Abstract
Sexing monomorphic birds by DNA and morphometrics allows researchers to study behavioural differences between the sexes. We aimed to verify the utility of the carpal spur to sex the monomorphic Blacksmith Lapwing or to determine a more suitable method. The Blacksmith Lapwing is a widespread resident of central and southern Africa. We used biometrics and carpal spur features of 116 Blacksmith Lapwings we ringed at five South African sites in 2008–2018 that were DNA-sexed from blood samples. Males were on average larger than females in most morphometrics, but all these measurements largely overlapped. Spur length was the most sexually dimorphic trait, then body mass and tarsus length. Male lapwings use their wing spurs in combat, thus the spurs’ length and sharpness, and body size, are likely subject to intrasexual selection. The male’s longer tarsus may be related to his role in scraping out the ground nest. Our backward stepwise analysis produced the discriminant function D1 = 0.366 tarsus length - 27.541 to determine this species’ sex, which correctly sexed 66% of our sample. If we allowed a 5% misclassification rate then 70% of individuals remained unsexed. Thus we recommend DNA analysis for studies that must accurately sex all Blacksmith Lapwings in a sample. All of the DNA-sexed adult males had sharp ivory tipped spurs but 36% had a spur shorter than 13 mm, proposed previously as the cut-off value between sexes. We suggest that Blacksmith Lapwings with sharp ivory-tipped spurs of 14 mm or longer can be safely sexed as males to reduce the need for expensive DNA tests.
Introduction
The activities of male and female birds often differ, so sex identification becomes an important aspect of behavioural studies (Dit Durell et al. Citation1993; Mathot & Elner Citation2004), migration phenology (Remisiewicz & Wennerberg Citation2006; Pinchuk et al. Citation2016; Meissner & Krupa Citation2017) and foraging strategy (Nebel Citation2005; Castro et al. Citation2009). Biological differences in the sexes are well described for species that show clear sexual dimorphism but are less known in species where the sexes do not differ noticeably in colour or size, which impedes studies of sex-specific patterns within a species (Newton Citation1998). Though DNA-based sexing methods (Griffiths et al. Citation1998; Kahn et al. Citation1998; Fridolfsson & Ellegren Citation1999) allow researchers to determine a bird’s sex accurately, this is a costly procedure, especially for large samples. The sexes differ in size in many species that exhibit no plumage dimorphism, which allows researchers to use discriminant functions derived from the biometrics of individuals of known sex to determine the sex of other measured birds (Dechaume-Moncharmont et al. Citation2011). This method is cheap, hardly invasive and may be applied to data collected in the past, so it is becoming increasingly popular in studies of monomorphic bird species (e.g. Sweeney & Tatner Citation1996; Dale & Robertson Citation2006; Poisbleau et al. Citation2010; Meissner et al. Citation2017), including waders (Meissner Citation2005; Meissner & Pilacka Citation2008; Scherer et al. Citation2014; Jiménez et al. Citation2015; Niemc et al. Citation2018; Witkowska & Meissner Citation2020).
The Blacksmith Lapwing Vanellus armatus is a common shorebird that inhabits short grasslands and mudflats from southern to central Africa (Turpie et al. Citation2005; BirdLife International Citation2016). Several studies on this species have focused on foraging ecology and other aspects of their behaviour (e.g. Hall Citation1964; Urban et al. Citation1986; Tree Citation1998; Calf Citation2002; Tjørve et al. Citation2008; Cantlay et al. Citation2019), but researchers were compelled to ignore any sexual differences because males and females have similar plumage (Turpie et al. Citation2005). Other Charadriidae do show sexual differences in their biology, such as the onset of primary moult (Walters Citation1984; Machín et al. Citation2018), foraging strategy (Castro et al. Citation2009), time spent on incubation (Byrkjedal Citation1985; Whittingham et al. Citation2000) and survival mechanisms (Eberhart-Phillips et al. Citation2017). Hence Blacksmith Lapwings probably show sexual differences in aspects of their behaviour. However sexual size dimorphism is less pronounced in Charadridae than in Scolopacidae (Jehl & Murray Citation1986), and Charadridae, including Blacksmith Lapwings, can seldom be sexed by body measurements or by mass (Zefania et al. Citation2010; Meissner et al. Citation2011). Though male Blacksmith Lapwings tend to be slightly larger than females (Turpie et al. Citation2005), it remains unknown if the degree of dimorphism is sufficient to derive a discriminant function that enables efficient sexing of this species in the field.
About half of the Vanellinae subfamily have a distinct carpal spur, a keratinous covering of a projecting bony core on the leading edge of the birds’ wing near the carpal joint (Rand Citation1954). The spur is well developed in both sexes, but is longer in males than in females for most species of this subfamily (Rand Citation1954; Tree Citation1999; Wakisaka et al. Citation2006; Cruz-Bernate et al. Citation2013). A long, sharp spur correlates with aggressiveness, especially of males (Piersma & Wiersma Citation1996). Tree (Citation1999) identified it as the most dimorphic measurement in the Blacksmith Lapwing, as did other authors for other species in the Vanellus genus (Wakisaka et al. Citation2006; Cruz-Bernate et al. Citation2013). We set out to verify the utility of the spur in sexing Blacksmith Lapwings, confirmed by DNA-sexing, and to check the cut-off values given by Tree (Citation1999) based on field observations. We also aimed to determine the extent of any sexual size dimorphism of the species and to determine any significant biometrical differences that could be used to sex this species in the field.
