Abstract
Feeding habitats and behavior are connected with various environmental conditions and anatomical structures of the hippocampus, altogether with proteins expression level that may enhance their cognitive memory abilities. The current study investigated the cytoarchitecture anatomical variations, the differences in the hippocampus volume, and the distribution of the proteoglycan agrin and ryanodine receptor protein expression; in the brain hippocampus complex of three avian species (the hooded crow (Corvus cornix, food caching), the cattle egret (Bubulcus ibis, non-food caching), and the rock dove pigeon (Columba livia, food caching). The histological results demonstrated that the anatomical brain structure and cytoarchitecture did not reveal specific characterizing features expect for the hooded crow with large-sized soma. Immunohistochemical staining of hippocampus complex for agrin proteoglycans and ryanodine receptor calcium channels proteins showed a significant increase among the hippocampus of Corvus cornix brain in the medial hippocampus (HCm) and lateral hippocampus (HCl) with value P < 0.001, respectively. The highly expressed agrin may play a major role in constructing the neuronal plasticity and activity of neurons. Moreover, there were significant difference in the concerning hippocampus volume but neurons count did not appeared with for the storing and non-storing behavior. Therefore, the present study revealed a significant difference between storing and non-storing species in the expression of proteoglycan and ryanodine receptor calcium channels proteins. Comprehensively, the hippocampal complex cytoarchitecture altogether with proteoglycan and ryanodine receptor calcium channel proteins are important to birds for fulfilling their cognitive needs such as food caching.
1. Introduction
Food production among animals confronting many problems that depending on feeding behavior and intense struggling for finding out their food stores, due to a wide range of environmental and seasonal factors. So, various behavioral tactics such as migration and hibernation have been developed or evolved by animals to defend these conditions and facilitate their ability to finding out new food-caching territories (Chancellor et al. Citation2011; Boussard et al. Citation2020).
Additionally, the hippocampus is considered one of the main important anatomical structures of the forebrain responsible for learning and memory (Brodin & Lundborg Citation2003). In birds, the hippocampus proper and the adjacent parahippocampus (APH) are frequently treated together as the hippocampal complex has large size among food-caching species in comparison to non-food caching species (Brodin & Lundborg Citation2003). Interestingly, birds characterized by their ability to memorize their food hoarding locations such as corvidae family (crows, jays, nutcrackers, magpies) and the paridae family (tits, titmice, and chickadees) identified by their specific large size and developed neuronal organization with long life survival rate (Huston et al. Citation2000; Brodin & Lundborg Citation2003).
Furthermore, agrin proteoglycans are important cell surface glycoproteins that improve axonal outgrowth modulating synaptogenesis and their expression in the brain is in high amounts during the period of synaptogenesis enhances synaptic plasticity. Noteworthy, Neves et al. (Citation2008) validated their hypothesis concerning the synaptic plasticity importance is necessary and sufficient for information storage in the rat brain hippocampus.
Besides, the neuron activity and survival rate depending upon the homeostatic activity of intracellular calcium (Ca2+) release that is carried out through ryanodine receptors expression in the brain (RyRs) and inositol (1.4.5)-trisphosphate (IP3Rs). In the brain, there are three RyR isoforms. Furthermore, RyR1 and RyR2 are important receptors expressed in the hippocampus with important functions for brain memory and long-term synaptic plasticity (Zhao et al. Citation2000).
Ryanodine receptors (RyRs) are enormous tetrameric channel proteins that discharge Ca2+ from intracellular stores inside the endoplasmic reticulum (ER) (Verkhratsky Citation2005). Three RyR isoforms are communicated in the mammalian brain (RyR1, RyR2, and RyR3) (Hertle & Yeckel Citation2007); while, the avian brain express two RyR isoforms (RyR1α-RyR and RyR2/3 β-RyR) (Baker et al. Citation2013). Moreover, their regulation in the hippocampus amplify calcium signals that trigger the postsynaptic pathways and per se synaptic plasticity, learning, and memory (Han et al. Citation2020).
So, the present study aimed to study the cytoarchitecture anatomical, hippocampus complex area, neurons count differences; in relation to the distribution of the proteoglycan agrin and ryanodine receptor protein expression in the brain hippocampus complex of three avian species hooded crow Corvus cornix, the cattle egret Bubulcus ibis, and the rock dove Columba livia and their role in the brain activity in food caching and retaining memorizing hoarding food.
2. Material and methods
2.1. Ethical statement
All experiments were conducted according to the Directive of the European Union 2010/63/UE and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Committee on the Ethics of Animal Experiments of the Suez Canal University, Egypt (Permit Number: 2,020,040). All surgeries were made to minimize animal suffering: animals were anesthetized and then sacrificed by cervical dislocation. Moreover, all bird species under study were not an endangered or rare species and did not require any special permission.
