?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The shovelnose guitarfish Pseudobatos productus is a major species in batoid catch on the north-western coasts of Mexico. Our goal was to describe the reproductive biology of this species, including morphometric and histological analysis of the gonads. Sampling was carried out on artisanal fishing camps near Bahía Tortugas, Baja California Sur, Mexico. A total of 147 organisms were collected, with a size of 58.9 to 162 cm of total length (TL). The size of first maturity (L50%) was 111.3 cm TL for females, and 102.2 cm TL for males. The sex ratio was 1.42 females per male, but 1:1 for embryos. Positive correlations were found between TL and the number of oocytes, the diameter of oocytes, the uterus height, the oviducal gland height, the oviducal gland width, and the clasper length. We describe four histological zones in the oviducal gland of females, with no sperm storage. Mature testicle lobes showed distinct spermatogenesis stages, ranging from spermatogonia to mature sperm cells.
Introduction
During the last decade, fisheries of batoids in Mexico and the world has acquired more importance, primarily in areas such as the west coast of the Baja California Peninsula and the Gulf of California (CONAPESCA-INP Citation2004). Due to the slow population replacement rates and particular lifecycles characteristics (e.g., slow growth, late maturity, and a small number of pups; Bejarano-Álvarez Citation2007), shark or ray populations impacted by fisheries will have a slow recovery and may need decades to reach previous population numbers (Pratt & Casey Citation1990; Hoening & Gruber Citation1990; Bejarano-Álvarez Citation2007; Downton-Hoffmann Citation2007).
Pseudobatos productus is one of the most abundant ray species in the artisanal fisheries of Northwest Mexico (CONAPESCA-INP Citation2004), and it has been catalogued as “near threatened” under the IUCN (International Union for Conservation of Nature) Red list (Farrugia et al. Citation2016). Previous studies addressing reproduction of P. productus along coastal zones, from California in the USA to the coasts of Sinaloa in Mexico, showed different reproductive biology patterns in three different zones: California in the USA (Talent Citation1985; Timmons & Bray Citation1997), the west coast of the Baja California peninsula (Villavicencio-Garayzar Citation1993c; Downton-Hoffmann Citation2007; Romo‐Curiel et al. Citation2017), and the Gulf of California in Mexico (Márquez-Farías Citation2007).
P. productus is an organism with an annual cycle with an aplacental viviparous reproduction whose embryonic development lasts 4–5 months. Females have two to 18 pups per litter; the size of the litter is directly related to the size of females (Talent Citation1985; Villavicencio-Garayzar Citation1993c; Timmons & Bray Citation1997; Downton-Hoffmann Citation2007; Márquez-Farías Citation2007; Romo‐Curiel et al. Citation2017). There is no histological description of the gonads of P. productus in the literature; only a rough characterization of the gonads has been reported (Márquez-Farías Citation2007; Romo‐Curiel et al. Citation2017).
The differences in reproductive biology parameters of P. productus in the northern area of where it is distributed (from the Pacific coasts of California in the USA, to the Gulf of California in the coasts of Sonora in Mexico) makes it necessary to establish continuous population monitoring to allow the study of local changes in the population, and therefore suggest appropriate management strategies; thus, the information on the life history parameters of a species that is directly targeted by artisanal fisheries in the north-western coasts of Mexico is important. The goal of this study was to describe the reproductive biology of P. productus in the Northwest coast of Baja California Sur, Mexico, including morphometric and histological analysis of the gonads and an estimation of the maturity of the population (L50%).
Material and methods
Fieldwork
Fieldwork was carried out on artisanal fishing camps near Bahía Tortugas, Baja California Sur, Mexico () from the summer of 2013 to the winter of 2017. Specimens of P. productus were obtained from artisanal fishing carried out in small boats called “pangas”; these boats have an outboard motor, and a gillnet of 8 inches mesh size. After samples were taken and measurements performed, organisms were not returned to the capture site; they were returned to the fishermen’s production line. No samples were obtained during the fishing ban season that runs from May first to July thirty-first; it was established by the government of Mexico in the regulation NOM-029-PESC-2006 (Diario Oficial de la Federación Citation2007). Each specimen was classified (Last et al. Citation2016), and total length (TL) and disc width were measured. The sex of the animals was determined by the presence of claspers in males. The weight of each organism was obtained using a commercial scale.
Laboratory analysis
Sexual maturity in males was determined through the calcification of the claspers and the presence or absence of semen in the rhipidion (Pratt Citation1979). In females, sexual maturity was determined after performing an incision in the abdominal cavity to examine the ovaries and oviducts (Simpfendorfer Citation1992). Female stages of maturity were determined according to the features of this species established by Downton-Hoffmann (Citation1996, Citation2007): the presence or absence of oocytes, the diameter of the oocytes, and the general condition of the uterus (width of the uterus in mm, ).
Table I. Description for maturity stages of male and female of Pseudobatos productus
For histological analysis 5 to 10 individuals of each sex were selected, depending on the abundance of organisms captured, specimens were divided into three size ranges: small (immature), medium (undergoing maturation), and large (mature). To observe sperm storage and to determine sexual maturity stages, histological analysis was performed in the middle part of the oviducal gland in females, and in the middle portion of the testicle, epididymis, vas deferens, and seminal vesicle in males (Pratt & Tanaka Citation1994).