Material and methods
Blacksmith Lapwings were caught with mist nets and walk-in traps at five locations in the South African provinces of North West (105 individuals, three sites) and KwaZulu-Natal (11 individuals, two sites) between 2008 and 2018. In North West Province, most birds (95) were caught at Barberspan Bird Sanctuary; the other 10 at two farms near the town of Potchefstroom. In KwaZulu-Natal Province the birds were trapped at Darvill Wastewater Works and at Eston Dam near the town of Pietermaritzburg. We analysed birds only in adult plumage for this study, measuring total head length, bill length and tarsus length with callipers to the nearest 0.1 mm, and wing length (maximum chord) and tarsus-plus-toe length with a stopped rule to the nearest 1 mm (Busse & Meissner Citation2015). Birds were also weighed by electronic balance with an accuracy of 1 g. The left and right spur lengths were measured with callipers from the base to the tip as described by Tree (Citation1999). We considered only the left spur in our analyses because the right spur of one bird had been damaged. The colour and the sharpness of the tip were noted because Tree (Citation1999) held that adult Blacksmith Lapwings have a sharp spur with an ivory tip (Tree Citation1999). Most birds (96%) were measured by the three authors (WM, MR, LP) and the accuracy and repeatability of the measurements were calibrated as described by Busse and Meissner (Citation2015).
The birds were definitively sexed molecularly. We drew 20–50 μl of blood from the brachial vein and preserved it in 70% ethyl alcohol. In the laboratory, after evaporating the ethanol we extracted the DNA using a Blood Mini DNA kit (A&A Biotechnology, Gdynia, Poland). Individuals were then sexed by the presence of one or two forms of the CHD genes in their sex chromosomes (Griffiths et al. Citation1998). The CHD gene was amplified with the 2550 F/2718 R pair of primers (Fridolfsson & Ellegren Citation1999), following the laboratory protocol of Remisiewicz and Wennerberg (Citation2006). The PCR products were separated with electrophoresis on 3.5% agarose gel stained with Midori Green Advanced DNA Stain (NIPPON Genetics). Samples that showed two bands on the gel, indicating two forms of the CHD gene, one from the W chromosome and the second from the Z chromosome, were sexed as females; single bands, indicating the same form of the CHD gene from ZZ chromosomes, were sexed as males (Fridolfsson & Ellegren Citation1999).
We measured and sexed 66 males and 50 females, but not all biometrics had been taken from each individual so sample sizes differed for certain measurements. Differences in morphological traits between males and females were tested with a two-sample t-test. A size dimorphism index (SDI) using a percentage scale was calculated as in Lovich and Gibbons (Citation1992); greater sexual dimorphism was indicated by a larger SDI value.
The spur lengths of 91 birds (51 males and 40 females) were measured. Fully developed spurs in lapwings should be ivory coloured at the tip (Marchant & Higgins Citation1993; Tree Citation1999). Rand (Citation1954) suggested that at least in some species, including plovers, moult of the outer layer of the horn covering the spurs results in shorter lengths after the outer cap-like layer is shed. Thus only spurs with an ivory tip, indicating adults, were analysed. That reduced our sample size to 28 males and 30 females.
Body mass is seldom included in discriminant analyses because of large seasonal fluctuations in migratory species on passage and over winter, especially during the birds’ pre-migratory preparations (Clark Citation1979; Scott et al. Citation1994). But the Blacksmith Lapwing is a sedentary or a nomadic species inhabiting the Afrotropical zone and its body mass only exceptionally shows much seasonal variability (Turpie et al. Citation2005). Hence we included body mass in our discriminant analysis as a measurement that may be significant, as reported for other sedentary species (e.g. Vögeli et al. Citation2007; Cardoni et al. Citation2009; Herring et al. Citation2010; Henry et al. Citation2015) including other Lapwings (Wakisaka et al. Citation2006; Cruz-Bernate et al. Citation2013).
Statistical analysis
All data met the assumptions of the homogeneity of variance (Brown–Forsyth test, P > 0.101) and normality (Shapiro–Wilk test, P > 0.070), except for body mass in males (Shapiro–Wilk test, W = 0.93, P = 0.001). Nevertheless, we did not transform this variable because discriminant analysis robustly withstands homogeneity, especially for minor deviations (Lachenbruch Citation1975; Tabachnick & Fidell Citation1996).