The animal model was selected according to different criteria: 1) all birds under the study have the ability to maintain their life in the same ecological urban and rural habitats; 2) their diets and their behavioral characters concerning food hoarding and caching (food and non-food storing). This experimental model included five individuals per bird species. Animal species involved in this study were the hooded crow (Corvus cornix, omnivorous food cacher), the rock dove pigeon (Columba livia, herbivorous food cacher), the cattle egret (Bubulcus ibis, carnivorous non-food cacher). All three species were captured and caged in order to be transported to Suez University alive during summer from rural habitats that happen around Al- Fayoum province, to ensure their wilderness nature Latitude: 29° 18ʹ 35.82” N Longitude: 30° 50ʹ 30.48” E.
The overall volume of the hippocampus and entire telencephalon (Krebs et al. Citation1989) were measured to determine the surface area of each section. Volumes were calculated by multiplying the surface area by the distance between the center planes of the measured sections.
2.2. Histological examination of the hippocampus complex anatomical structure
2.2.1. Histological and histochemical studies
Animals were anesthetized with intraperitoneal injection with 80 mg/kg sodium pentobarbital and followed by transcardial perfusion using normal saline and 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). After perfusion, brains were removed and subjected to tissue fixation and paraffin blocks preparation. The brains were removed from the skull and, dehydrated in an ascending alcohol series (50%, 70%, 90%, 100% ethanol), finally cleared in xylene, and embedded in paraffin wax. Coronal serial sections (5 μm thickness) were cut with a rotatory microtome. Sections were mounted on glass slides, deparaffinized in xylene, dipped in a descending alcohol series (absolute, 90%, 70%, 50%, 30%), and then stained with hematoxylin and eosin, and toluidine blue stain according to the protocol of Lynch et al. (Citation1969). Additionally, the sections were dehydrated in an ascending alcohol series, cleared in xylene, and finally mounted on slides using DPX mounting medium.
2.2.2. Immunohistochemistry
Sections were mounted on positive charge glass slides sequentially treated with 0.3% H2O2 in PBS for 30 min and 10% goat serum in PBST for 30 min. The sections were then incubated with mouse anti-Ryanodine receptors and Mouse anti-agrin (2–5 ug/ml, Developmental Studies Hybridoma Bank DSHB, the University of Iowa, USA) overnight at room temperature and subsequently exposed to biotinylated goat anti-mouse IgG (1:200) and streptavidin peroxidase complex (1:200).
3. RNA extraction and cDNA synthesis
The total RNA extracted from brain homogenate with TRIzol Reagent following the standard manufacturer’s protocol and assessed using 1.0% agarose gel for electrophoresis, the RNA concentration and purity were determined using spectrophotometer at 260/230 and 260/280 absorbance ratios. First strand of cDNA (GE27926101, Sigma)) was synthesized using 1 mg/ml of purified RNA. The produced cDNA strand was used to form target gene and analyzing their expression. Primers used for the amplification for mt cyt b gene:L14827:CCACACTCCACACAGGCCTAATTAA; H16065, GGAGTCTTCAGTCTCTGGTTTACAAGAC-3′ (Helm-Bychowski & Cracraft Citation1993). PCR products used to amplify the target specific gene and standardized against β-actin and quantified using GelQuantNET software to calculate its gene expression.
3.1. Quantification and statistical analysis
Serial coronal sections of brain tissue were examined. All quantification was performed with the Image J software, processed in Prism 7 software (GraphPad, San Diego, CA, United States). Statistical analysis of densitometry proteins expression was carried out using t’ student test of SPSS statistics software (Version 16.0). All data were presented as means ± SE standard errors for each protein distribution in the hippocampus of the bird’s brain. For all analyses P-value < 0.05 was considered to be statistically significant. Species differences for relative hippocampus volume were analyzed using with a one-way ANOVA on log telencephalon.
4. Results
The lateral hippocampus (HCl) of the hooded crow Corvus cornix was appeared to be occupied with a large number of glial cells that found and distributed in the mainline, and scattered in clusters parallel to ventricles separating the lateral and ventral regions of the hippocampus and ventral hippocampus (). Additionally, well-developed neurons were found to be distributed in a few numbers in a scattered manner as shown in . The medial hippocampus (HCm) was mainly occupied with a large number of large-sized neurons. These neurons are differentiated into mono and bi-tufted shapes with several types like multipolar neurons, pyramidal-like neurons, and pyramidal neurons. ().