For histological analysis, tissues were cut into 3 µm sections, and different staining techniques were performed (hematoxylin-eosin, Mallory’s trichrome staining, periodic acid–Schiff (PAS), and Feulgen technique) (Martoja & Martoja-Pierson Citation1970). These staining techniques were selected because of their ability to react with specific tissues and cells of the reproductive system. We followed the methodology of Juaristi-Videgaray (Citation2016) for staining times and other details.
Data analysis
An analysis of variance was applied to compare differences in size between sexes. Also, for each sex an exponential correlation between the TL and weight was performed, to establish the type of growth of this species (isometric or allometric). The sex proportion of adults and embryos was calculated, and the 1:1 ratio evaluated using a Chi-Square test.
Analysis of the population’s first maturity (L50%) was performed adjusting a logistic function to the proportion of mature individuals relative to TL, according to Mollet et al. (Citation2000) and Walker (Citation2005). The following equation was used: , where a and b are constants; Pm represents the sex proportion of mature organisms which was converted to binomial values (immature organisms were assigned the value of 0, and mature ones the value of 1).
A comparison between gonad structures was also performed. In females, the height and width of different structures (uterus, oviducal gland, and ovary) were measured. In males, the height and width of the testis and epididymis were measured. Structures were compared between the different sides of the gonads using t-Student tests for parametric analysis, and with U Mann-Whitney tests for non-parametric analysis.
For statistical analysis, we used the software SigmaPlot 12.0 (Citation2011), as well as the “R” statistical package (R Core Team, Citation2012) and StatSoft Statistica Version 10 (Citation2011).
Results
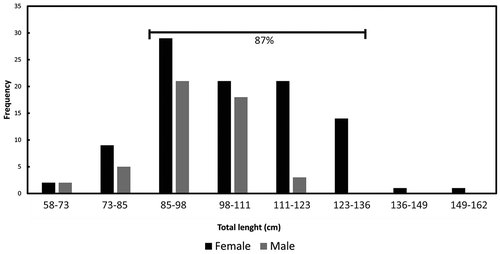
A total of 147 organisms were collected for analysis; animals ranged from 58.9 to 162 cm TL (mean was 101.54 ± 16.08 cm). There were 98 females with a mean of 105.13 ± 17.27 cm TL (range 60.2 cm to 162 cm); there were 49 males with a mean of 94.37 of ± 10.24 cm TL (range 58.9 cm to 115 cm). TL of 87% of the organisms was between 85 and 136 cm TL (). There were significant differences in TL between females and males (F (1,145) = 16.14; p < 0.05). After performing an exponential correlation analysis between TL and weight by sexes, an exponent of 2.79 was observed for females and 2.22 for males, which is indicative of allometric growth for both sexes.
Figure 2. Total length frequency distribution by sexes of Pseudobatos productus in Bahía Tortugas, Mexico. Organisms collected ranged between 58.9-cm to 162-cm-TL (mean 101.54 - SD± 16.08-cm-TL), the 87% of the organisms sampled oscillated between 85-cm to 136-cm-TL
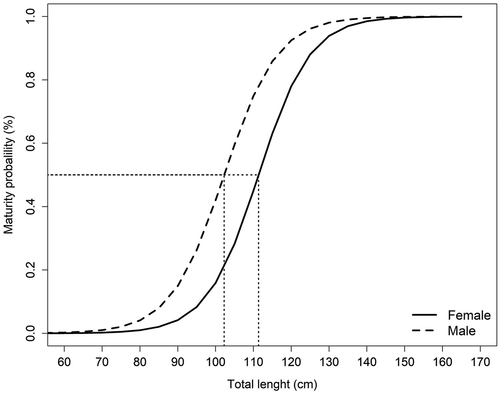
The female:male proportion of sexes was 1.42:1; there was dominance of female specimens, and the difference was statistically significant (χ2 = 16.33; p < 0.05). In the case of embryos, no significant differences were found (χ2 = 1.92; p = 0.17). The calculated L50% was 111.3 cm TL (CI95%, 106.07 to 116.66 cm TL) for females, and 102.2 cm TL (CI95%, 95.45 to 109.09 cm TL) for males ().
Figure 3. Size of first maturity L50% of females and males of Pseudobatos productus, females represented by solid line and males in long dashed lines
Macroscopic analysis of the gonads
The reproductive apparatus of females showed two functional ovaries and uteri. No significant differences were observed in height and width between the left and right sides of the gonadal structures (uterus, ovaries, and oviducal glands) (). Males also presented two-sided functional gonads. No significant differences were observed in height and width between the left and right sides of the gonadal structures (testicles and the epididymis) ().
Table II. Comparison between measurements of both sides of the gonads (males and females) of Pseudobatos productus. Marked with a circle (°) correspond to a t of Student analysis, while the rest correspond to a non-parametrical analysis of U Mann-Whitney. Right and left measurements are expressed in millimeters, mean ± standard deviation
In females, there was a positive correlation between TL and the various measurements of the different structures of the reproductive apparatus: length of the uterus r = 0.65 (p = 0.0000); length of the oviducal gland r = 0.76 (p = 0.0000), and width of the oviducal glans r = 0.77 (p = 0.0000). In males, there was only a significant positive correlation between the length of the clasper and TL, r = 0.44 (p = 0.0082) (Juaristi-Videgaray Citation2016).