A backward stepwise discriminant function analysis was performed to determine which set of variables best classified the sexes in our sample. In the first approach, we excluded spur length from the stepwise procedure because few birds in our sample had sharp, ivory-tipped spurs. We did include spur length in our second approach, because it was the most sexually dimorphic trait though this reduced our sample size. The inclusion of a morphometric into the model was based on the Wilk’s Lambda ratio, with a default minimum partial F to enter the model equal to F = 3.84 and maximum partial F to remove: 2.71 (Tacq Citation1997). We recognise criticisms of automated stepwise selection of variables (Whittingham et al. Citation2006; Mundry & Nunn Citation2009, but see: Dechaume-Moncharmont et al. Citation2011), so we have presented the full model except for the equation selected in the stepwise procedure and models that include combinations of measurements with the highest SDI value before comparing their effectiveness.
As in other studies (Bluso et al. Citation2006; Shealer & Cleary Citation2007; Gates et al. Citation2013; Meissner & Krupa Citation2016) prior probabilities were considered equal for both groups despite the slightly male-biased sex ratio in the sample. Discriminant equations presented in this paper are based on unstandardized canonical discriminant function coefficients, where D < 0 indicates a female and D > 0 indicates a male. We validated our discriminant functions with a jackknife procedure, as suggested by Dechaume-Moncharmont et al. (Citation2011), where each case except the instance being examined is classified using a discriminant function based on all the other cases (Miller Citation1964). To show the degree of overlap, biomorphic measurements of the sexes at 95% prediction intervals are presented, a range of values likely to contain the value of any single new observation given the settings of the predictors (Patel Citation1989). The statistical analyses were performed using Statistica 13.1 software (Dell Inc.) with an additional Statistica macro file for the jackknife procedure that was downloaded from http://sdn.statsoft.com.
Results
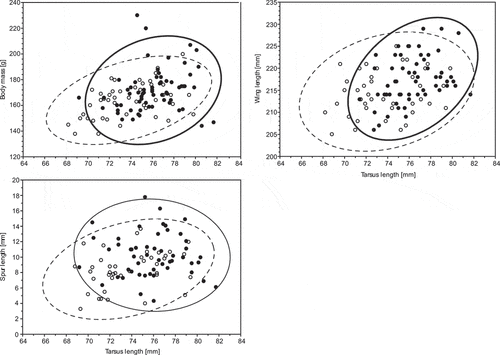
Males were larger than females in all measurements except bill length, which did not differ significantly between the sexes (). The most sexually dimorphic trait was spur length, followed by body mass, tarsus and tarsus-plus-toe length (). The tarsus length was highly correlated with the tarsus-plus-toe length (r = 0.81) because the second measurement contains the first. Hence only tarsus length, which also showed greater sexual dimorphism, was used in further analyses to avoid violating the multicollinearity assumption of independent variables in discriminant functions (Tabachnick & Fidell Citation1996). The two most dimorphic features, spur length and body weight, had much larger coefficients of variation than the other measurements (), which may affect their discriminatory power. Measurements overlapped extensively, so most birds were located in the overlapping zone of 95% prediction intervals, with similar proportions of males and females ().
Table I. Differences in mean linear measurements between male and female adult Blacksmith Lapwings, with the sexual dimorphism index (SDI) according to Lovich and Gibbons (Citation1992) expressed in percent and the coefficient of variation (CV)
Figure 1. Relationship of the most dimorphic traits in male (black dots) and female (white dots) Blacksmith Lapwings against the tarsus length. The ellipses show the 95% prediction intervals for a single observation, given the parameter estimates for the bivariate distribution computed from the data for males (solid line) and females (dashed line)
In the stepwise procedure only the tarsus length was selected, producing the equation D1 (). The full model with four measurements (D2) was only slightly more effective. The model including only spur length reduced the sample size to 28 males and 30 females but provided the equation (D5), which produced the lowest misclassification rate and correctly sexed 76% of the sample. Combining tarsus length with spur length did not improve the effectiveness of discriminant function (D6) (). Equations D3 and D4 had lower discriminant power than D5 and D6 (). Equation D1 is preferable because it contains only one measurement and has similar discriminant power as the equations with more measurements. The discriminant function using spur length alone (D5) provided the highest efficiency, but should be used with caution because of our small sample considering that the power of a discriminant analysis is lowered by small samples (Tabachnick & Fidell Citation1996). Moreover, only spurs with a sharp ivory tip should be considered Tree (Citation1999) which further limits the use of this equation because only 64% of Blacksmith Lapwings caught for this study had such a spur.
Table II. Equations for calculating discriminant scores. The percentage of birds correctly sexed is given from the jackknife procedure. TL – tarsus length, THL – total head length, WL – wing length, BM – body mass. SL – spur length
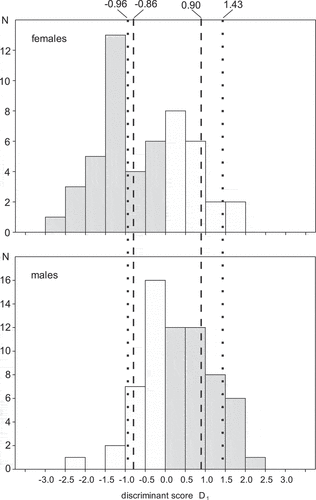
Using these equations to sex Blacksmith Lapwings produces a slightly male-biased sex ratio because more birds from the overlapping zone are classified as males. If birds with D1 < −0.96 were identified as females and those with D1 > 1.43 as males, only 5% of males and 5% of females would be misclassified (), but would leave 70% of the individuals unsexed. Allowing a misclassification of 10% would shift the cut-off values to −0.86 for females and 0.90 for males, but reduce the number of unsexed birds to 55% ().