Figure 1. Photomicrograph of the hippocampal complex of Corvus cornix stained by hematoxylin and eosin stain showing the distribution of neurons throughout the hippocampal complex as glial cells are distributed laterally, while multipolar neurons are found medially (a) rostral, (b), (c) lateral hippocampus, (c) medial hippocampus. Bar: A = 200 µm, B = 100 µm, C-D = 50 µm
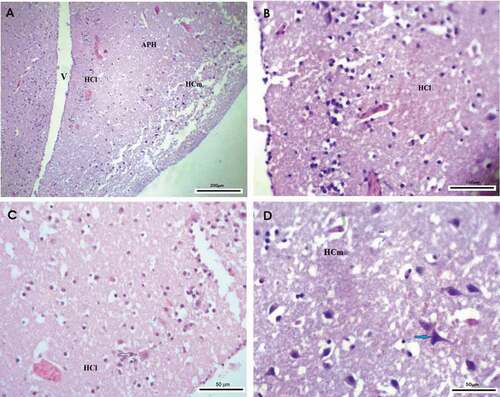
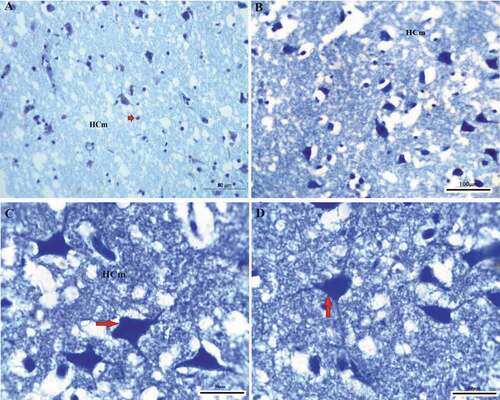
Figure 2. Photomicrograph of the hippocampal complex from of Corvus cornix stained by toluidine blue stain showing the shape of neurons distributed in different positions throughout the medial hippocampus as where large multipolar neurons (red arrows) are found associated with pyramidal, and pyramidal-like neurons (a), (b), (c), (d) medial hippocampus. Bar: A-C-D = 50 µm, B = 100 µm
Cattle egret (Bubulcus ibis) medial hippocampus (HCm) showed somewhat similar anatomical structure to that of pigeon in the overall organization, with very little difference in the distribution of lower numbers and small-sized neurons in the hippocampus compared to that of crow as shown in . But the HCm in cattle egret was organized in the same manner of that of pigeon HCm, their neurons distributed inline form shape starting from the rostral area of the hippocampus, with different well-developed neurons differentiated into multipolar, pyramidal-like, and pyramidal neurons. Their lateral hippocampus (HCl) contains less number of neurons that were randomly scattered within small size () & ). The glial cells were randomly distributed in the whole hippocampus complex area.
Figure 3. Photomicrograph of the hippocampal complex of Bubulcus ibis stained by hematoxylin and eosin stain which shows an overall organization of neurons similar to that of the Columba livia as all types of neurons is present (a) rostral, (b) intermediate position of the hippocampus, (c), (d) lateral hippocampus. Bar: A = 200 µm, B-C-D = 50 µm
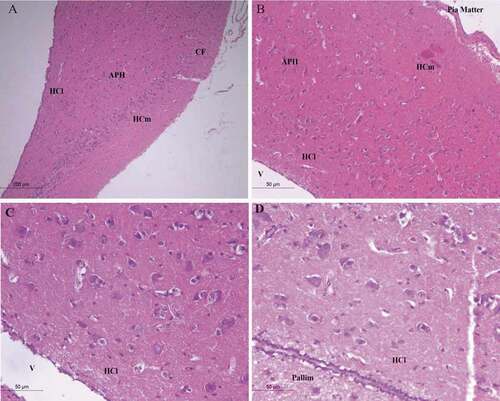
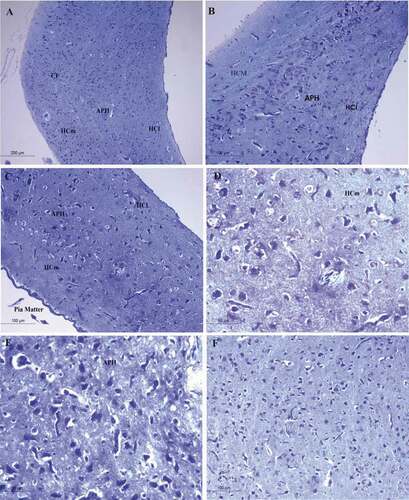
Figure 4. Photomicrograph of the hippocampal complex of Bublucus ibis stained by toluidine blue stain showing different shapes of neurons like multipolar neurons, pyramidal, pyramidal-like neurons in the hippocampus with large dendritic connectivity (a), (c) Intermediate positions of the hippocampus, (b) rostral hippocampus, (d), (f) medial hippocampus, (e) Parahippocampus. Bar: A = 200 µm, B-C-F = 100 µm, D-E = 50 µm. HCl: lateral hippocampus; APH: parahippocampus; CF: area of the crescent-shaped field; HCm: medial hippocampus; V: ventricle
The medial hippocampus (HCm) of pigeon (Columba livia) was characterized by distributed neurons with a long line shape parallel to the cortical line from the rostral area and running parallel to the frontal cortex to the lateral cortex ()). The glial cells were presented but in lower numbers in pigeon hippocampus and they were found randomly scattered clusters ().This line consisted of well-developed neurons and differentiated into a large number of multipolar neurons, pyramidal, and pyramidal-like neurons. Moreover, the lateral hippocampus (HCl) showed that the neurons uniformity disappears and they were scattered throughout the hippocampal complex randomly and all types of neurons pyramidal, pyramidal-like, and multipolar neurons .