Microscopic analysis of the gonad
Histological analysis of the gonads of immature females revealed the presence of germinal cells and primary follicles. Histological sections of the ovaries of mature females showed germinal cells in more advanced stages of follicular growth, and even oocytes in advanced stages of vitellogenesis. A clear histological difference was noted between zones; mature oocytes were found in the upper area of the ovary (anteroposterior axis), while only germinal cells and primordial follicles were observed in the inferior part of the ovary.
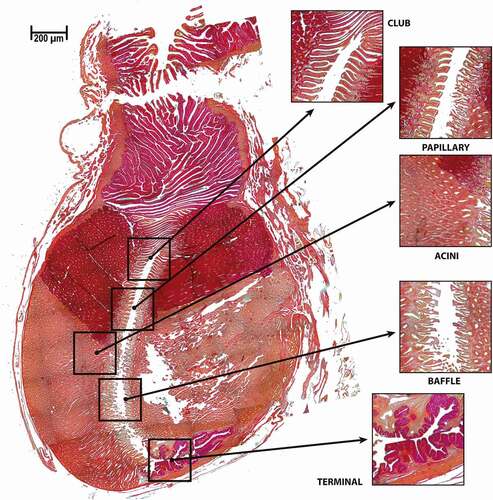
Four zones of the oviducal gland were identified based on the description of Hamlett (Citation2005). A compilation of 108 photographs of one specimen showed an array that represents the complete oviducal gland with clear differences between zones (). The four distinctive zones of the oviducal gland were observed microscopically over longitudinal sections throughout the entire gland. The “club one”, which is the first zone posterior to the oviduct, was characterized by an epithelial structure and a positive reaction to PAS. Next, the “papillary zone” in the lumen of the gland had a different epithelial structure; the papillary zone was also positive for PAS staining. Further down in the oviducal gland, there was a change in the structure of the epithelium of the lumen; a colour change was also noted in the acini, which signalled the start of the “baffle zone”. Finally in the “terminal zone”, there was a change in the structure of the epithelium in the lumen, with PAS staining acquiring darker tones, which may be related to the quantity of mucus present in this zone ().
Figure 4. Longitudinal section of an oviducal gland of Pseudobatos productus. Showing the four zones of the oviducal gland based on the description made by Hamlett (Citation2005) including a close-up to the acini. PAS stain technique 2.5X
In males, histological sections of the testicle were made dorso-ventrally. Microscopic anatomy with a compound form was observed; it was characterized by the presence of multiple germinal zones on the surface of the testicle. Spermatocytes derived from each germinal zone were fixed in columns that radiated from the centre, and in transversal form in the testicle ().
Figure 5. Different stages of spermatogenesis were identified, and the stages of development of spermatogenesis were distinguished in the testicular lobe of a mature testicle of Pseudobatos productus. Feulgen stain technique, 2.5X
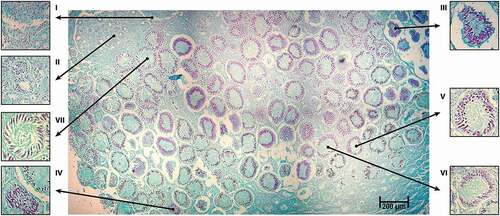
The development of sperm occurred within each lobe of the testicles and at the same time towards the periphery of the testicle (Pratt Citation1988). Differences between immature and mature organisms were observed in transversal histological sections. It was not possible to distinguish an ordered structure in the cells from immature testicular sections, where only germinal cells were found. In sections of mature testicles, various stages of spermatogenesis were identified, and the stages of development of spermatogenesis were distinguished. At stage I, gonocytes in the first stage of spermatogenic development were observed in the periphery of the testicular lobe without any apparent order. At stage II, Sertoli cells initiated their migration into the periphery of the spermatocyte; spermatogonia were aligned and supported by the basal membrane, and spermatocytes increased in size. At stage III, primary spermatocytes that resulted from the first miotic division of the spermatogonia were observed together with larger nuclei. In this stage, Sertoli cells were ordered in the periphery. At stage IV, secondary spermatocytes with smaller and rounded nuclei were observed. Stage V was characterized by the formation of spermatids from the second meiotic division of secondary spermatocytes; elliptic nuclei and the appearance of a flagellum were also observed. However, spermatozoids were not ordered inside the spermatocyst. At stage VI, immature long sperm cells were observed; they were more ordered with the acrosome directed to the basal membrane of the spermatocyst and the flagellum directed towards the lumen. Finally, stage VII consisted of mature spermatozoids organized in clusters compacted towards the periphery with the acrosomes directed towards the basal membrane of the spermatocyst ().
Fecundity
Intra-ovarian fecundity varied from four to 44 oocytes, while real fecundity was seven to 18 embryos. Embryos with the biggest size were observed in August, with a TL of 190 mm. There was a positive significant correlation between TL of the females and the number (r = 0.65, p = 0.00000) and diameter of the oocytes (r = 0.50, p = 0.0001).