Figure 2. Distribution of the discriminant score D1 calculated for females and males sexed molecularly. Grey bars – correct, white bars – incorrect classification. Dotted lines show D1 with cut-off values of −0.96 and 1.43; dashed lines indicate cut-off values of −0.86 and 0.90, which enabled correct classification for 95% of males and 90% of females
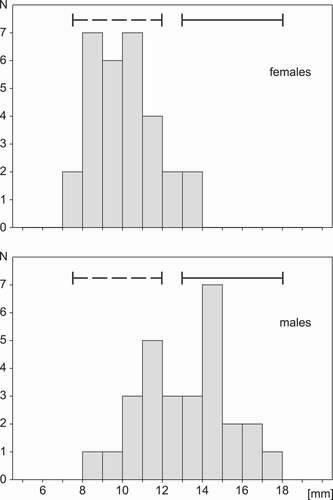
In the sample of molecularly sexed Blacksmith Lapwings only one female of 30 (i.e. 3%) had a spur length within the range for males given by Tree (Citation1999), whereas 10 of 28 males (36%) fell in the range of females (). Hence, though males on average have longer spurs than females, many males had spurs within the range typical of females, though all these males had ivory tips indicating adults. Moreover, 57% of females with an ivory spur tip also had a sharp tip, but all adult males had spurs with sharp ivory tips.
Figure 3. Distribution of the spur length in adult female and male Blacksmith Lapwings sexed molecularly, showing the range of spur lengths for females (dashed line) and males (solid line) given by Tree (Citation1999)
Discussion
The small sexual size dimorphism we found in most measurements of the Blacksmith Lapwing, except for a much longer spur in males than in females, may be explained in the context of sexual selection, considering the type of displays and competition between males in this territorial species. Wader size dimorphism is an effect of sexual selection that would affect the males’ mating success and the females’ fecundity. Some studies have suggested that in waders the magnitude of size dimorphism is related to the mating system: males are larger in polygynous species and females are larger in polyandrous species (Jehl & Murray Citation1986; Jönsson & Alerstam Citation1990). Moreover, in species with acrobatic aerial displays, selection favours smaller males with more rounded wings for agility and manoeuvrability, but this selection pressure does not apply to males that perform simple displays in the air or on the ground (Figuerola Citation1999; Székely et al. Citation2000). The Blacksmith Lapwing is socially monogamous and territorial (Turpie et al. Citation2005). Their courtship takes place mostly on the ground with some non-acrobatic aerial displays by both partners (Hall Citation1964). Male and female Blacksmith Lapwings share parental care throughout incubation and chick rearing (Hall Citation1964; Turpie et al. Citation2005). Therefore, the limited dimorphism we found in most morphometrics of this species may be related to the weak competition between the sexes and thus with weak intersexual selection. However, male Blacksmith Lapwings are generally slightly larger than females, as in other closely related species, which would favour them in physical competition with rivals. Male Blacksmith Lapwings are indeed more involved in aggressive behaviour and attack other bird species twice as often as females, which spend more time with the chicks (Hall Citation1964). Similar patterns have been found in other Lapwings (Ward Citation1989).
In both our measurements of the leg – tarsus and tarsus-plus-toe length – sexual dimorphism was more pronounced than in bill length. Blacksmith Lapwings mostly take invertebrate prey from hard or soft surfaces or from just below the surface of mud or water and only occasionally forage in shallow water (Hall Citation1964). A similar feeding pattern was found in the Southern Lapwing Vanellus chilensis (Cruz-Bernate et al. Citation2013) and in the Common Sandpiper Actitis hypoleucos (Meissner & Krupa Citation2016), which also take prey mainly from the surface and show little sexual dimorphism in bill length. Hence a lack of sexual size dimorphism in bill length would be expected in the Blacksmith Lapwing, as suggested by Székely et al. (Citation2000) for the whole suborder Charadrii, because there is no niche segregation between males and females. The significant sexual difference in the tarsus and tarsus-plus-toe length could be an effect of the sexes’ different roles in the scraping ceremony during courtship, which involves kicking sand from the emerging scrape (Turpie et al. Citation2005). In related African species, such as African Wattled Lapwing Vanellus senegallus, Kittlitz’s Plover Charadrius pecuarius and Three-banded Plover Charadrius tricollaris, the male usually prepares a scrape for the nest (Turpie et al. Citation2005). In Blacksmith Lapwings both sexes are involved in the scraping ceremony (Hall Citation1964), but Turpie et al. (Citation2005) suggest that the behaviour is mostly confined to the males.
