Abstract
The mite family Syringophilidae (Acariformes: Prostigmata) includes permanent parasites of birds exclusively, occupying their quill feathers where they reproduce and feed on living tissue fluids, piercing the quill-wall with their long and stiletiform chelicerae. The Boreal Owl, Aegolius funereus (L.) (Strigiformes: Strigidae) is parasitised by the Bubophilus aegolius, which is a new species of syringophilid mite described herein. In our study a total of 55 Boreal Owls were examined, including birds from Poland, Austria, Switzerland, Germany, Czechia, Slovakia, Sweden, Finland, and Norway. A total of four host specimens from Poland, Switzerland, and Finland were infested by Bubophilus aegolius (index of prevalence IP = 7.3; 95% confidence interval for prevalence (Sterne’s method) CI = 2.5–17.9). In the material consisting of 14 juvenile (in the 1st calendar year) and 41 adults (in the 2nd calendar year or older) birds, only juvenile hosts were infested (IP = 28.6%; CI = 10.4–57.4). Among 3,190 examined feathers (primaries (N = 1,100), secondaries (N = 1,430), rectrices (N = 660), quill mites were found only in four feathers, each feather from a single host specimen: in two small secondaries, S12, S13, and in two middle rectrices R1. This low prevalence may be related to the solitary behaviour of owls and the marginal role of horizontal transfers of parasites between hosts. Hypothetically, the low number of infested feathers on the host indicates low effectiveness (= high mortality) during transmission, where – in each case – only one fertilised female foundress (dispersal stage) was able to transfer from the parent to the offspring and enter a new quill feather.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:66025B6E-A126-48FF-8B1A-F618265CA34D
1. Introduction
The Boreal Owl, Aegolius funereus (Linnaeus) (Strigiformes: Strigidae) belongs to the Siberian-Canadian faunal type. Its general distribution is circumpolar holarctic, in boreal climatic zones and mountains. In Northern Europe, the Boreal Owl is distributed in a more or less regular belt, but there is a number of small isolated breeding areas in the mountains south of the continuous range, being relics from certain periods of the post-glacial epoch (Glutz Von Blotzheim & Bauer Citation1980; Mikkola Citation1983; Cramp & Brooks Citation1985; Korpimäki & Hakkarainen Citation2012).
Although syringophilid mites (Acariformes: Prostigmata: Syringophilidae) have not been recorded on the Boreal Owl so far, representatives of this family are widely distributed on strigiform birds. Currently, the fauna comprises seven species grouped in three genera, i.e., Bubophilus (four species), Neobubophilus (2), and Megasyringophilus (1) collected from 11 owl species both from the family Strigidae (10 species) and Tytonidae (1) (Philips & Norton Citation1978; Skoracki & Bochkov Citation2002; Skoracki & Dabert Citation2002; Nattress & Skoracki Citation2009; Skoracki et al. Citation2016).
Mites from this family are obligatory and permanent parasites, living and reproducing inside hollow quills (calamus) of the wing, tail, and contour feathers (Kethley Citation1971; Skoracki Citation2011). At all stages, these mites are able to feed on fluids of the soft tissue of their hosts by piercing the quill-wall with their needle-like chelicerae forming a cheliceral tube (Kethley Citation1971; Filimonova & Mironov Citation2010). Fertilised females are dispersal propagules and invade newly forming feathers, where they start to produce eggs, one at the time (Kethley Citation1971). The dispersion of syringophilids is strictly linked to the biology and ecology of their hosts and occurs at different organisational levels: First, among individual birds, when quill mites infest newly developed feathers during the autumn moult (moulting passage) (Casto Citation1975a, Citation1975b, Citation1976); then, within bird species during the breeding season, when mites move from infested parents to their nestlings (vertical transmission); finally, some indirect data suggest horizontal transmission of syringophilid mites from one individual adult host to another of the same species, either during the moult of gregarious birds or during copulation (Skoracki Citation2011; Hromada et al. Citation2016). Finally, the transfer of syringophilid mites can take place between “normal” host species and other host species (e.g. its avian predator), which is known as host switching (Nattress Citation2011). This paper describes a new species of the quill mite Bubophilus aegolius sp. nov. parasitising the Boreal Owl, and examines the distribution of this mite species in flight feathers, i.e. primaries and secondaries of the wing and rectrices of the tail.
2. Material and methods
Feather samples of the Boreal Owl Aegolius funereus used in this study come from a private collection of feathers of Western Palearctic birds of prey and owls collected by Professor Cieślak and Dul (Citation2006), Cieślak (Citation2017), Kwieciński (Citation2017). This feather material is currently deposited in the Department of Avian Biology and Ecology, Faculty of Biology, Adam Mickiewicz University, Poznań, Poland (AMU-DABE).
Each of the 55 bird specimens was represented by whole dry wings or the full complement of the wing (primaries, secondaries) and tail feathers (rectrices) ().