Figure 5. Photomicrograph of the hippocampal complex of Columba livia stained by hematoxylin and eosin showing the organization and distribution in all of the hippocampus as all types of neurons like multipolar, pyramidal, pyramidal-like, and other types of neurons were found distributed (a) lateral hippocampus, (b) rostral hippocampus, (c), (d) medial hippocampus. Bar: A = 100 µm, B = 200 µm, C-D = 50 µm
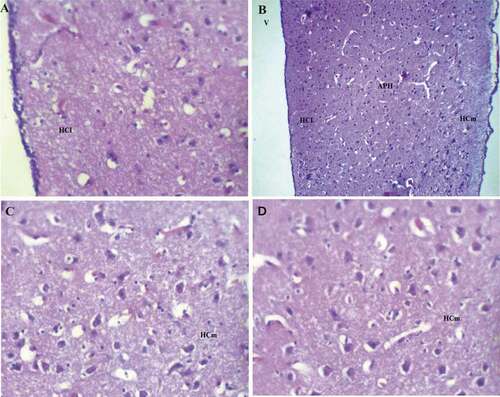
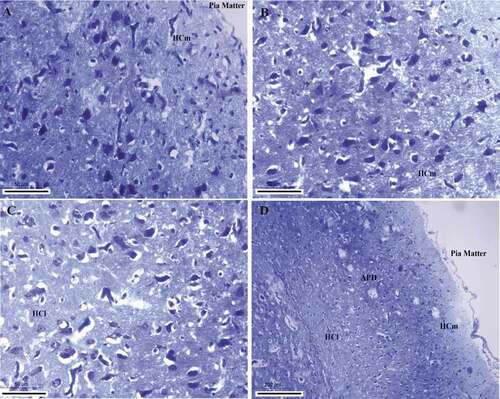
Figure 6. Photomicrograph of the hippocampal complex of Columba livia stained by toluidine blue stain showing the different structures and their dendritic connections throughout the hippocampal complex (a), (b) medial hippocampus, (c) lateral hippocampus, (d) intermediate hippocampal position. Bar: A-B-C = 50 µm, D = 200 µm
5. Agrin proteoglycan and ryanodine receptors immune-reactivity
The studied species showed specific anatomical variations in the expression and immune-reactivity with agrin proteoglycan and ryanodine receptor protein in the different areas of the hippocampus.
On one side, the agrin proteoglycan showed high distribution and high immunoreaction expression among Corvus cornix hippocampus complex area with a high level in the medial hippocampus (HCm) followed by scattered immunoreaction in the lateral hippocampus (HCl) and very little expression in the parahippocampus area (APH). While agrin proteoglycan revealed low immunoreaction and expression in the pigeon hippocampus followed by the cattle egret with only distribution in the medial hippocampus area (HCm) for both species ().
Figure 7. Photomicrograph of Corvus cornix hippocampus stained by 6D2 antibody showing the expression of proteoglycan protein (a) rostral, (b) medial hippocampus. Bar: A-B = 100 µm
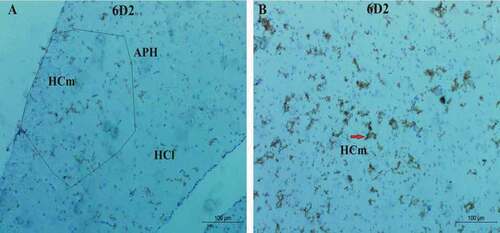
Figure 8. Photomicrograph of Corvus cornix hippocampus immunoreactivity stain showing the expressional pattern of ryanodine receptor (a, b) is the lateral hippocampus (HCl). Bar: A-B = 100 µm
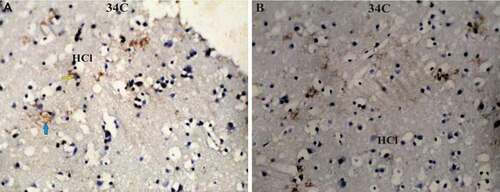
Figure 9. Photomicrograph of Cattle egret (Bublucus ibis) hippocampus immunoreaction stain (a) reveals the low reaction with agrin proteoglycan in the medial hippocampus (HCm); (b) the density reaction level of the ryanodine receptor. Bar: A-B = 100 µm
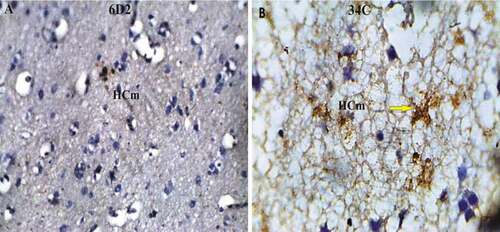
Figure 10. Photomicrograph of Pigeon (Columba livia) medial hippocampus immunoreaction; (a) showed the agrin proteoglycan expression; (b) reveal the ryanodine receptor expression. Bar: A-B = 20 µm
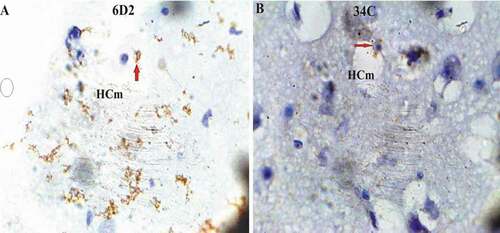
On the other side, ryanodine receptor protein revealed high expression level in only the lateral hippocampus area (HCl) of hooded crow (Corvus cornix), followed by cattle egret and pigeon in the medial hippocampus area (HCm) ( & )
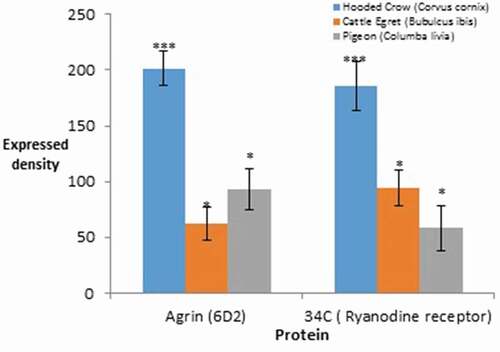
The expressional level using densitometry quantitative analysis of agrin proteoglycan and ryanodine receptor proteins revealed highly significant expression with a value P < 0.001 in the brain hippocampus of Corvus cornix more than other species; while agrin and ryanodine receptor protein showed an interchangeable increase between both cattle egret and pigeon with low significant value of P < 0.05 ().