Spur length showed the greatest sexual dimorphism, despite the high coefficient of variation, which was much higher than for any other trait (). Many (46%) of the males had sharp ivory-tipped spurs shorter than 13 mm, the lower limit for males given by Tree (Citation1999). The spur grows slowly up to the fourth year, especially in males, and the sharp tip assumes the ivory colour only during their third year of life (Tree Citation1999). The spur is an epidermal structure that may moult, causing periodic shortening, as Rand (Citation1954) observed in White-headed Lapwing Vanellus albiceps and in Blacksmith Lapwing. Though evidence is scanty, slow growth and moult may cause the short spurs we found in so many males and thus the wide variation of this measurement. Many male Blacksmith Lapwings in our study had spur lengths within the range given by Tree (Citation1999) for females. That suggests he may have treated individuals with short spurs as females, especially because he could not sex birds molecularly. The females’ spurs in our study fitted much better in the range of lengths for their sex provided by Tree (Citation1999). Wing spurs are used in intrasexual combat in some lapwings (Cramp & Simmons Citation1983; Wakisaka et al. Citation2006) and may therefore be subjected to intrasexual selection, as are leg spurs in many Galliform species (von Schantz et al. Citation1989; Stettenheim Citation2000), because their length is positively correlated with physical condition and dominance (Wittzell Citation1991; Mateos & Carranza Citation1996). Morphological features used in fights between males showed the greatest sexual dimorphism in the Common Crane Grus grus (Alonso et al. Citation2019), who suggested that the species’ male-biased sexual size dimorphism had evolved through intra-male competition. We thus suggest that the spur in the Blacksmith Lapwing reflects the quality of the male, hence the largest sexual dimorphism in this trait.
We recommend that Blacksmith Lapwings with sharp ivory-tipped spurs of at least 14 mm should be sexed as males. From our results the spur length of 13 mm proposed by Tree as the lowest value for sexing males would produce a misclassification of 7%. Birds with blunt ivory-tipped spurs may be sexed as females. However, considering the small sample of birds with fully developed spurs that we sexed molecularly, we recommend a cautious application of these criteria. The large overlap in morphometrics of the sexes limits the accuracy of the discriminant function derived in this study, as found in other Lapwing species (Wakisaka et al. Citation2006; Cruz-Bernate et al. Citation2013). Hence, only molecular analysis achieves accurate sexing of all individual Blacksmith Lapwings in any study. The discriminant function developed in this study may be an effective tool for sexing this species if individuals with a discriminant score from D1 between −0.86 and 0.90 are treated as unsexed, but from our study this would fail to sex 55% of the individuals. No geographical variation in size has been reported for the Blacksmith Plover (Turpie et al. Citation2005). Therefore our results likely apply to all populations that inhabit southern Africa, but we recommend using them with caution because only limited data is available on the biometrics of this species throughout its breeding range.
Authors’ contributions
Design and methodology: W.M., M.R., L.P. Data collection: W.M., M.R., L.P. Data analysis: W.M. Writing original draft: W.M., M.R. Writing review and editing: all authors.
Availability of data and material
The data analysed in this study are available from the corresponding author upon reasonable request.
Ethics approval
Bird ringing and data collection was conducted by experienced, qualified ringers with valid licences and the permission of Safring, the South African bird ringing authority. All the fieldwork was undertaken to the ethical standards recommended by Safring and the University of Gdańsk. Blood samples were collected with the required permits (NW Province DACERD Permit 000146 NW-09).
Geolocation information
South Africa.
Acknowledgements
We are grateful to the Animal Demography Unit, University of Cape Town and the team of the Barperspan Bird Sanctuary, especially to Sampie van der Merwe, Andrew Mvundle, Amos Koloti, Lebo Moeti and Sydwell Setuki, for their help in fieldwork over 2009–2018. Tadeusz Stawarczyk, Tadeusz Namiotko, Krzysztof Stępniewski, Katarzyna Stępniewska, Jarosław Nowakowski, Aleksandra Mazur, Justyna Szulc and Tomasz Maciąg assisted in fieldwork. Barry Taylor organised our fieldwork in KZN Province in 2009, and Henk Bouwman organised fieldwork in Potchefstroom (NW Province) in 2011. Izabela Fischer and Marta Witkowska helped with laboratory work. Joel Avni greatly helped in fieldwork, commented on and edited the manuscript.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Alonso JC, Bautista LM, Alonso JA. 2019. Sexual size dimorphism in the common crane, a monogamous, plumage-monomorphic bird. Ornis Fennica 96:194–204.
- BirdLife International. 2016. Vanellus armatus. The IUCN Red List of Threatened Species 2016:e.T22693978A93432296. Accessed Apr 2020 17. DOI: 10.2305/IUCN.UK.2016-3.RLTS.T22693978A93432296.en.
- Bluso JD, Ackerman JT, Takekawa JY, Yee JL. 2006. Sexing Forster’s terns using morphometric measurements. Waterbirds 29:512–517. DOI: 10.1675/1524-4695(2006)29[512:SFTUMM]2.0.CO;2.