Figure 1. The Boreal Owl (male; specimen no. AMU–DABE AF45 from Finland), an open wing with secondary feather S12 (marked by arrow) infested by quill mites, Bubophilus aegolius sp. n
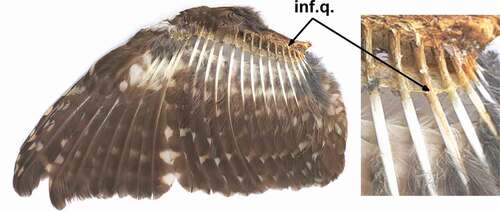
Figure 2. The Boreal Owl (male; specimen no. AMU–DABE AF45 from Finland) with secondary feather S12 (middle feather) infested by quill mites, Bubophilus aegolius sp. n

Figure 3. The Boreal Owl (female; specimen AMU–DABE AF24 from Switzerland), tail feathers (rectrices). Feather R1 (marked by arrow) infested by Bubophilus aegolius sp. n

Mites were collected according to the protocol introduced by Skoracki (Citation2011). Each quill feather was examined for the evidence of quill mites with a dissecting microscope. Infested feathers were opened and mites were extracted with fine sharp tweezers via a longitudinal cut made in the quill by a scalpel. Before mounting, mites were softened and cleared in Nesbitt’s solution at 50°C for one hour. Then, mites were mounted on slides in Faure’s medium. Identifications and drawings of mite specimens were carried out with a ZEISS Axioscope2 light microscope (Carl-Zeiss AG, Germany), equipped with DIC optics and a camera lucida.
All measurements are given in micrometers. Measurements (ranges) of paratypes are given in brackets following data on the holotype. Idiosomal setation follows Grandjean (Citation1939) as adapted for Prostigmata by Kethley (Citation1990). The nomenclature of leg chaetotaxy follows Grandjean (Citation1944). The morphological terminology used in the paper is consistent with Skoracki (Citation2011).
Specimen depositories and reference numbers include the following abbreviations: AMU, Adam Mickiewicz University, Department of Animal Morphology, Poznań, Poland; and ZSM, Bavarian State Collection of Zoology, Munich, Germany.
Prevalence and 95% confidence interval (Sterne) were calculated with Quantitative Parasitology 3.0 (Rózsa et al. Citation2000; Reiczigel et al. Citation2019).
3. Results
3.1. Prevalence and feather infestation
A total of 55 specimens of Boreal Owls were examined, including birds originating from Poland, Austria, Switzerland, Germany, Czechia, Slovakia, Sweden, Finland, and Norway (host reg. nos. AMU-DABE AF1-55).
Four of the inspected hosts were infested by quill mites (prevalence = 7.3; CI = 2.5–17.9; N = 55 Boreal owls), all of which were juvenile (prevalence = 28.6; CI = 10.4–57.4; N = 14 juvenile Boreal owls): One female from Switzerland (host reg. no. AMU-DABE AF-24) and three males from Poland (host reg. no. AMU-DABE AF-25), Switzerland (host reg. no. AMU-DABE AF-29), and Finland (host reg. no. AMU-DABE AF-45).
Among 3,190 examined feathers (1,100 primaries, 1,430 secondaries, 660 rectrices), quill mites were found only in four feathers, each from a single host specimen: In a small secondary (S12) of host reg. no. AMU-DABE AF-45 and (S13) of host reg. no. AMU-DABE AF-29, and in the middle rectrix (R1) of hosts AMU-DABE AF-24 and AMU-DABE AF-25 ().
The number of quill mites inside quills suggests that all infested feathers represent old infestation with the second generation, because more than 50 mite specimens, abundant excrements, and decanting material were found in each of the four infested feathers. Neither feather with a single female foundress nor the one with the first generation of infrapopulation were found.
The syringophilids collected from all of the infested feathers is a species new to the science.
3.2. Systematics
Family Syringophilidae LavoipierreSubfamily Syringophilinae LavoipierreGenus Bubophilus Philips and Norton
Bubophilus aegolius Skoracki, Kosicki & Kwieciński sp. nov.