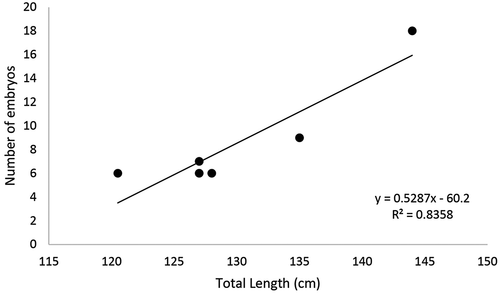
There was a positive correlation between TL and the number of embryos in the uterus of the females with pups. The smallest pregnant female was 120 cm TL and had a total of six pups in the uterus, while the biggest pregnant female had 144 cm TL and a total of 18 pups ().
Discussion
Little is known about the migratory patterns of P. productus; however, changes in size have been recorded throughout the eastern Pacific. In this study, a size range between 58.9 and 162 cm TL was registered. We recorded the maximum sizes recorded in the area: in males 115 cm TL and in females 162 cm TL. Castro-Aguirre (Citation1965) reported a maximum size of 80 cm TL in specimens from zones ranging from San Francisco to Isla Cedros. Talent (Citation1985) and Timmons (Citation1991) reported organisms from 90 cm TL to 150 cm TL in Monterey Bay, California. Villavicencio-Garayzar (Citation1993c) reported maximum sizes of 98 cm TL in males and 136 cm TL in females in Bahía Almejas, BCS Mexico. Downton-Hoffmann (Citation2007) in the zone of Bahía Almejas reported 112 cm TL and 141.5 cm TL for males and females, respectively. In the Gulf of California, Márquez-Farías (Citation2007) observed organisms with smaller sizes, 66.4 cm TL for males and 105 cm TL for females.
A recent study by Rutledge (Citation2019) suggested the possibility that organisms collected inside the Gulf of California corresponded to another species of the genus Pseudobatos, which is very similar to P. productus. Rutledge (Citation2019) suggests that Pseudobatos buthi captured inside the Gulf of California may be misidentified as P. productus. This could somehow explain the differences in the life history of organisms found at different areas of the pacific coast (Talent Citation1985; Villavicencio-Garayzar Citation1993c; Timmons & Bray Citation1997; Downton-Hoffmann Citation2007; Romo‐Curiel et al. Citation2017) and the organisms collected by Márquez-Farías (Citation2007) inside the Gulf of California. Nevertheless, Rutledge (Citation2019) implies that future DNA sequencing of the organisms under study will yield more conclusive results.
In this study females were not only more frequent but also significantly larger; Downton-Hoffmann (Citation2007) indicates that this pattern is common in elasmobranchs. After analyzing 230 populations of sharks, Cortés (Citation2000) determined that females mature to larger sizes and live longer than males. Downton-Hoffmann (Citation2007) reported that females collected in Bahía Almejas in BCS were 20% larger than males. This difference in sizes also has been reported worldwide for several species of rays (e.g., Rhinoptera bonasus, Myliobatis californica, Narcine brasiliensis, Gymnura marmorata, Dipturus oxyrinchus and Raja polistigma) (Smith & Merriner Citation1987; Martin & Cailliet Citation1988; Villavicencio-Garayzar Citation1993a, Citation1993b; Bellodi et al. Citation2017; Porcu et al. Citation2020).
In our present study, the potential “Total weight–Total length” relationship showed negative allometric growth in both sexes. P. productus showed a greater increase in length relative to weight. These results differ from the report by Downton-Hoffmann (Citation1996; Citation2007) for populations of Bahía Almejas BCS and San Ignacio BCS Mexico; for the exponential correlation curve, this author reported exponent values between 2.99 and 3.14 (b), which correspond to isometric growth.
From the differences in the proportion of sexes we infer potential spatial segregation by sex (e.g., the presence of a nursery area where females and males do not share the space, Grijalba-Bendeck et al. Citation2008; Soto-López Citation2014), which could be a direct response to a phase of the reproductive cycle. Wourms (Citation1977) reports the propensity of elasmobranchs to segregate after reaching sexual maturity (Downton-Hoffmann Citation2007). According to Downton-Hoffmann (Citation2007), this kind of segregation has already been observed in P. productus from different zones, such as California (Herald & Dempster Citation1952; Herald Citation1952; Herald et al. Citation1960) and Bahía Almejas, Mexico (Villavicencio-Garayzar Citation1993c).
In the present study, a higher number of females was found in Bahía Tortugas, which suggests segregation by sex. This segregation is possibly associated with depth and nourishment, which has been reported for P. productus and other batoids (Farrugia et al. Citation2011; Bellodi et al. Citation2017; Porcu et al. Citation2020). For instance, Farrugia et al. (Citation2016) reported that, in Baja California, gravid females move to shallow bays and estuaries to give birth during spring and summer, while males arrive to mate in late summer. Thus, the proportion of sexes could change depending on the sampling season, and sex segregation could not be attributed to the selectivity of the fishing gear as suggested by Márquez-Farías (Citation2007); this author suggests that this kind of nets function as a “passive” capture system whose selectivity is the result of the probability of encounter and the probability of retention (Regier & Robson Citation1966; Hamley & Regier Citation1973; Hamley Citation1975; Kirkwood & Walker Citation1986). Therefore, if there were a similar proportion of sexes in the fishing area, this proportion would be reflected in the catch.
