The abdominal segments are white, with a small proportion of gray scales.
Description of genitalia
Male genitalia (prep. gen. no. YPO 19/2016, ,d)).
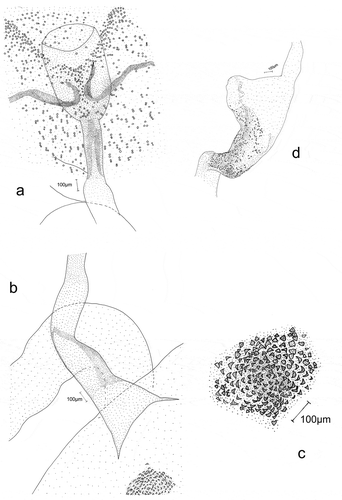
Figure 5. Male genitalia: Eidophasia hufnagelii: a – general view, b – aedeagus; Eidophasia insulella: c – general view, d – aedeagus.
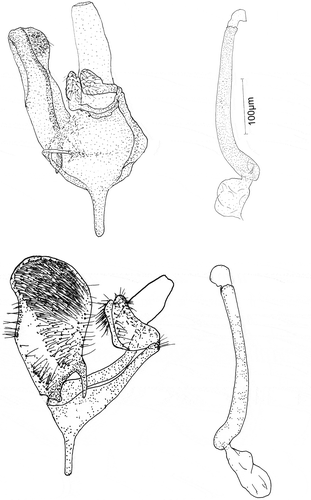
Figure 6. Male genitalia: Eidophasia hufnagelii: a – separated valva, b – part of aedeagus, c – end of aedeagus, d – sacculus.
The anal tube is wide, short, and membranous. Socii are wide, with scanty hair-like spinules. The valva is elongated, with a gently rounded rectangular apex. Its outer side (ventral margin) is markedly concave near the apex, its distal part straight, and its margin strengthened with a small number of long, hairlike bristles. On the ventral side the terminal part of valva, with a large, strongly sclerotized sacculus, is covered by a group of long, thick spines. Its central and apical parts, have conspicuous groups of hair-like bristles. The -processus basalis is broad, short, curved, and gently narrowed apically. The costal margin of the valva is strengthened with long, single spines. The apical part of the valva is covered with long, hair-like bristles. At the valva apex on the ventral side, there is a small membranous, rounded sacculus with some large spines ()). The vinculum is narrow. The saccus is short and wide, markedly narrowing apically. The aedeagus ()) is membranous. and is only slightly longer than valva. It is of even width, although slightly wider near its base. It is straight up to half of its length. The remaining part is strongly curved near its base and it is more gently curvedthereafter. The vesica has no cornuti (spines). The bulbus ejaculatorius is membranous with a triangular outline, and the ductus ejaculatorius is thin and membranous.
Figure 7. Male genitalia: Eidophasia insulella: a – separated valva, b – part of aedeagus, c – end of aedeagus, d – sacculus.
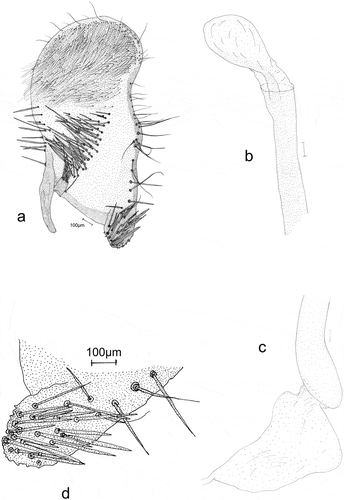
Female genitalia (prep. gen. no. YPO 18/2016, ))
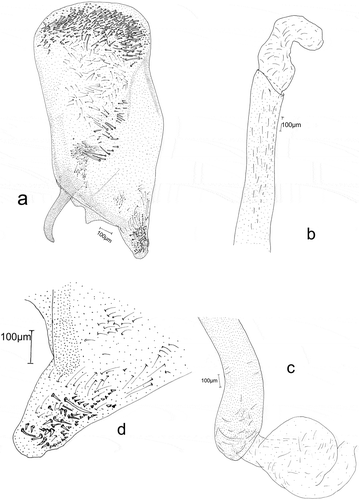
Figure 9. Female genitalia: Eidophasia hufnagelii: a – antrum, b – ductus bursae near inception to bursa copulatrix, c – signum, d – bulla seminalis.
In female genitalia, the labii are elongated, and covered with very small, scanty hair-like bristles. Apophyses posteriores and anteriores are short and thin. The antrum ()) is large, wide, and resembles an elongated cup. The ductus bursae are present only adjacent to markedly narrower and more sclerotized parts of the antrum. The remaining part is wider and membranous ()). The bursa copulatrix is small, oval, and membranous, and its wall is strengthened by very small, fine sclerites. Signum a single dentate sclerite, situated at a distance from the entrance of ductus bursae ()). The ductus bullae emerge from the bursa copulatrix near the entrance of ductus bursae. Ductus bullae are short, broad, and membranous. The bulla seminalis is oval and membranous. It is strengthened with very small sclerites in its posterior part only near the ductus bullae. The ductus seminalis is narrow and membranous ()).