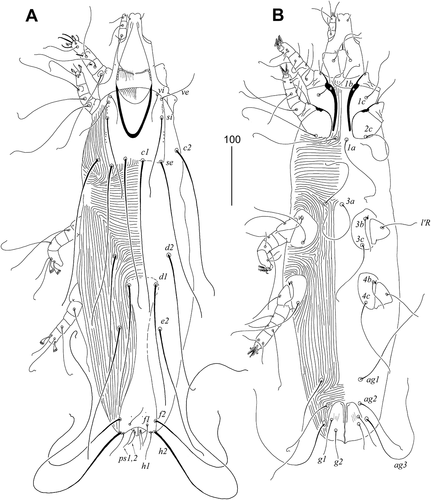
Female (). Total body length 720 in the holotype (665–790 in 24 paratypes). Gnathosoma. Stylophore constricted posteriorly, apunctate, 210 (205–225) long. Each medial branch of peritreme with two chambers, each lateral branch with four chambers. Infracapitulum apunctate. Movable cheliceral digit 165 (160–165) long. Idiosoma. Propodonotal shield entire, slightly concave on anterior margin, bases of setae vi, ve, si, se, and c1 situated on this shield. Length ratio of setae vi:ve:si 1:1.8–1.9:2.4–3. Setae c1 and se subequal in length. Hysteronotal shield fused with pygidial shield, apunctate, tapered in the middle, bases of setae d1 situated on the anterior margin of this shield. Setae d2 slightly (1.2 times) longer than d1. Setae f1 and h1 equal in length. Setae h2 2–2.4 times longer than f2. Genital plate weakly developed, only bases of genital setae situated on this plate. Pseudanal setae ps1 1.5 times longer than ps2; g1 1.5–1.7 times longer than g2. Length ratio of setae ag1:ag2:ag3 1.5–2:1:2.2–2.7. Coxal fields well sclerotized, apunctate. Setae 3 c about twice as long as 3b. Legs. Fan-like setae p’ and p” of legs III and IV with 9–11 tines. Lengths of setae: vi 45 (40–50), ve 80 (70–80), si 135 (105–130), c1 (255–260), c2 255 (235–255), se 260 (255–270), d1 200 (190–200), d2 (230–240), e2 225 (195–225), f1 40 (40–45), f2 210 (210–235), h1 40 (40–45), h2 (505–515), ag1 160 (160–190), ag2 105 (90–110), ag3 235 (220–250), g1 60 (60–65), g2 40 (40–50), ps1 30 (30–35), ps2 20 (20–25), l’RI 20 (20–25), l’RII 50 (50–55), l’RIII 75 (65–75), l’RIV 65 (55–65), 3b 50 (50–55), 3 c 135 (110–130).
Figure 5. Bubophilus aegolius sp. n. Female (a–d). (a) – peritremes; (b) – fan–like seta p’ of leg III; (c) – pseudanal setae ps1 and ps2; (d) – solenidia of leg I. Male (e–h). (e) – peritremes; (f) – fan–like seta p’ of leg III; (g) – opisthosoma in ventral view; (h) – body in dorsal view
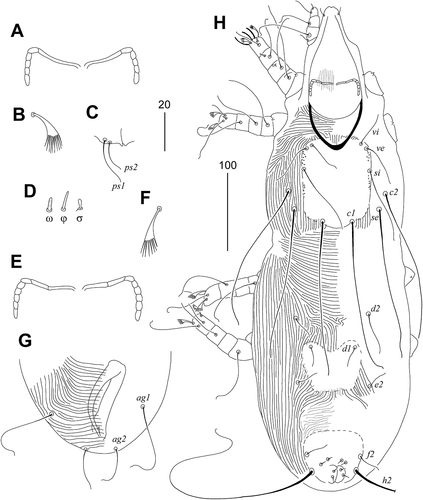
Male ()). Total body length 590–620 in 8 paratypes. Gnathosoma. Stylophore apunctate, covered by striae ornament in medial part, 180–185 long. Each medial branch of peritremes with three chambers, each lateral branch with five chambers. Movable cheliceral digit 160–170 long. Idiosoma. Propodonotal shield entire, punctate laterally and covered by minute granulations; bases of setae vi, ve, si, and c1 situated on this shield. Length ratio of setae vi:ve:si 1:1.3–1.5:2.3–2.4. Setae c1, c2, and se subequal in length. Bases of setae se situated anterior to level of setal bases c1. Hysteronotal shield apunctate, with bases of setae d1. Setae d2 1.5–2 times longer than d1 and e2. Pygidial shield not fused with hysteronotal shield bears bases of setae f2 and h2. Agenital setal series with two pairs, setae ag1 about twice as long as ag2. Legs. Fan-like setae p’ and p” of legs III and IV with 8–9 tines. Lengths of setae: vi 40–50, ve 50–65, si 80–90, c1 185–195, c2 190, se 190–195, d1 25–30, d2 45–65, e2 25–30, f2 30–35, h2 325–340, ag1 100–110, ag2 50–55, l’RI–II 30, l’RIII 50–55, l’RIV 30–45, 3b and 4b 30–35, 3c and 4c 85–90.
Type material. Female holotype and paratypes: 24 females, 8 males, 6 tritonymphs, 3 protonymphs, 4 larvae from the quill of rectrix (R1) of the Boreal Owl Aegolius funereus (Linnaeus) (Strigiformes: Strigidae) [host reg. no. AMU-DABE AF25, male]; POLAND: Zielona Góra, Stary Kisielin, 30 November 2005, coll. M. Cieślak. Numerous ethanol preserved paratypes, same data.
Type material deposition. Holotype and most of the paratypes are deposited in the AMU (reg. no. AMU-SYR.890A), 10 female and 4 male paratypes in the ZSM (reg. no. ZSMA-20190425).