In our study, the length values of size at first maturity (L50%) for both sexes (111.3 and 102.2 cm TL for females and males, respectively) are in keeping with those reported by Timmons and Bray (Citation1997) for P. productus in the coasts of California USA (99 cm for females and 91–100 cm TL for males). Also, Downton-Hoffmann (Citation2007) calculated L50% of P. productus in Laguna San Ignacio and Bahía Almejas BCS, Mexico, reporting L50% of 100 cm TL and 80 cm TL for females and males, respectively. Márquez-Farías (Citation2007) reported an L50% of 57 cm TL for females and 53 cm TL for males at the coasts of Sonora, inside the Gulf of California. Sandoval-Castillo et al. (Citation2004) found geographical variations in the maximum size and L50%; they also observed a significant genetic difference between populations of the Gulf of California and the Baja California Peninsula on the Pacific Coast (Kume et al. Citation2009).
Macroscopic analysis of the gonad
In the macroscopic analysis of female gonads, two functional ovaries were identified with developmental features characteristic of batoids (i.e., external development, according to Pratt (Citation1988). Downton-Hoffmann (Citation2007) and Márquez-Farías (Citation2007) also reported that oocytes were present at different stages of development, which indicates that they have an asynchronous development (Soto-López Citation2014). Romo-Curiel et al. (Citation2017) reported activity in the ovaries throughout the year, with larger oocytes found in June. Two oviducts were present; through these oocytes pass to the oviducal gland where fertilization takes place; these oviducts are connected to the uterus ending in the cloaca.
Downton-Hoffmann (Citation2007) reported that maturity stages in females are directly associated with the development of oocytes in the ovary and that P. productus oocytes larger than 21 mm are indicative of sexual maturity. Márquez-Farías (Citation2007) implied that the sole natural presence of an intense yellow colour and “clearly” vascularized ova were indications of mature females.
Based on our results, we believe that the maturity classification of females of Downton-Hoffmann (Citation2007) for this species could conceal some mature females during some seasons. For instance, during ovulation, females loose the largest oocytes of the ovary, which could directly affect the classification of maturity stage: larger females that have already partaken in the reproductive period could be considered immature. In our study, some large females lacked large oocytes, despite showing a well-developed and distended uterus. We thus recommend using the uterus width as an additional feature to classify the maturity state of this species ().
Both sides of the gonad were functional and symmetrical, with no size differences between sides. In females, different reproduction stages can be analyzed from any side of the gonad using the length and width of the uterus, ovaries, and oviducal gland. This is of great benefit given that handling captured organisms in fishing camps does not always allow the collection of a complete gonad. In our study, symmetrical testicles, epididymis, and vas deferens were identified in males. Downton-Hoffmann (Citation2007) and Márquez-Farías (Citation2007) reported the presence of lobed testicles (a feature that is accentuated in mature organisms), which is in keeping with our findings.
There was a low correlation between males’ TL and the length of the clasper. After reaching sexual maturity, the organism as a whole and the clasper grow at different rates, which may directly affect the value of the correlation.
Microscopic analysis of the gonad
In the present study, histological sections of different zones of the ovary showed oocytes in different developmental stages; yet, there was a large difference in oocytes size, even in the same ovary. We observed that organisms with the same TL exhibited ovaries with different states of maturity. Assuming that some organisms have the corresponding TL of a mature organism, the ovary may show oocytes in a very early stage of development. In batoids, it is common to find females at the onset of oogenesis, therefore, initial stages of oogenesis can be observed in immature females and mature females starting the annual cycle (Serra-Pereira et al. Citation2011; Soto-López Citation2014).
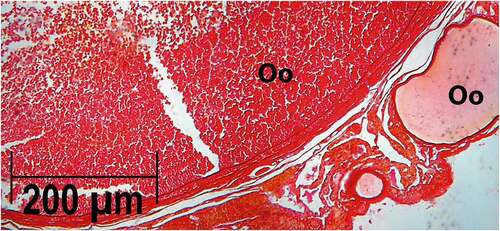
In our analysis of the ovaries, oocytes with a small number of yolk granules were observed at the initial stages of vitellogenesis; larger oocytes with abundant yolk were also observed. Mature females showed the largest number of vitelogenic follicles (). Neither a corpus luteum or atresic follicles were observed. This observation suggests that females had not spawned recently, or that the histological sections did not correspond to a zone containing atresic follicles. The lack of an organization pattern could be a flaw of the histological section analyzed, or a specific characteristic of this species. We observed no sperm stored in the oviducal gland, which could also be related to the specific area of the histological section, or rather to the sampling season.
Figure 7. Histological section of the ovary of Pseudobatos productus with oocytes in different stages of development. PAS stain technique, 2.5X
In the present study, the testicles were characterized histologically by distinct stages of spermatogenesis, ranging from spermatogonia to mature sperm (stages defined by Pratt (Citation1988) for this species). These features have been also reported for the family Rajidae and in oviparous sharks (Stehmann Citation2002; Meza-Castillo et al. Citation2012; Soto-López Citation2014).
We found that the body of the epithelium of the testicles consisted of a simple columnar epithelium, which has also been observed in other species of rays (Maruska et al. Citation1996; Hamlett Citation2005; Soto-López Citation2014; Pellamati Citation2015). A simple columnar epithelium was also found in the vas deferens, but there were also ciliated cells. A certain organization pattern of sperm cells has been observed in other species in the conducts inside the testicle (Pratt & Tanaka Citation1994); however, we did not observe a clear organization pattern, neither as spermatophores, or spermatozeugmata (Pratt & Tanaka Citation1994). This observation could be the result of a section that was cut from the vas deferens, or as a specific characteristic of the species.