Figure 11. The densitometry quantitative analysis of Agrin proteoglycan and ryanodine receptor proteins in the hippocampus. Error bars represent ±S.E, *p < 0.05, **p < 0.01, ***P < 0.001 Student’s t-test
The overall number of neurons distributed in the hippocampus showed significant value of difference among the three species. The lowest number of neurons was appeared in the Corvus cornix with significant difference from other two species with P < 0.01, neurons showed distribution area of 2.953% of the total hippocampal complex region ()). While, there was no significant difference of neuron counts among pigeon (Columba livia) and cattle egret (Bubulcus ibis). The highest number of neurons with lowest occupied area was in the brain of Cattle egret Bubulcus ibis with occupation area of 1.953% from the total hippocampus complex area that appeared their distribution with concentrated manner.
Figure 12. The variation differences among hippocampus of the three species; (a), the neuronal count, (b), the Hp/telencephalon column, C, COI gene expression
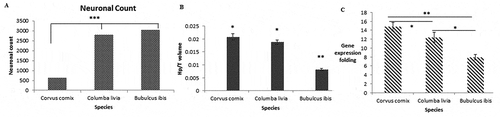
Additionally, the overall volume of hippocampus in relation to telencephalon of hooded crow Corvus cornix was significant higher than pigeon Columba livia with significant value with P < 0.05; while both species were significantly higher than those of Cattle egret Bubulcus ibis p < 0.01 as shown in . The COI gene expression level of isolated hippocampus of the three species revealed increased expression among Corvus cornix with a value higher than both pigeon Columba livia and Bubulcus ibis with a value of P < 0.05 and P < 0.01, respectively as shown in ).
6. Discussion
Food-storing birds recover their food caches with memory abilities for spatial locations with specific cognitive mechanisms that may depend upon the chance for the particular kinds of cache sites (Sherry & Vaccarino Citation1989). So, the main anatomical structure and their expressed proteins in the bird’s brain that is responsible for memorization of the stored food locations and is still points of mystery.
The present study showed that the medial hippocampus area (HCm) of the hooded crow Corvus cornix showed different types of multipolar neurons and mono and bi-tufted neural cells with various distribution rates more than in the medial hippocampus area of each of pigeon Columba livia and cattle egret Bubulcus ibis. On the contrary, Atoji et al. (Citation2002) showed that the cytoarchitectural anatomical shape of hippocampus neurons of Corvus splendens and the common pigeon Columba livia have characterized by their widely distributed rate of the multipolar neurons controlling the ability of food storing behavior that followed by memorizing the different stored locals. Additionally, the present study showed that Corvus cornix hippocampus has various shapes and structures of neuron types that may be considered as one of the differentiation factors that may govern the activity in their ability to impose the food-storing behavior. In agreement with Singh et al. (Citation2014) who stated the presence of varied subtypes of neuronal classes within the food-storing Corvus splendens and proposed it could be a specialization over nonfood-storing birds. The neuronal soma is believed to be governed or controlled by the biosynthetic and metabolic requirements of a cell, including the dendritic field and axons (Kaas Citation2000).