Figure 10. Female genitalia: Eidophasia insulella: a – antrum, b – ductus bursae near inception to bursa copulatrix, c – signum, d – bulla seminalis.
Biology
: unknown
Distributional area
: Corsica – endemic species (Agassiz & Friese Citation1996; Agassiz Citation2013)
Discussion
The two analyzed species have similar forewing patternwhich differ from those of other members of Plutellidae (Moriuti Citation1977; Baraniak Citation2007). The banded pattern is characteristic for the genus Eidophasia. The two species differ slightly from other members of genus Eidophasia because the bands are oblique and extend from the dorsal margin to the costal margin, in contrast to the perpendicular bands in E. messingiella (Fischer von Röslerstamm 1840), E. albifasciata Issiki 1930, E. infuscata (Staudinger [1871] 1870), E. tauricella (Staudinger, 1880) (Moriuti Citation1977; Baraniak & Sohn Citation2015, Citation2016; Baraniak & Walczak, Citation2015; Sohn & Baraniak Citation2016), and the other known European species.
The examination of the male genitalia revealed high similarity between E. hufnagelii and E. insulella in the rectangular shape of the valvae, and the concavity of the ventral margin near the apex. Simultaneously, the concavity of the margins of the valvae distinguishes them from E. syenitella (Herrich-Schäffer 1854) and E. zukowskyi Amsel 1939, (Baraniak & Sohn Citation2015, Citation2016). The shape of the valva shape in the other European species of this genus is obovate (Moriuti Citation1977; Baraniak Citation2010; Sohn & Baraniak Citation2016). Differences in the female genitalia are found in the structure of the signum in the bursa copulatrix and the shape of the antrum narrow in the two speciesconsidered in this study but wide in the others (Baraniak Citation2010; Baraniak & Walczak, Citation2015, Sohn & Baraniak Citation2016). Thus, the morphology of the male and female genitalia, despite some differences, is consistent with that of species currently included in Eidophasia (Dugdale et al. Citation1999).
Acknowledgements
Sincere thanks are due to R. Gaedike, Ch. Kutzcher (Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany), P. Leraut, J. Minet (Museum national d’Histoire naturelle, Paris, France), M. Lödl (Naturhistorisches Museum, Vienna, Austria), W. Mey (Museum für Naturkunde, Humboldt-Universität, Berlin, Germany), L. Ronkay (Hungarian Natural History Museum, Budapest, Hungary), S. Sinev, L. Lvovsky (Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia), A. Hausmann (Zoologische Staatssammlung, Munich, Germany), and O. Karsholt (Zoologisk Museum, Copenhagen, Denmark), for allowing me to examine the collections under their care.
I am also very grateful to David Agassiz for his valuable comments on the manuscript and his correction of English language. I would like to express special thanks to Eric J. van Nieukerken and Leif Aarvik for valuable comments on the manuscript. I am also indebted to an anonymous reviewer and the two editors Alessio De Biasse and Stefano Fenoglio.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Agassiz DJL. 2013. Fauna Europaea: Plutellide. In: Karsholt O, van Nieukerken EJ, editors. Fauna Europaea: Lepidoptera, Moths. Fauna Europaea version 2017.06. https://fauna-eu.-org.
- Agassiz DJL, Friese G. 1996. Plutellidae. In: Karsholt O, Razowski J, editors. The Lepidoptera of Europe. Stenstrup: Apollo Books. pp. 59–60.
- Baraniak E. 2007. Taxonomic revision of the genus Plutella Schranck, 1802 (Lepidoptera, Plutellidae) from the Palaearctic region with notes on its phylogeny. Polish Journal of Entomology 76(Suppl 1):1–122.
- Baraniak E. 2010. Klucze do oznaczania owadów Polski, część XXVII, Motyle – Lepidoptera, 20: Tantnisiowate – Plutellidae. Polskie Towarzystwo Entomologiczne, Poznań. pp. 1–52.
- Baraniak E. 2016. Rhigognostis senilella (Zetterstedt, 1839) and R. marmorosella (Wocke, 1849): Two valid species distinguishable in genitalia (Lepidoptera, Plutellidae). Zootaxa 4084:348–360. DOI:10.11646/zootaxa.4084.3.2.
- Baraniak E, Sohn J-C. 2015. Redescription of Eidophasia syenitella (Herrich-Schaffer, [1854]) (Lepidoptera, Plutellidae). Zootaxa 4057:585–589. DOI:10.11646/zootaxa.4057.4.9.