Fecundity
The observed value of intra-ovarian fecundity was four to 44 oocytes; a positive correlation was observed between the size of the organism and either the number of oocytes or their diameter; Pratt and Casey (Citation1990) suggest that this positive correlation exists in various species of elasmobranchs (see for example Downton-Hoffmann Citation2007). A similar correlation was found by Márquez-Farías (Citation2007) in specimens from the coast of Sonora.
In terms of real fecundity, seven to 18 embryos were found with a sex ratio of 1:1, which supports the idea that adults segregate by sex in the area. Downton-Hoffmann (Citation2007) reported four to 18 embryos. We found a direct positive relation between the number of embryos and the TL of females; these results are in keeping with the observations of Downton-Hoffmann (Citation2007), and Romo‐Curiel et al. (Citation2017). The direct correlation between size and the number of embryos has also been observed in species from the family Rhinobatidae, such as R. horkelii in Brazil (Lessa et al. Citation1986), R. annulatus in South Africa (Rossouw Citation1984), and R. hynnicephalus in China (Zheng & Qiu Citation1993).
Conclusion
Our results show that P. productus is a species with a small number of pups and late maturity, which reveals a significant vulnerability of this population if unmanaged fisheries are conducted; especially, because this species is considered “near threatened” in the Red List of the IUCN (Farrugia et al. Citation2016).
Acknowledgements
RIOB, FGM, AHH and MVF wish to thank the Instituto Politécnico Nacional for the fellowships (COFAA, EDI) DJV received a schoolarship provided by CONACYT.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bejarano-Álvarez M. 2007. Biología reproductiva del tiburón martillo Sphyrna lewini (Griffith y Smith, 1834) en Salina Cruz, Oaxaca, México. Master Thesis. La Paz, Baja California Sur, México: CICIMAR-IPN. p. 74. Available: http://repositoriodigital.ipn.mx/handle/123456789/13829
- Bellodi A, Porcu C, Cannas R, Cau AL, Marongui MF, Mulas A, Vittori S, Follesa MC. 2017. Life-history traits of the long–nosed long-nosed skate Dipturus oxyrinchus. Journal of Fish Biology 90(3):867–888. DOI: 10.1111/jfb.13205.
- Castro-Aguirre JL. 1965. Peces sierras, rayas, mantas y peces afines de México. Anales del Instituto Nacional de Investigaciones Biológico Pesqueras 1:171–256.
- CONAPESCA-INP. 2004. Plan de Acción Nacional para el Manejo y Conservación de Tiburones, Rayas y Especies Afines en México. Comisión Nacional de Acuacultura y Pesca e Instituto Nacional de la Pesca, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. México: Mazatlán. p. 80.
- Cortés E. 2000. Life-history patterns and correlations in sharks. Reviews in Fisheries Science 8(4):299–344. DOI: 10.1080/10408340308951115.
- Diario Oficial de la Federación. 2007. NOM-029-PESC-2006, Pesca responsable de tiburones y rayas, especificaciones para su aprovechamiento. Publicada el 14 de Febrero del 2007. Ciudad de México. México.
- Downton-Hoffmann CA. 1996. Estrategia reproductiva de la guitarra Rhinobatos productus (Ayres 1856) en la costa Occidental de Baja California Sur, México. Bachelor thesis. México: UABCS. La Paz, B.C.S. p. 51. Available: http://biblio.uabcs.mx/tesis/te148.pdf
- Downton-Hoffmann CA. 2007. Biología del pez guitarra Rhinobatos productus (Ayres, 1856), en Baja California Sur, México. IPN Doctoral thesis. p. 213. Available: http://repositoriodigital.ipn.mx/handle/123456789/13732
- Farrugia TJ, Espinoza M, Lowe CG. 2011. Abundance, habitat use and movement patterns of the shovelnose guitarfish (Rhinobatos productus) in a restored southern California estuary. Marine & Freshwater Research 62(6):648–657. DOI: 10.1071/MF10173.
- Farrugia TJ, Márquez-Farías F, Freedman RM, Lowe CG, Smith WD, Bizzarro JJ. 2016. Pseudobatos productus. The IUCN Red List of Threatened Species 2016 e.T60171A104004394. Accessed July 2020. DOI: 10.2305/IUCN.UK.2016-3.RLTS.T60171A104004394.en.
- Grijalba-Bendeck M, Acero AP, González E. 2008. Biología reproductiva de Rhinobatos percellens (Walbaum, 1792) (Batoidea: Rajiformes) en el Caribe colombiano. Revista de biología marina y oceanografía 43(3):469–481. DOI: 10.4067/S0718-19572008000300006.
- Hamlett WC. 2005. Reproductive biology and phylogeny of Chondrichthyes: Sharks, batoids and chimaeras, Volume 3. 1st ed. Science Publishers Inc. Enfield, NH, USA. p. 576. DOI: 10.1201/9781439856000.
- Hamley JM. 1975. Review of gillnet selectivity. Journal of the Fisheries Research Board Canada 32(11):1943–1969. DOI: 10.1139/f75-233.