- Busse P, Meissner W. 2015. Bird ringing station manual. Warsaw: De Gruyter Open Ltd.
- Byrkjedal I. 1985. Time-activity budget for breeding greater golden plovers Pluvialis apricaria in Norwegian mountains. Wilson Bulletin 97:486–501.
- Calf KM. 2002. Predation on a Kittlitz’s Plover chick by a Blacksmith Plover. Wader Study Group Bulletin 98:47.
- Cantlay JC, Portugal SJ, Martin GR. 2019. Visual fields and foraging ecology of Blacksmith Lapwings Vanellus armatus. Ibis 161:895–900. DOI: 10.1111/ibi.12725.
- Cardoni DA, Maldonado JE, Isacch JP, Greenberg R. 2009. Subtle sexual dimorphism in the bay-capped wren-spinetail (Spatonoica maluroides; Furnariidae) uncovered through molecular sex determination. Ornitologia Neotropical 20:347–355.
- Castro M, Masero JA, Pérez-Hurtado A, Amat JA, Megina C. 2009. Sex-related seasonal differences in the foraging strategy of the Kentish Plover. Condor 111:624–632. DOI: 10.1525/cond.2009.080062.
- Clark Jr GA. 1979. Body weights of birds: A review. Condor 81:193–202. DOI: 10.2307/1367288.
- Cramp S, Simmons KEL. 1983. Handbook of the birds of Europe the Middle East and North America, vol. 3, Waders to gulls. Oxford: Oxford University Press.
- Cruz-Bernate L, Riascos Y, Barreto G. 2013. Sexual size dimorphism and DNA sex determination in Southern Lapwing (Vanellus chilensis). Ornitologia Neotropical 24:433–444. (in Spanish with English summary).
- Dale AR, Robertson BC. 2006. DNA sexing of weka (Gallirallus australis). Notornis 53:385–386.
- Dechaume-Moncharmont FX, Monceau K, Cezilly F. 2011. Sexing birds using discriminant function analysis: A critical appraisal. Auk 128:78–86. DOI: 10.1525/auk.2011.10129.
- Dit Durell SEALV, Goss-Custard JD, Caldow RWG. 1993. Sex-related differences in diet and feeding method in the Oystercatcher Haematopus ostralegus. Journal of Animal Ecology 62:205–215. DOI: 10.2307/5495.
- Eberhart-Phillips LJ, Küpper C, Miller TEX, Cruz-López M, Maher KH, Dos Remedios N, Stoffel MA, Hoffman JI, Krüger O, Székely T. 2017. Sex-specific early survival drives adult sex ratio bias in snowy plovers and impacts mating system and population growth. Proceedings of the National Academy of Sciences of the United States of America 114:E5474–E5481. DOI: 10.1073/pnas.1620043114.
- Figuerola J. 1999. A comparative study on the evolution of reversed size dimorphism in monogamous waders. Biological Journal of the Linnean Society 67:1–18. DOI: 10.1111/j.1095-8312.1999.tb01926.x.
- Fridolfsson A-K, Ellegren H. 1999. A simple and universal method for molecular sexing of non-ratite birds. Journal of Avian Biology 20:116–121. DOI: 10.2307/3677252.
- Gates HR, Yezerinac S, Powell AN, Tomkovich PS, Valchuk OP, Lanctot RB. 2013. Differentiation of subspecies and sexes of Beringian Dunlin using morphometric measures. Journal of Field Ornithology 84:389–402. DOI: 10.1111/jofo.12038.
- Griffiths R, Double MC, Orr K, Dawson RJG. 1998. A DNA test to sex most birds. Molecular Ecology 7:1071–1075. DOI: 10.1046/j.1365-294x.1998.00389.x.
- Hall KRL. 1964. A study of the Blacksmith Plover Hoplopterus armatus in the Cape Town area: II. Behaviour. Ostrich 35:3–16. DOI: 10.1080/00306525.1964.9633828.
- Henry L, Biquand V, Craig AJFK, Hausberger M. 2015. Sexing adult Pale-Winged Starlings using morphometric and discriminant function analysis. PLoS ONE 10:e0135628. DOI: 10.1371/journal.pone.0135628.
- Herring G, Ackerman JT, Eagles-Smith CA, Takekawa JY. 2010. Sexing California Gulls using morphometrics and discriminant function analysis. Waterbirds 33:79–85. DOI: 10.1675/063.033.0109.
- Jehl Jr JR, Murray Jr. BG. 1986. The evolution of normal and reverse sexual size dimorphism in shorebirds and other birds. In: Johston R, editor, Current ornithology. Vol. 3. New York: Plenum Press, pp. 1–86.
- Jiménez A, García-Lau I, Gonzalez A, Acosta M, Mugica L. 2015. Sex determination of least Sandpiper (Calidris minutilla) and Western Sandpiper (Calidris mauri): Comparing methodological robustness of two morphometric methods. Waterbirds 38:10–18. DOI: 10.1675/063.038.0103.