Furthermore, the hippocampus volume size among the food storing species Corvus cornix and Columba livia revealed significant difference from the non-storing species Bubulcus ibis. Therefore, the present study showed that the hippocampus volume relative to the telencephalon volume may be considered vital anatomical structure for birds with food caching and storing behavior. On one side, our study was consistent with Krebs et al. (Citation1989) who revealed that there is interspecific relation between hippocampus volume and food hoarding behavior that may face various restriction factors for executing their behavior (Hampton et al. Citation1995On the other side, in opposite. Brodin and Lundborg (Citation2003) reported that no significant interspecific relationship between food hoarding and the relative volume of the hippocampus area.
Garamszegi and Eens (Citation2004) showed that the relative size of the overall brain was positively and significantly related to food hoarding among birds did not affected by the captivity behavior.
Therefore, food storing, caching, and hoarding may be considered as important factors that trigger the evolutionary mechanisms for increasing the hippocampus size and all other large size brain areas. Gould et al. (Citation2001) revealed that food storing birds like black capped chickadees (Parus atricapillus) has significantly large relative hippocampus volume than blue tit (Parus caeruleus) and great tit (Parus major) as to the non-storing dark-eyed junco (Junco hyemalis). Additionally, the overall distribution of the neuropeptide Y cells immune-reactivity and fibers in the songbird species with different food storing behavior in consistent with that found in pigeon with clear demonstrating conservative distribution among avian brain and no species differences in NPY cells.
In contrast, Boussard et al. (Citation2020) stated that variation in brain size and neuron number is important for variation in learning performance and memory, but these differences are not great enough to cause the larger differences in efficient learning strategies observed at higher taxonomic levels.
Noticeably, the well-distributed neurons with obvious big-soma diameters among the HCm of Corvus cornix were more than that of the common pigeon (Columba livia) and cattle egret (Bubulcus ibis). Srivastava et al. (Citation2007) reported that the soma size may be an effective anatomical architecture that is important for birds with food storing behavior according to their anatomical results among Corvus splendens (food-storing) and Taeniopygia guttata (Montagnese et al. Citation1996) and Estrilda amandava (Srivastava et al. Citation2007) (both being nonfood-storing in nature) that the greater soma size in Corvus splendens suggests the intense or more metabolic requirements of neurons in Corvus splendens for larger dendritic and axonal projection and in turn extended neuronal networking. Similarly, a higher spine density of pyramidal neurons of the APH region contributes to better functionality of the the parahippocampus area (APH) region and can be correlated with better cognition and memory (Elston Citation2003).
Furthermore, Corvus cornix medial hippocampus area showed an increased number of mono and bi-tufted neurons more than other species may exert specific food-storing and caching abilities with increased neuronal activity spiking. Srivastava et al. (Citation2011) revealed that the spine of dendrites numbers density of neurons in the hippocampus of food-storing birds is greater than those of nonfood-storing bird species; therefore, the hippocampal area was reported to be associated with performance and use of spatial memory, higher spine density in Corvus splendens might help Corvus splendens in acquiring enhanced spatial memory, thereby helping in food-cache retrieval.
The present study accessed the activity of the cell surface heparin sulfate proteoglycan agrin protein expression and their role in the activity of the birds in the process of food storing and per se food caching. Corvus cornix revealed varietal levels of expression for the protein in the area HCm, APH, and HCl with a localized increased level in the HCm according to Erich et al. (Citation2005) nomenclature. Moreover, the total expression level in the hippocampus was significantly greater than both species pigeon and cattle egret.
The predominant form of agrin expressed by neurons in the brain is a type II transmembrane proteoglycan (Neumann et al. Citation2001). Additionally, there is strong evidence that agrin regulates formation or stabilization of a subclass of excitatory synapses in the murine brain, and that also regulates the excitatory synaptic activity and synaptic plasticity in areas including the hippocampus and cortex. Besides the agrin density and regional distribution in the different anatomical areas of the hippocampus of hooded crow Corvus cornix, common pigeon Columba livia, and cattle egret Bubulcus ibis, we studied the ryanodine receptors expression and regional distribution density as one of the most important factors that increase the release level of the intracellular calcium Ca2+ and the memory behavior.