- Hamley JM, Regier HA. 1973. Direct estimates of gillnet selectivity to walleye (S. vitreum). Journal of the Fisheries Research Board 30(6):817–830. DOI: 10.1139/f73-137.
- Herald ES. 1952. The 1952 Shark derbies at Elkhorn slough, Monterey Bay, and at Coyote Point, San Francisco Bay. California Fish and Game 2:237–243.
- Herald ES, Dempster RP. 1952. The 1951 Shark Derby at Elkhorn Slough. California Fish and Game 38(1):133–134.
- Herald ES, Schneebeli W, Green N, Innes K. 1960. Catch records for seventeen shark derbies held at Elkhorne Slough Monterey Bay, California. California Fish and Game 1:59–67.
- Hoening JM, Gruber SH (1990) Life-history patterns in the elasmobranchs: Implications for fisheries management. NOAA technical report NMFS. NMFS 90:1–16.
- Juaristi-Videgaray D. 2016. Biología reproductiva del pez guitarra Pseudobatos productus (Ayres, 1856), en Bahía Tortugas, Baja California Sur, México. Master thesis. México: CICIMAR-IPN. Available: http://www.biblioteca.cicimar.ipn.mx/oacis/Medios/tesis/juaristivi1.pdf
- Kirkwood GP, Walker TI. 1986. Gill net mesh selectivities for gummy shark, Mustelus antarcticus Günther, taken in south-eastern Australian waters. Marine and Freshwater Research 37(6):689–697. DOI: 10.1071/MF9860689.
- Kume G, Furumitsu K, Tanaka S, Yamaguchi A. 2009. Reproductive biology of the guitarfish Rhinobatos hynnicephalus (Batoidea: Rhinobatidae) in Ariake Bay, Japan. Environmental Biology of Fishes 85(4):289. DOI: 10.1007/s10641-009-9487-2.
- Last P, Naylor G, Séret B, White W, de Carvalho M, Stehmann M, Eds. 2016. Rays of the world. CSIRO Publishing, Clayton South VIC, Australia. p. 800.
- Lessa RT, Vooren CM, Lahaye J. 1986. Desenvolvimiento e ciclo sexual das Fêmeas, migraçôes e fecundidade de viola Rhinobatos horkelii (Müler y Henle, 1841) Do Sul Do Brasil. Atlántica, Río Grande 8:5–34.
- Márquez-Farías JF (2007) Demografía del pez guitarra Rhinobatos productus. Doctoral thesis. México: Centro de investigaciones Biológicas del Noroeste. La Paz, Baja California Sur. p. 147. Available: http://dspace.cibnor.mx:8080/handle/123456789/169
- Martin LK, Cailliet GM. 1988. Age and growth determination of the Bat Ray, Myliobatis californica Gill, in central California. Copeia 3(3):762–773. DOI: 10.2307/1445399.
- Martoja R, Martoja-Pierson M. 1970.Técnicas de histología animal. 1ra ed. Toray-Masson SA Editores. Barcelona, España. p. 350.
- Maruska KP, Cowie EG, Tricas TC. 1996. Periodic gonadal activity and protracted mating in elasmobranch fishes. The Journal of Experimental Zoology 276:219–232.
- Meza-Castillo JH, Carrera-Fernández M, Galván-Magaña F. 2012. Descripción morfológica del aparato reproductor de la raya Zapteryx exasperata (Jordan and Gilbert, 1880) en Bahía Tortugas, Baja California Sur. In: Díaz Sánchez AW, Aguilar CG, Mendoza Vargas OU, editors. Libro de Resúmenes V Simposium Nacional de Tiburones y Rayas. México: SOMEPEC. pp. 10- 12.
- Mollet H, Cliff J, Pratt Jr. H, Stevens J. 2000. Reproductive biology of the female shortfin mako, Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of Lamnoids. Fishery Bulletin 98(2):299–318. Available: http://hdl.handle.net/102.100.100/208936?index=1
- Pellamati T (2015) Reproductive biology of the Bat Ray Myliobatis californica off the west coast of Baja California Sur, México. Master Thesis. Università Politecnica delle Marche. p. 101
- Porcu C, Bellodi A, Cau A, Cannas R, Marongui MF, Mulas A, Follesa MC. 2020. Uncommon biological patterns of a little known endemic Mediterranean skate, Raja polystigma (Risso, 1810). Regional Studies in Marine Science 34:101065. DOI: 10.1016/J.RSMA.2020.101065.
- Pratt HL. 1979. Reproduction in the blue shark, Prionace glauca. Fishery Bulletin 77(2):445–470.
- Pratt Jr. HL. 1988. Elasmobranch gonad structure: A descriptive survey. Copeia 3(3):719–729.
- Pratt HL, Casey JG. 1990. Shark reproductive strategies as a limiting factor in directed fisheries, with a review of Holden’s method of estimating growth parameters. In: Pratt HL, Gruber SH, Taniuchi T. editors, Elasmobranchs as living resources: Advances in the biology, ecology, systematic, and the status of the fisheries. NOAA Technical Report NMFS 90. pp. 97–110.
- Pratt HL, Tanaka S. 1994. Sperm storage in male elasmobranch: A description and survey. Journal of Morphology 219(3):297–308. DOI: 10.1002/jmor.1052190309.