Additional material. 10 females, 4 males (reg. no. AMU-SYR.890B) from the quill of rectrix (R1) of same host species [host reg. no. AMU-DABE AF24]; SWITZERLAND: Zurich, Aldisberg, 24 October 2005, coll. M. Cieślak. 12 females, 5 males (reg. no. AMU-SYR.890C) from quill of secondary (S13) same host species [host reg. no. AMU-DABE AF29]; SWITZERLAND: Zurich, Moosholzweiher, 20 November 2007, coll. M. Cieślak. 10 females, 5 males (reg. no. AMU-SYR.890D) from quill of secondary (S12) same host species [host reg. no. AMU-DABE AF45]; FINLAND: Oulu, Varepudas, 10 September 2014, coll. M. Cieślak. The slide-mounted mite specimens and numerous ethanol preserved specimens are deposited in the AMU.
Etymology. The name aegolius is taken from the specific name of the host.
Differential diagnosis. Bubophilus aegolius sp. n. is morphologically similar to two species in which setae si are longer than 100 µm, i.e. Bubophilus ascalaphus Philips and Norton, 1975 known from owls of the genus Bubo: B. virginianus (Gmelin), B. africanus (Temminck), and B. bubo (Linnaeus), and Bubophilus aluconis Nattress and Skoracki, Citation2009 known from owl of the genus Strix: S. aluco Linnaeus and S. woodfordii (Smith) (Philips & Norton Citation1978; Nattress & Skoracki Citation2009; Skoracki et al. Citation2016). This new species differs from these both species by the following features: in females of B. aegolius, the length of stylophore is 205–225 µm (vs. 149–165 µm in B ascalaphus and 180 µm in B. aluconis); the length ratios of setae ps1:ps2 and g1:g2 is 1:1.5 and 1:1.5–1.7, respectively (vs. the length ratios of setae ps1:ps2 and g1:g2 are 1:1 in B ascalaphus and B. aluconis); length of setae g1 and ag2 are 60–65 µm and 90–110 µm, respectively (vs. 41–54 µm and 56–77 µm, respectively, in B ascalaphus and 45–50 µm and 55–65, respectively, in B. aluconis).
4. Discussion
4.1 Prevalence
Prevalence is rarely reported in the literature on syringophilid mites and their hosts. So far, it has been calculated for birds collected in the wild (Doster et al. Citation1980; Casto Citation1974a, Citation1974b, Citation1975a, Citation1975b; Skoracki et al. Citation2003; Citation2010; Órdenes et al. Citation2005; Goulart et al. Citation2011; Moraes et al. Citation2011; Hache et al. Citation2017; Skirnisson & Nielsen Citation2019; Grossi & Proctor Citation2020), kept in farm households (Gritsenko Citation1973; Pires & Daemon Citation2007; Skirnisson & Palsdottir Citation2020) or in a zoological garden (Jardim et al. Citation2012), as well as the ones deposited in ornithological collections housed in museums or scientific institutions (Skoracki et al. Citation2001, Citation2017, Citation2018, Citation2019; Kaszewska et al. Citation2018). In all of the above-mentioned studies, quill mites were collected on the basis of an examination of all flight feathers, random feathers taken from live hosts or from museum specimens. Prevalence (IP) calculated through an examination of the whole plumage was recorded only for a few host species: (i) gregarious house sparrows Passer domesticus infested by Syringophiloidus minor (Berlese), where the IP = 82% (N = 492) (Casto Citation1975a); (ii) domestic chickens Gallus gallus domesticus kept in crowded conditions and infested by Syringophilus bipectinatus Haller (IP = 75%; N = 1,500) (Rebrassier & Martin Citation1932); (iii) ovenbirds Seiurus aurocapilla infested by Betasyringophiloidus seiuri (Clark) (IP = 42.9%; N = 21) (Grossi & Proctor Citation2020); and (iv) rock ptarmigans Lagopus muta infested by Mironovia lagopus Bochkov et Skirnisson (IP = 7.3%; N = 1,209) (Skirnisson & Nielsen Citation2019). As we can see, the highest prevalence was noted among social and domestic birds, where a horizontal transfer may play an important role in the migration of quill mites. On the other hand, our results show relatively low prevalence (7.3%; CI 2.5–17.9), where mites Bubophilus aegolius use only a small fraction of available host individuals of the Boreal Owl in the environment. This observation can be connected with the non-social behaviour of this host. The male usually spends winter alone in his territory, waiting for spring and a new female to breed. The female incubates eggs alone, and after the breeding season leaves the male’s territory and starts migration (Mikkola Citation1983; Cramp & Brooks Citation1985; Korpimäki & Hakkarainen Citation2012). Thus, we speculate that if a horizontal transfer occurs in the mite Bubophilus aegolius – Boreal Owl system, its role is marginal.