The present study revealed that ryanodine receptors have shown highly significant expression in the HCl in the brain of Corvus cornix followed by Bubulcus ibis and Columa livia in the HCm. The high expressed density of calcium (Ca2+) release receptor imposes cellular functions in the brain cognition and behavior through their activity in increased synaptic processes thought to underlie both learning and memory (Eckel-Mahan et al. Citation2008). So, this may be one of the triggering factors that enhanced Corvus cornix food storing and caching behavior. In the chick brain, RyRs are found in the hippocampus and the mesopallium, although expressed at a lower level than in the mammalian brain (Ouyang et al. Citation1993). Moreover, the crosslinking between agrin and calcium expression appears in the elevation of the ryanodine receptors which increase the intracellular (Ca2+) and agrin protein expression in the Corvus cornix brain. The latter effect of agrin was triggered by an elevation of intracellular (Ca2+) but also required Ca2+ entry through voltage-sensitive Ca2+ channels (Hilgenberg et al. Citation2006).
The highly distributed agrin proteoglycan and ryanodine receptors may be responsible for the release of the intracellular Ca2+ in the hippocampus of the Corvus cornix as one of the most common birds with food storing and caching behavior. Grodzinski and Clayton (Citation2010) stated that food-storing corvids face many challenges in hiding and retrieving their caches and in protecting their caches from competitors and pilfering others’ caches themselves. Interestingly, the close difference of the forebrain/brain stem ratio among corvids was compared to other avian species and similar to the difference of neocortex: brainstem ratio between the great apes and other primates and insectivores (Clayton & Dickinson Citation1999)
7. Conclusion
Based on the present research results it can be concluded that the anatomical brain structure and cytoarchitecture with the regional distribution of different specific proteins may shed light on the specific brain bird’s activity. Being, food caching and hoarding is considered one of the most complex cognitive memory behaviors characterizing animals and birds in specific, the brain cytoarchitecture did not reveal specific characterizing structures but showed significant differences in the hippocampus volume among food storing and non-storing species. Birds were triggering these mechanisms depending upon their environmental needs and feeding habitats. The present study revealed the importance of the expressed proteins such as agrin proteoglycan and ryanodine receptor proteins specific regulatory mechanisms to enhance the brain learning actions for food caching and memorizing their various localities.
Therefore, the question that should be asked, what are the triggering factors that lead corvids to be considered as one of the most important species with high learning, memory, and intelligence?; are these behavioral characters are generated through innate memory behavior and complemented with the acquired learning behaviors?.
Author contributions
All authors have contributed equally for the production of the present research paper: Associate professor Hafez with Mr. Hamza have carried out the practical work from animal brain extraction to data acquisition, analysis, and writing the manuscript draft. Prof. Mobarak and Prof. Hafez have designed the hypothesis of the present research paper and full revision of the final manuscript draft.
Acknowledgements
Authors wish to thank the staff at the zoology department, Faculty of Science, Suez University for their valuable assistance and Dr. Ahmed Bahaa Eldine Darwish for his special assistance in animal handling.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atoji Y, Wild JM, Yamamoto Y, Suzuki Y. 2002. Intratelencephalic connections of the hippocampus in pigeons (Columba livia). The Journal of Comparative Neurology 447:177–199. DOI:10.1002/cne.10239.
- Baker KD, Edwards TM, Rickard NS. 2013. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neuroscience and Biobehavioral Reviews 37(7):1211–1239.
- Boussard A, Buechel SD, Amcoff M, Kotrschal A, Kolm N. 2020. Brain size does not predict learning strategies in a serial reversal learning test. Experimental Biology 1(15):223.
- Brodin A, Lundborg K. 2003. Is hippocampal volume affected by specialization for food hoarding in birds? Proceedings of the Royal Society of London Series B: Biological Sciences 270(1524):1555–1563. DOI:10.1098/rspb.2003.2413.
- Chancellor LV, Roth TC, LaDage LD, Pravosudov VV. 2011. The effect of environmental harshness on neurogenesis: A large‐scale comparison. Developmental Neurobiology 71:246–252. DOI:10.1002/dneu.20847.
- Clayton NS, Dickinson A. 1999. Memory for the contents of caches by Scrub jays. Journal of Experimental Psychology Animal Behavior Processes 25:82–91.
- Eckel-Mahan KL, Phan T, Han S, et al.. 2008. Circadian oscillation of hippocampal MAPK activity and cAmp: Implications for memory persistence. Nature Neuroscience 11(9):1074–1082. DOI:10.1038/nn.2174.
- Elston GN. 2003. Cortex, cognition and the cell: New insights into the pyramidal neuron and prefrontal function. Cerebral Cortex 13:1124–1138. DOI:10.1093/cercor/bhg093.
- Erich DJ, Onur G, Laura B, et al. 2005. Avian brains and a new understanding of vertebrate brain evolution. Nature Reviews Neuroscience 6:151–159. DOI:10.1038/nrn1606.
- Garamszegi LZ, Eens M. 2004. The evolution of hippocampus volume and brain size in relation to food hoarding in birds. Ecology Letters 7:1216–1224. DOI:10.1111/j.1461-0248.2004.00685.x.