- R Core Team. 2012. R: A language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org/
- Regier HA, Robson DR. 1966. Selectivity of gillnets, especially to the lake whitefish. Journal of the Fisheries Research Board of Canada 23(3):423–454. DOI: 10.1139/f66-034.
- Romo‐Curiel AE, Sosa‐Nishizaki O, Pérez‐Jiménez JC, Rodríguez‐Medrano MC. 2017. Reproductive cycle and maternal–embryonic maternal-embryonic nutritional relationship of shovelnose guitarfish Pseudobatos productus in the Gulf of California. Journal of Fish Biology 90(3):889–905. DOI: 10.1111/jfb.13204.
- Rossouw GJ. 1984. Age and growth of the sand shark, Rhinobatos annulatus, in Algoa Bay, South Africa. Journal of Fish Biology 25(2):213–222. DOI: 10.1111/j.1095-8649.1984.tb04868.x.
- Rutledge KM. 2019. A new guitarfish of the genus Pseudobatos (Batoidea: Rhinobatidae) with key to the guitarfishes of the Gulf of California. Copeia 107(3):451–463. DOI: 10.1643/CI-18-166.
- Sandoval-Castillo J, Rocha-Olivares A, Villavicencio-Garayzar C, Balart E. 2004. Cryptic isolation of Gulf of California shovelnose guitarfish evidenced by mitochondrial DNA. Marine Biology 145(5):983–988. DOI: 10.1007/s00227-004-1378-7.
- Serra-Pereira B, Figueiredo I, Serrano LG. 2011. Maturation of the gonads and reproductive tracts of the thornback Ray Raja clavata, with comments on the development of a standardized reproductive terminology for oviparous elasmobranchs, marine and coastal fisheries. Dynamics, Management, and Ecosystem Science 3(1):160–175. DOI: 10.1080/19425120.2011.555707.
- SigmaPlot for Windows Version 12.0 Build 12.2.0.45 copyright 2011 Systat Softwares, Inc.
- Simpfendorfer CA. 1992. Reproductive strategy of the Australian sharpnose shark, Rhizoprionodon taylori (Elasmobranchii: Carcharhinidae), from Cleveland Bay, northern Queensland. Marine & Freshwater Research 43(1):67–75. DOI: 10.1071/MF9920067.
- Smith JW, Merriner JV. 1987. Age and growth, movements and distribution of the cownose ray, Rhinoptera bonasus, in Chesapeake Bay. Estuaries 10(2):153–164. DOI: 10.2307/1352180.
- Soto-López K. 2014. Biología reproductiva de la raya Raja velezi en el sureste de la Costa Occidental de Baja California Sur. Master thesis. UNAM. México. p. 100. DOI: 10.13140/RG.2.2.14000.87042.
- StatSoft, Inc. 2011. STATISTICA (data analysis software system), version 10. Available: www.statsoft.com
- Stehmann M. 2002. Proposal of a maturity stages scale for oviparous and viviparous cartilaginous fishes (Pisces, Chondrichthyes). Archive of Fishery and Marine Research 50:23–48.
- Talent LG. 1985. The occurrence, seasonal distribution, and reproductive condition of elasmobranch fishes in Elkhorn Slough, California. California Fish and Game 71(4):210–219.
- Timmons M. 1991. Age, growth and sexual maturity of the shovelnose guitarfish, Rhinobatos productus (Ayres). California. State University. Master’s Thesis. California: Long Beach. p. 84
- Timmons M, Bray NB. 1997. Age, growth and sexual maturity of the shovelnose guitarfish, Rhinobatos productus (Ayres). Fishery Bulletin 94:349–359.
- Villavicencio-Garayzar CJ. 1993a. Observaciones sobre la biología reproductiva de Narcine brasiliensis (Olfers) (Pisces: Narcinidae), en Bahía Almejas, Baja California Sur, México. Rev Inv Cient UABCS 4(1):95–99.
- Villavicencio-Garayzar CJ. 1993b. Notas sobre Gymnura marmorata (Cooper) (Pisces: Dasyatidae), Bahía Almejas, B.C.S., México. Revista de investigación científica de la Universidad Autónoma de Baja California Sur 4(1):91–94.
- Villavicencio-Garayzar CJ. 1993c. Biología reproductiva de Rhinobatos productus (Pisces: Rhinobatidae), en Bahía Almejas, Baja California Sur, México. Revista De Biologia Tropical 41(3):441–446.
- Walker TI. 2005. Reproduction in fisheries science. In: Hamlett WC, editor. Reproductive biology and phylogeny of Chondrichthyes: Sharks, batoids and chimaeras. Enfield, NH: Science Publishers Incorporated, vol. 3. USA. pp. 81–127.
- Wourms JP. 1977. Reproduction and development in chondrichthyan fishes. American Zoologist 17(2):379–410. DOI: 10.1093/icb/17.2.379.
- Zheng W, Qiu S. 1993. Reproductive biology of the guitarfish, Rhinobatos hynnicephalus. In: Demski L.S., Wourms J.P. editors. The reproduction and development of sharks, skates, rays and ratfishes. Dordrecht: Springer. pp. 81–93. DOI: 10.1007/978-94-017-3450-9_8.