- Jönsson PE, Alerstam T. 1990. The adaptive significance of parental role division and sexual size dimorphism in breeding shorebirds. Biological Journal of the Linnean Society 41:301–314. DOI: 10.1111/j.1095-8312.1990.tb00838.x.
- Kahn NW, St John J, Quinn TW. 1998. Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk 115:1074–1078. DOI: 10.2307/4089527.
- Lachenbruch PA. 1975. Discriminant analysis. New York: Hafner Press.
- Lovich E, Gibbons JW. 1992. A review of techniques for quantifying sexual size dimorphism. Growth, Development and Aging 56:269–281.
- Machín P, Remisiewicz M, Fernández-Elipe J, Jukema J, Klaassen R. 2018. Trade-offs of moulting and breeding at the same time: The case of the Eurasian Golden plover Pluvialis apricaria. Journal of Avian Biology 49:9, e01709. DOI: 10.1111/jav.01709.
- Marchant S, Higgins PJ, editors. 1993. Handbook of Australian, New Zealand & Antarctic Birds, vol 2, Raptors to lapwings. Melbourne: Oxford University Press.
- Mateos C, Carranza J. 1996. On the intersexual selection for spurs in the ring-necked pheasant. Behavioral Ecology 7:362–369. DOI: 10.1093/beheco/7.3.362.
- Mathot KJ, Elner RW. 2004. Evidence for sexual partitioning of foraging mode in Western Sandpipers (Calidris mauri) during migration. Canadian Journal of Zoology 82:1035–1042. DOI: 10.1139/z04-080.
- Meissner W. 2005. Sex determination of juvenile Dunlins migrating through the Polish Baltic region. Journal of Field Ornithology 76:368–372. DOI: 10.1648/0273-8570-76.4.368.
- Meissner W, Kośmicki A, Niemczyk A, Fischer I. 2017. Development of sexual dimorphism and sexing of Baltic herring gull Larus argentatus argentatus in successive age classes. Waterbirds 40:24–32. DOI: 10.1675/063.040.0104.
- Meissner W, Krupa R. 2016. Identifying the sex of the common sandpiper (Actitis hypoleucos) by linear measurements. Annales Zoologici Fennici 53:175–182. DOI: 10.5735/086.053.0406.
- Meissner W, Krupa R. 2017. Sex-related differences in autumn migration timing of adult common sandpipers Actitis hypoleucos (Linnaeus, 1758) (Charadriiformes: Scolopacidae). The European Zoological Journal 84:136–140. DOI: 10.1080/11250003.2016.1278474.
- Meissner W, Pilacka L. 2008. Sex identification of adult Dunlins Calidris alpina alpina migrating in autumn through Baltic region. Ornis Fennica 85:135–138.
- Meissner W, Remisiewicz M, Pilacka L. 2011. Investigations of sexual dimorphism in live Kittlitz’s Plover Charadrius pecuarius from inland South Africa. Ostrich 82:135–139. DOI: 10.2989/00306525.2011.603470.
- Miller RG. 1964. A trustworthy jackknife. The Annals of Mathematical Statistics 35:1594–1605. DOI: 10.1214/aoms/1177700384.
- Mundry R, Nunn CL. 2009. Stepwise model fitting and statistical inference: Turning noise into signal pollution. The American Naturalist 173:119–123. DOI: 10.1086/593303.
- Nebel S. 2005. Latitudinal clines in bill length and sex ratio in a migratory shorebird: A case of resource partitioning? Acta Oecologica 28:33–38. DOI: 10.1016/j.actao.2005.02.002.
- Newton I. 1998. Population limitation in birds. London: Academic Press. DOI:10.1016/s0006-3207(99)00105-6.
- Niemc A, Remisiewicz M, Avni J, Underhill LG. 2018. Sexual dimorphism in adult Little Stints (Calidris minuta) revealed by DNA sexing and discriminant analysis. PeerJ 6:e5367. DOI: 10.7717/peerj.5367.
- Patel JK. 1989. Prediction intervals - a review. Communications in Statistics - Theory and Methods 18:2393–2465. DOI: 10.1080/03610926.2018.1563181.
- Piersma T, Wiersma P. 1996. Family Charadriidae (Plovers). In: Del Hoyo J, Elliott A, Sargatal J, editors. Handbook of the birds of the world, vol. 3, Hoatzin to Auks. Barcelona: Lynx Edicions. pp. 384–442.
- Pinchuk P, Karlionova N, Meissner W. 2016. Biometry indicates sexual differences in spring migration strategy in Ringed Plovers Charadrius hiaticula tundrae captured in the southern Belarus. North-Western Journal of Zoology 12:319–324.
- Poisbleau M, Demongin L, van Noordwijk HJ, Strange IJ, Quillfeldt P. 2010. Sexual dimorphism and use of morphological measurements to sex adults, immatures and chicks of rockhopper penguins. Ardea 98:217–224. DOI: 10.5253/078.098.0212.
- Rand AL. 1954. On the spurs on birds’ wings. Wilson Bulletin 66:127–134.