- Baraniak E, Sohn J-C. 2016. Revised taxonomic status of Eidophasia zukowskyi Amsel, 1938 (Lepidoptera, Plutellidae) with first description of its male and female genitalia. Zootaxa 4162:164–172. DOI:10.11646/zootaxa.4162.1.8.
- Baraniak E, Walczak U. 2015. Redescription of Eidophasia tauricella Staudinger, 1880 (Lepidoptera, Plutellidae) with first description of female genitalia. Zootaxa 3956:445–448. DOI:10.11646/zootaxa.3956.3.10.
- Dugdale JS, Kristensen NP, Robinson GS, Scoble MJ. 1999. The Yponomeutoidea. In: Kristensen NP, editor. Lepidoptera, Moths and Butterflies. Vol. 1: Evolution, Systematics and Biogeography, Handbook of Zoology 4, Arthropoda: Insecta 35. Berlin, New York: Walter de Gruyter. pp. 119–130.
- Fletcher TB. 1929. A list of the generic named used for Microlepidoptera. Memoirs of the Department of Agriculture in India. Entomological Series 11:1–246.
- Kaila L. 1999. Phylogeny and classification of the Elachistidae s. s. (Lepidoptera: Gelechioidea). Systematic Entomology 24:139–169. DOI:10.1046/j.1365-3113.1999.00069.x.
- Klots AB. 1970. Lepidoptera. In: Tuxen SL, editor. Taxonomist’s Glossary of genitalia in insects. Munksgaard, Copenhagen: Skandinavian University Books. pp. 115–129.
- Landry JF. 1991. Systematic of Nearctic Scythrididae (Lepidoptera: Gelechioidea): Phylogeny and classification of superspecific taxa, with a review of described species. Memoirs of the Entomological Society of Canada 160:1–341.
- Leraut P. 1980. Liste systematique et synonymique des Lepidopteres de France. Belgique et Corse. Alexanor 1–336.
- Moreno AV. 1992. Catalogo sistematico y sinonimico de los lepidopteros de la Penisula Iberica y Baleares (Insecta: Lepidoptera). Ministerio de Agricultura, Pesca y Alimentation, Madrid 1–378.
- Moriuti S. 1977. Fauna Japonica. Yponomeutidae s. lat. (Insecta: Lepidoptera). Tokyo: Keigaku Publishing Co. pp. 1–327.
- Nye IWB, Fletcher DS. 1991. The generic names of moths of the world, vol. 6: Microlepidoptera. London: British Museum (Natural History). pp. i–xxx, 1–368.
- Razowski J. 2008. Tortricidae (Lepidoptera) of the Palaearctic Region. General Part and Tortricini 1:1–152. Frantisek Slamka, Kraków - Bratislava.
- Robinson G, Sattler K. 2001. Plutella in the Hawaiian Islands: Relatives and host-races of the Diamondback Moth (Lepidoptera: Plutellidae). Bishop Museum Occasional Papers 67:1–27.
- Schȕtze K. 1931. Die biologie der Kleinschmeterlinge. Frankfurt a. M. pp. 1–325. Verlag des Internationalen Entomologischen Vereins E. V.
- Sinev S. 2008. Plutellidae 49-50. In: Sinev SY, editor. Catalogue of the Lepidoptera of Russia. St. Petersburg-Moscow: KMK Scientific Press Ltd. pp. 1–424 (in russian).
- Sohn J-C, Baraniak E. 2016. Eidophasia infuscata Staudinger, 1870, status nova, with the first description of male and female genitalia. (Lepidoptera, Plutellidae). SHILAP Revista de lepidopterología 44:259–264.
- Sohn J-C, Regier JC, Mitter C, Davis D, Landry J-F, et al. 2013. A molecular phylogeny of Yponomeutoidea (Insecta, Lepidoptera, Ditrysia) and its implications for classification, biogeography and the evolution of host plant use. PLoS ONE 8(1):e55066. DOI: 101371/journal.pone.0055066.
- Ulenberg SA. 2009. Phylogeny of the Yponomeuta species (Lepidoptera, Yponomeutidae) and the history of their host plant associations. Tijdschrift Voor Entomologie 152:187–207, Figs 1-5, Tables 1-5. DOI: 10.1163/22119434-900000275.
- van Nieukerken EJ, Kaila L, Kitching IJ, Kristensen NP, Lees DC, et al. 2011. Order Lepidoptera Linnaeus, 1758. In: Zhang Z-Q, editor. Animal biodiversity: An outline of higer-level classification and survey of taxonomic richeness. Zootaxa 3148:212–221.
- Wallengren HDJ. 1880. Skandinaviens Aṙter at Tineidgruppen Plutellidae (Staint.). Entomologisk Tidskrift 1:53–63.
- Walsingham L. 1900. New Corsican and French micro-lepidoptera. Entomologists Monthly Magazine 34:152–153.
- Zeller PC. 1839. Versuch einer naturgemassen Einteilung der Schaben. Isis von Oken. 32:161–220.