What is worth noticing is the fact that in our host sample only juvenile birds (1st CY) were parasitised by quill mites (N = 14; IP = 28.6%; CI = 10.4–57.4), whereas none of the 41 examined adult birds (2nd CY or older) were infested. This situation may be due to high mortality of quill mites during the moulting period (in May/June) of the Boreal Owl. Typically, during the moulting passage adult mite females emerge from mature feather quills (adult birds), disperse, and enter new feathers (newly grown) of nestlings. Between these periods, the growth and dispersal of the population of quill mites must be synchronised with these critical events in the host’s life cycle in order to minimise the loss of dispersants (Casto Citation1976). The absence of infested adult birds indicates that the replacement of feathers can have a significant negative effect on the number of mite dispersants which successfully enter newly developed feathers.
4.2 Habitat preference
Syringophilids live in quills of various parts of plumage (primaries, secondaries, alulars, coverts, tail-feathers and contour feathers), and particular species differ in the kind of inhabited plumage preferences (niche specificity). This division in the habitat preference is visible only on the subfamily level, where representatives of the subfamily Picobiinae inhabit exclusively contour feathers (except monotypic genus Calamincola Casto), whereas mites of the subfamily Syringophilinae occupy mainly quills of flight feathers. Among Syringophilinae, there are species that prefer only secondaries, primaries or coverts even on the same bird host (Kethley Citation1971). The kind of a niche where particular mite taxa can live is basically determined by two feather characteristics, such as i) the volume of the quill, and ii) its wall thickness (Kethley Citation1971; Casto Citation1974b; Grossi & Proctor Citation2020). Females with short chelicerae inhabiting large quills are not able to pierce the quill-wall and inevitably die. On the other hand, when quills are too small, mites cannot produce an adequate number of propagules to disperse to a new host. Due to the fact that there is a correlation between the volume of the quill and the thickness of its wall, and between the size of the mite and the length of its chelicerae, an optimal niche is the one that can house a relatively large number of mites (even more than 200 individuals per quill), and its wall thickness does not interfere with foraging (Kethley Citation1971; Casto Citation1974a, Citation1974b). An earlier study on the whole (wing and tail) plumage showed niche specificity of a few mite species, e.g. Betasyringophiloidus seiuri inhabiting mainly the ovenbird’s primaries (P1–P8), all secondaries (S1–S8), and sporadically rectrices (R1) (Grossi & Proctor Citation2020); Mironovia lagopus parasitising the rock ptarmigan mainly on secondaries, in the adjacent large coverts, and sporadically on primary flight feather (Skirnisson & Nielsen Citation2019); and Syringophiloidus minor infesting the house sparrow on all its secondaries, and a part of primaries (Casto Citation1974b).
How dispersants are able to find and enter feathers which provide the most advantageous harbourage is unknown, but in contrast to the above-mentioned examples, all of the four infested specimens of juvenile Boreal Owls used in our examination had only a single feather but fully filled by quill mites (secondaries S12, S13 or 2 × R1). This observation indicates low effectiveness of the nestling passage where only a single fertilised female (dispersal stage and foundress of the infrapopulation) was able to transfer from the parent to the offspring and enter a new quill feather. Nevertheless, the question why the Bubophilus aegolius selects these feathers is more of a challenge.
Literature on the moulting of the Boreal Owl in the wild reports two to six outer primaries replaced in the 2nd CY (P5,7 to P10), usually all rectrices, as well as parts of secondaries (Glutz Von Blotzheim & Bauer Citation1980; Cramp & Brooks Citation1985; Hornfeldt et al. Citation1988; Martínez et al. Citation2002; Cieślak & Kwieciński Citation2006; Korpimäki & Hakkarainen Citation2012; Mikkola & Lamminmäki Citation2014; Cieślak Citation2017). For instance, according to Zuberogoitia et al. (Citation2018), inner secondaries (S12, S13) of a nestling stop growing first, and they are the only subset of relatively small juvenile wing feathers that are retained at least until after the third prebasic moult, i.e. the moult during the 3rd CY in winter or the 4th CY in spring. Perhaps this could help understand why the Bubophilus aegolius was found in S12 and S13 feathers of young individuals, and the observed prevalence in adults was zero. Basically, during the “nestling passage”, the B. aegolius is likely to select S12–13 feathers of the nestling due to a combination of feather size suitability (relatively small wing feathers) and the expected juvenile feather lifespan (at least 3 years). Most importantly, however, these feathers grow the fastest, and therefore, it can be assumed that the direct reason for these feathers to be infested is their rapid growth on a young individual, and consequently optimal conditions for the parasites. On the other hand, we cannot exclude a notion that all primaries and secondaries from S1 to S11 represent a habitat inadequate for quill mites, because the quill-wall is too thick and they are not able to feed. Finally, the absence of quill mites in adults could be partly explained by the loss of the B. aegolius as part of the moulting event involving S11–13 feathers, i.e. affecting bird individuals older than 4 years.
Unfortunately, no similar analogy can be found among the infested middle rectrices (R1) in two owl specimens. Since the feathers grow at the same rate and morphology is essentially the same (Cieślak & Kwieciński Citation2006, Citation2009; Jenni & Winkler Citation2020), all rectrices should be potentially available for invasion and entered with an equal frequency.