- Remisiewicz M, Wennerberg L. 2006. Differential migration strategies of the Wood Sandpiper (Tringa glareola) – Genetic analyses reveal sex differences in morphology and spring migration phenology. Ornis Fennica 83:1–10.
- Scherer AL, Scherer JFM, Petry MV, Valiati VH. 2014. Sexual dimorphism and body condition of wintering White-rumped Sandpipers in southern Brazil. Wilson Journal of Ornithology 126:553–561. DOI: 10.1676/13-121.1.
- Scott I, Mitchell PI, Evans PR. 1994. Seasonal changes in body mass, body composition and food requirements in wild migratory birds. Proceedings of the Nutrition Society 53:521–531. DOI: 10.1079/pns19940062.
- Shealer DA, Cleary CM. 2007. Sex determination of adult Black Terns by DNA and morphometrics: Tests of sample size, temporal stability and geographic specificity in the classification accuracy of discriminant function models. Waterbirds 30:180–188. DOI: 10.1675/1524-4695(2007)30[180:sdoabt]2.0.co;2.
- Stettenheim PR. 2000. The integumentary morphology of modern birds—an overview. American Zooologist 40:461–477. DOI: 10.1093/icb/40.4.461.
- Sweeney JJ, Tatner P. 1996. Sexing Wrens Troglodytes troglodytes indigenus using morphological measurements and discriminant analysis. Bird Study 43:342–350. DOI: 10.1080/00063659609461027.
- Székely T, Reynolds JD, Figuerola J. 2000. Sexual size dimorphism in shorebirds, gulls and alcids: The influence of sexual and natural selection. Evolution 54:1404–1413. DOI: 10.1111/j.0014-3820.2000.tb00572.x.
- Tabachnick BG, Fidell LS. 1996. Using multivariate statistics. New York: Harper Collins College Publishers.
- Tacq J. 1997. Multivariate analysis techniques in social science research: From problem to analysis. Thousand Oaks: SAGE Publications Inc.
- Tjørve KMC, Underhill LG, Visser GH. 2008. The energetic implications of precocial development for three shorebird species breeding in a warm environment. Ibis 150:125–138. DOI: 10.1111/j.1474-919x.2007.00752.x.
- Tree AJ. 1998. Movements of Blacksmith Plover in south-central Africa. Honeyguide 44:199–203.
- Tree AJ. 1999. Ageing and sexing the Blacksmith Plover in the hand. Safring News 28:27–28.
- Turpie JK, Ryan PG, Tree AJ. 2005. Blacksmith Lapwing. In: Hockey PAR, Dean WRJ, Ryan PG, editors. Roberts birds of Southern Africa. 7th ed. Cape Town: Trustees of the John Voelcker Bird Book Fund. pp. 408–410.
- Urban EK, Fry CH, Keith S. 1986. The birds of Africa. Vol. 2. London: Academic Press. DOI: 10.5040/9781472926999.
- Vögeli M, Serrano D, Tella JL, Méndez M, Godoy JA. 2007. Sex determination of Dupont’s Lark Chersophilus duponti using molecular sexing and discriminant functions. Ardeola 54:69–79.
- von Schantz T, Göransson G, Andersson G, Fröberg I, Grahn M, Helgée A, Wittzell H. 1989. Female choice selects for a viability-based male trait in pheasants. Nature 337:166–169. DOI: 10.1038/337166a0.
- Wakisaka H, Nakagawa M, Wakisaka K, Itoh M. 2006. Molecular sexing and sexual difference in carpal spur length of the Gray-headed Lapwing Vanellus cinereus (Charadriidae). Ornithological Science 5:133–137. DOI: 10.2326/osj.5.133.
- Walters J. 1984. The onset of primary moult in breeding Charadrius plovers. Bird Study 31:43–48. DOI: 10.1080/00063658409476814.
- Ward D. 1989. Behaviour associated with breeding of Crowned, Black-winged and Lesser Black-winged Plovers. Ostrich 60:141–150. DOI: 10.1080/00306525.1989.9633746.
- Whittingham MJ, Percival SM, Brown AF. 2000. Time budgets and foraging of breeding golden plover Pluvialis apricaria. Journal of Applied Ecology 37:632–646. DOI: 10.1046/j.1365-2664.2000.00519.x.
- Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP. 2006. Why do we still use stepwise modelling in ecology and behaviour? Journal of Animal Ecology 75:1182–1189. DOI: 10.1111/j.1365-2656.2006.01141.x.
- Witkowska M, Meissner W. 2020. Sexual dimorphism in size and plumage in adult Curlew Sandpipers (Calidris ferruginea) migrating in autumn through the Baltic Sea region. Ornis Fennica 97:186–199.
- Wittzell H. 1991. Directional selection on morphology in the pheasant, Phasianus colchicus. Oikos 61:394–400. DOI: 10.2307/3545247.
- Zefania S, Emilienne R, Faria PJ, Bruford MW, Long PR, Székely T. 2010. Cryptic sexual size dimorphism in Malagasy plovers Charadrius spp. Ostrich 81:173–178. DOI: 10.2989/00306525.2010.519909.