- Gould KL, Newman SW, Tricomi EM, DeVoogd TJ. 2001. The distribution of substance P and neuropeptide Y in four songbird species: A comparison of food-storing and non-storing birds. Brain Research 918(1–2):80–95. DOI:10.1016/S0006-8993(01)02961-4.
- Grodzinski U, Clayton NS. 2010. Problems faced by food-caching corvids and the evolution of cognitive solutions. Philosophical Transactions of the Royal Society B: Biological Sciences 365(1542):977–987. DOI:10.1098/rstb.2009.0210.
- Hampton RR, Sherry DF, Shettleworth SJ, Khurgel M, Ivy G. 1995. Hippocampal volume and food-storing behavior are related in Parids. Brain, Behavior and Evolution 45:54–61. DOI:10.1159/000113385.
- Han R-W, Liu Z-P, Lin H-R, et al. 2020. Role of lateral amygdala calstabin2 in regulation of fear memory. Molecular Brain 13:35. Published online Mar 2020 9. DOI:10.1186/s13041-020-00576-7.
- Helm-Bychowski, K, Cracraft, J. 1993. Recovering phylogenetic signal from DNA sequences: relationships within the corvine assemblage (class aves) as inferred from complete sequences of the mitochondrial DNA cytochrome-b gene. Molecular Biology and Evolution 10: 1196–1214. DOI:10.1093/oxfordjournals.molbev.a040072.
- Hertle DN, Yeckel MF. 2007. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience 150:625–638. DOI:10.1016/j.neuroscience.2007.09.058.
- Hilgenberg LGW, Su H, Gu H, O’Dowd DK, Smith MA. 2006. Alpha3Na+/K+-ATPase is a neuronal receptor for agrin. Cell 125:359–369. DOI:10.1016/j.cell.2006.01.052.
- Huston JP, Weth K, Silva ADS, Junghans U, Müller HW, Hasenöhrl RU. 2000. Facilitation of learning and long-term ventral pallidal–cortical cholinergic activation by proteoglycan biglycan and chondroitin sulfate C. Neuroscience 100(2):355–361. DOI:10.1016/S0306-4522(00)00270-0.
- Kaas JH. 2000. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller. Brain and Mind 1:7–23. DOI:10.1023/A:1010028405318.
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. 1989. Hippocampal specialization of food-storing birds. Proceedings of the National Academy of Sciences 86(1989):1388–1392. DOI:10.1073/pnas.86.4.1388.
- Lynch MJ, Raphael SS, Mellor LD, Spare PD, Inwood MJ. 1969. Medical laboratory technology and clinical pathology. 2nd ed. Philadelphia: WB Saunders Co. London Toronto LG Luna, Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, third edition, McGraw Hill.
- Montagnese CM, Krebs JR, Meyer G. 1996. The dorsomedial and dorsolateral forebrain of the zebra finch (Taeniopygia guttata): A Golgi study. Cell and Tissue Research 283:263–282. DOI:10.1007/s004410050537.
- Neumann FR, Bittcher G, Annies M, Schumacher B, Kroger S, Ruegg MA. 2001. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Molecular and Cellular Neuroscience 17:208–225. DOI:10.1006/mcne.2000.0932.
- Neves G, Cooke S, Bliss T. 2008. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nature Reviews Neuroscience 9:65–75. DOI:10.1038/nrn2303.
- Ouyang Y, Deerinck TJ, Walton PD, Airey JA, Sutko JL, Ellisman MH. 1993. Distribution of ryanodine receptors in the chicken central nervous system. Brain Research 620:269–280. DOI:10.1016/0006-8993(93)90165-J.
- Sherry DF, Vaccarino AL. 1989. Hippocampus and memory for food caches in black-capped chickadees. Behavioral Neuroscience 103(2):308. DOI:10.1037/0735-7044.103.2.308.
- Singh D, Kumar P, Srivastava, UC. 2014. Neuronal specialization in hippocampal complex of food storing Indian bird corvus splendens. National Academy Science Letters 37:233–235. DOI:10.1007/s40009-014-0226–7.
- Srivastava UC, Singh S, Gaur P. 2011. Seasonal plasticity in avian hippocampus. In: Srivastava UC, Kumar S, editors. Emerging trends in zoology. New Delhi, India: Narendra Publishing House. pp. 13–34.
- Srivastava UC, Chand P, Maurya RC. 2007. Cytoarchitectonic organization and morphology of the cells of hippocampal complex in strawberry finch, Estrilda amandava. Cellular and Molecular Biology 53:103–120.
- Verkhratsky A. 2005. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiological Reviews 85:201–279. DOI:10.1152/physrev.00004.2004.
- Zhao W, Meiri N, Xu H, et al.. 2000. Spatial learning induced changes in expression of the ryanodine type II receptor in the rat hippocampus. The FASEB Journal 14:290–300. DOI:10.1096/fasebj.14.2.290.