Although the relatively low prevalence of the Bubophilus aegolius can be a consequence of simultaneous factors, e.g. biology of the host’s moult, feather structure, specific breeding, and behaviour of the Boreal Owl, an alternative explanation cannot be disregarded as a system composed of the mite Bubophilus aegolius and the host Aegolius funereus represents an evolutionarily young and not very stable system. In this scenario, the B. aegolius as a straggler (species arriving to a new host) has a limited ability to survive, reproduce, and disperse (see Rózsa Citation1993; Doña et al. Citation2019).
To conclude, the issue requires further studies aimed at the quill mite fauna of four other representatives of the genus Aegolius, i.e. A. acadicus, A. gradyi, A. ridgwayi, and A. harrisii.
Compliance with ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Authors’ contributions
Design and methodology: MS, JZK, ZK. Data collection: MS, JZK, ZK. Data analysis: MS, ZK. Original draft: MS, ZK.
Review and editing: all authors.
Ethics approval consent to participate
All the fieldwork was done according to the ethical standards recommended by those institutions.
Geolocation information
Wielkopolska, Poznań, Poland, Central Europe
Acknowledgements
The article is in memory of a prominent Polish ornithologist Professor Marian Cieślak. We would like to thank Paweł Podkowa for taking photographs for the manuscript. This manuscript benefited from invaluable comments of the reviewers XY and XX.
Data availability statement
The data analyses in this study are available from the corresponding author upon reasonable request.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Casto SD. 1974a. Entry and exit of syringophilid mites (Acarina: Syringophilidae) from the lumen of the quill. Wilson Bulletin 86:272–278.
- Casto SD. 1974b. Quill wall thickness and feeding of Syringophiloidus minor (Berlese) (Acarina: Syringophilidae). Annals of the Entomological Society of America 67:824. DOI: 10.1093/aesa/67.5.824.
- Casto SD. 1975a. The effect of the post juvenal molt in the house sparrow on infestations of the quill mite, Syringophiloidus minor (Berlese) (Acarina: Syringophilidae). Journal of Medical Entomology 12(1):23–27. DOI:10.1093/jmedent/12.1.23.
- Casto SD. 1975b. Mortality in the quill mite, Syringophiloidus minor (Acarina: Syringophilidae). Annals of the Entomological Society of America 68(3):551–552. DOI:10.1093/aesa/68.3.551.
- Casto SD. 1976. Dispersal of the Quill Mite Syringophiloidus minor (Berlese) (Agarina: Syringophilidae). Journal of Medical Entomology 13(3):357–360. DOI:10.1093/jmedent/13.3.357.
- Cieślak M. 2017. Feathers of European owls. Insights into species ecology and identification. In: Sterno A, Kwieciński Z, Grudzińska-Sterno M, editors. Editorial team into English version. Uppsala, Sweden: Oriolus Publishing House. p. 208.
- Cieślak M, Dul B. 2006. Feathers: Identification for bird conservation. Warszawa: Natura Publishing House.
- Cieślak M, Kwieciński Z. 2006. Moulting of Tengmalm’s owls in the Poznań Zoological Garden. Notatki Ornitologiczne 47:241–251. (In Polish).
- Cieślak M, Kwieciński Z 2009. Moult and breeding of captive Northern Hawk Owls Surnia ulula. In: Johnson DH, Van Nieuwenhuyse D, Duncan JR, editors. Proceedings of the 4th World Owl Conference. Oct–Nov 2007, Groningen, The Netherlands. Ardea 97:571–579.
- Cramp S, Brooks DJ. 1985. Handbook of the birds of Europe, the Middle East and North Africa: The birds of the Western Palearctic. Vol. IV. New York: Oxford University Press.
- Doña J, Serrano D, Mironov S, Montesinos-Navarro A, Jovani R. 2019. Unexpected bird-feather mite associations revealed by DNA metabarcoding uncovers a dynamic ecoevolutionary scenario. Molecular Ecology 28(2):379–390. DOI:10.1111/mec.14968.
- Doster GL, Wilson N, Kellogg FE. 1980. Ectoparasites collected from Bobwhite Quail in the Southeastern United States. Journal of Wildlife Diseases 16(4):515–519. DOI:10.7589/0090-3558-16.4.515.
- Filimonova SA, Mironov SV. 2010. Functional morphology of the gnathosoma in the quill mite Syringophilopsis fringilla Fritsch (Acari: Prostigmata: Syringophilidae). Zoologischer Anzeiger - A Journal of Comparative Zoology 249(3–4):165–180. DOI:10.1016/j.jcz.2010.08.002.
- Glutz Von Blotzheim UN, Bauer KM. 1980. Handbuch der Vögel Mitteleuropas. Vol. 9. Aula-Verlag: Wiesbaden.
- Goulart TM, Moraes DL, Prado AP. 2011. Mites associated with the eared dove, Zenaida auriculata (Des Murs, 1847), in São Paulo State, Brazil. Zoosymposia 6(1):267–274. DOI:10.11646/zoosymposia.6.1.36.
- Grandjean F. 1939. Les segments postlarvaires de l’hysterosoma chez les oribates (Acariens). Bulletin de la Société Zoologique de France 64:273–284.
- Grandjean F. 1944. Observations sur les acariens de la famille des Stigmaeidae. Archives des Sciences Physiques et Naturelles 26:103–131.
- Gritsenko EF 1973. The biology and ecology of the quill mite Syringophilus bipectinatus Heller, 1880. In: Milan D, Rosicky B editors. Proceedings of the 3rd International Congress of Acarology. Prague, Czechoslovakia: Czechoslovak Academy of Sciences. pp. 515–516.
- Grossi AA, Proctor HC. 2020. The distribution of quill mites (Betasyringophiloidus seiuri) among flight feathers of the Ovenbird (Seiurus aurocapilla). Journal of Parasitology 106(1):82–89. DOI:10.1645/18-160.
- Hache S, Bayne EM, Villard M-A, Proctor HC, Davis CS, Stralberg D, Janes JK, Hallworth MT, Foster KR, Chidambara-Vasi E, Grossi AA, Gorrell JC, Krikun R. 2017. Phylogeography of a migratory songbird across its Canadian breeding range: Implications for conservation units. Ecology and Evolution 7(16):6078–6088. DOI:10.1002/ece3.3170.
- Hornfeldt B, Carlsson BG, Nordstrom A. 1988. Molt of primaries and age determination on Tengmalm’s Owls (Aegolius funereus). Auk 105:783–789.
- Hromada M, Klimovicova M, Unsold M, Skoracki M. 2016. Host-parasite relationships in the system composed by cuckoos and quill mites. Systematic & Applied Acarology 21(4):528–536. DOI:10.11158/saa.21.4.11.
- Jardim CC, Cunha LM, Calmo Rezende L, Teixeira CM, Silva Martins NR, Oliveira PR, Leite RC, Faccini JLH, Leite RC. 2012. Quill mites in Brazilian psittacine birds (Aves: Psittaciformes). Journal of Zoo and Wildlife Medicine 43(3):511–516. DOI:10.1638/2011-0232R1.1.
- Jenni L, Winkler R. 2020. The biology of moult in birds. London: Helm.
- Kaszewska K, Skoracki M, Hromada M. 2018. A review of the quill mites of the genus Gunabopicobia Skoracki and Hromada (Acariformes: Prostigmata: Syringophilidae) associated with birds of the order Columbiformes. International Journal of Acarology 44(7):288–299. DOI:10.1080/01647954.2018.1515982.
- Kethley JB. 1971. Population regulation in quill mites (Acarina: Syringophilidae). Ecological Society of America 52:1113–1118.
- Kethley JB. 1990. Acarina: Prostigmata (Actinedida). In: Dindal DL, editor. Soil biology guide. New York, NY: Wiley and Sons. pp. 667–754.
- Korpimäki E, Hakkarainen H. 2012. The Boreal Owl. Ecology, behaviour and conservation of forest-dwelling predator. Cambridge UK: Cambridge University Press.
- Kwieciński Z. 2017. Obituary. Marian Cieślak, PhD (1950–2016). In: Sterno A, Kwieciński Z, Grudzińska-Sterno M, editors. Cieślak M. Feathers of European owls. Insights into species ecology and identification. Editorial team into English version. Uppsala, Sweden: Oriolus Publishing House. pp. 7–8.
- Martínez JA, Zuberogoitia I, Alonso R. 2002. Determinación del sexo y la edad de las rapaces nocturnas ibéricas. Madrid: Editorial Monticola.
- Mikkola H. 1983. Owls of Europe. Calton: T&AD Poyser.
- Mikkola H, Lamminmäki J. 2014. Moult, ageing and sexing of Finnich owls. Saarijärvi, Finland: Suomenselän Lintutieteellinen Yhdistys.
- Moraes DL, Goulart TM, Prado AP. 2011. Mites associated with the ruddy ground dove, Columbina talpacoti (Temminck, 1810), in São Paulo State, Brazil. Zoosymposia 6(1):275–281. DOI:10.11646/zoosymposia.6.1.37.
- Nattress B. 2011. Horizontal transmission of Syrngophilopsis kirgizorum (Acari: Cheyletoidea: Syringophilidae). Acarina 19:270.
- Nattress B, Skoracki M. 2009. A new species and further records of quill mites (Acari: Cheyletoidea: Syringophilidae) parasitic on birds (Aves) in England. Zootaxa 2133(1):49–54. DOI:10.11646/zootaxa.2133.1.4.
- Órdenes JSM, Ibáñez CB, Contreras LR, Schmäschke R, Daugschies A, González-Acuña D. 2005. Ectoparasitism in the common chimango caracara Milvago chimango chimango (Vieillot, 1816) (Aves, Falconidae) in the Ñuble Area, Chile. Lundiana 6:49–55. (in Spanish with English abstract).
- Philips JR, Norton RA. 1978. Bubophilus ascalaphus gen. et sp. n. (Acarina: Syringophilidae) from the quills of a Great Horned Owl (Bubo virginianus). Journal of Parasitology 64(5):900–904. DOI:10.2307/3279528.
- Pires EO, Daemon E. 2007. Biological and ecological aspects of quill mites, parasites of domestic hen Gallus gallus domesticus (Aves, Phasianidae) from rusting breeding locations in the municipality of Juiz de Fora, Minas Gerais, Brasil. Revista Brasileira de Zoociencias 9:95–102.
- Rebrassier RE, Martin ED. 1932. Syringophilus bi-pectinatus a quill mite of poultry. Science 76(1962):128. DOI:10.1126/science.76.1962.128.
- Reiczigel J, Marozzi M, Fábián I, Rózsa L. 2019. Biostatistics for parasitologists – A primer to quantitative parasitology. Trends in Parasitology 35(4):277–281. DOI:10.1016/j.pt.2019.01.003.
- Rózsa L. 1993. Speciation patterns of ectoparasites and “straggling” lice. International Journal for Parasitology 23(7):859–864. DOI:10.1016/0020-7519(93)90050-9.
- Rózsa L, Reiczigel J, Majoros G. 2000. Quantifying parasites in samples of hosts. Journal of Parasitology 86(2):228–232. DOI:10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2.
- Skirnisson K, Nielsen ÓK. 2019. Quill mite infestation of rock ptarmigan Lagopus muta (Aves: Phasianidae) in relation to year and host age, sex, body condition, and density. Parasitology Research 118(9):2643–2650. DOI:10.1007/s00436-019-06380-0.
- Skirnisson K, Palsdottir GR. 2020. Past and present status of poultry parasites in Iceland. Icelandic Agricultural Sciences 33:3–14. DOI: 10.16886/IAS.2020.01.
- Skoracki M. 2011. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2840(1):1–414. DOI:10.11646/zootaxa.2840.1.1.
- Skoracki M, Bochkov AV. 2002. A new quill mite species Bubophilus asiobius sp. n. (Acari: Syringophilidae) from the long-eared Owl Asio otus (Strigiformes: Strigidae). Genus 13:141–144.
- Skoracki M, Dabert J. 2002. A review of parasitic mites of the family Syringophilidae (Acari: Prostigmata) from African birds, with description of four new species. Acta Parasitologica 47:137–146.
- Skoracki M, Hromada M, Prevuznakova P, Wamiti W. 2019. Mites of the family Syringophilidae (Acariformes: Cheyletoidea) parasitizing waxbills of the genus Estrilda (Passeriformes: Estrildidae). Systematic & Applied Acarology 24(9):1799–1808. DOI:10.11158/saa.24.9.15.
- Skoracki M, Hromada M, Sikora B. 2017. Castosyringophilus meropis sp. n. (Acariformes: Syringophilidae) - a new quill mite species parasitising the world population of Merops apiaster Linnaeus (Coraciiformes: Meropidae). Folia Parasitologica 64:24. DOI: 10.14411/fp.2017.024.
- Skoracki M, Hromada M, Tryjanowski P. 2001. Description of a new species of quill mite Syringophiloidus weiszii sp. n. (Acari, Prostigmata, Syringophilidae) from Great Grey Shrike Lanius excubitor. Acta Parasitologica 46:30–34.
- Skoracki M, Hromada M, Zmudzinski M, Unsoeld M, Sikora B. 2018. Parasitic quill mites of the family Syringophilidae (Acariformes: Prostigmata) associated with Sub-Saharan Sunbirds (Passeriformes: Nectariniidae): Species composition and host-parasite relationships. Journal of Medical Entomology 55(6):1464–1477. DOI:10.1093/jme/tjy106.
- Skoracki M, Michalik J, Sikora B. 2010. Prevalence and habitat preference of quill mites (Acari, Syringophilidae) parasitizing forest passerine birds in Poland. Acta Parasitologica 55(2):188–193. DOI:10.2478/s11686-010-0021-7.
- Skoracki M, Møller AP, Tryjanowski PT. 2003. A new species of parasitic mites of the genus Syringophiloidus Kethley 1970 (Acari: Syringophilidae) from the Barn Swallow Hirundo rustica Linnaeus, 1758. Parasite 10(1):17–20. DOI:10.1051/parasite/2003101p17.
- Skoracki M, Unsoeld M, Marciniak N, Sikora B. 2016. Diversity of quill mites of the family Syringophilidae (Acari: Prostigmata) Parasitizing Owls (Aves: Strigiformes) with remarks on the host–parasite relationships. Journal of Medical Entomology 53(4):815–826. DOI:10.1093/jme/tjw065.
- Zuberogoitia I, Zabala J, Martínez JE. 2018. Moult in birds of prey: A review of current knowledge and future challenges for research. Ardeola 65(2):183–207. DOI:10.13157/arla.65.2.2018.rp1.