Abstract
The status of the genera in the small microtrombidiid mite tribe Hexathrombiini is reevaluated. Type specimens representing all genera were studied and diagnostic characters for Hexathrombiini are reviewed, summarized, and new data and a key to the genera in the tribe are provided: Alhamitrombium, Beronium, Hexathrombium and Hoplothrombium. Hexathrombium is the most speciose genus in the tribe and species recorded from South America are compared, as well as those with a divided pygidial plate. A provisional key to species assigned to Hexathrombium is provided. Finally, Hexathrombium abirami was captured in Peru parasitizing a bright metallic tiger beetle (Tetracha fulgida). A total of 361 larvae were removed parasitizing a single carabid host; this is the highest load of parasites reported in terrestrial Parasitengona mites associated with arthropods. This capture represents a new record of Hexathrombiini mites for Peru. A redescription of He. abirami using all specimens available to date is included.
Introduction
The tribe Hexathrombiini Fain & Drugmand, Citation1993 (see Discussion section for authorship) was erected within Eutrombidiinae Thor, 1935 to accommodate Hexathrombium Cooreman, Citation1944, Beronium Southcott, Citation1986 and Hoplothrombium Ewing, Citation1925. Later, Mayoral & Barranco (Citation2005a) described Alhamitrombium in Hexathrombiini. Fifteen species, variously distributed within the six zoogeographic regions, have been described in the tribe to date. All remain known exclusively from larvae that parasitize carabids (including formerly distinguished Cincindelidae) and staphylinid beetles. There are two exceptions: Hoplothrombium quinquescutatum Ewing, Citation1925 reported on a single specimen adhering to an Oribatida mite taken from the stomach of a toad (Ewing Citation1925), and Hexathrombium southcotti Zheng, Citation1997 captured from ichneumonid wasps (Zheng Citation1997; Felska et al. Citation2018). Haitlinger (Citation1999) considered the latter to be probably an accidental host. Most species are known only from few specimens, and they have been recorded from single localities; as an exception, more than 40 specimens of Beronium laemostenis Mayoral & Barranco, 2005 have been collected from 10 different localities (caves) in Spain (Mayoral & Barranco Citation2005b; Mayoral Citation2013).
The most speciose genus is Hexathrombium and it contains the species: Hexathrombium cicindelae (Floch & Abonnenc, Citation1941), Hexathrombium abirami Haitlinger, Citation1997, and Hexathrombium marittae Haitlinger, Citation1994 – from the Neotropical region; Hexathrombium lubomirae (Haitlinger, Citation1994) – from the Afrotropical and Oriental Region; Hexathrombium fageli Fain & Drugmand, Citation1993 and Hexathrombium spatuliferum Cooreman, Citation1944 – from the Afrotropical region; Hexathrombium willisi Southcott, Citation1993 – with Nearctic distribution; Hexathrombium sorayae Haitlinger, Citation1994 and He. southcotti – with Palaearctic distribution; and Hexathrombium mamerti Haitlinger, Citation1999 – from the Australian region. Two other findings from South America refer to uncertain identifications of specimens assigned to He. cf. marittae and He. cf. cicindelae (Pérez-Espinoza & Moreno Salas Citation2016; Almada & Cédola Citation2017).
The genus Beronium Southcott, Citation1986 was erected by Southcott (Citation1986) to accommodate Hoplothrombium coiffaiti Beron, Citation1973. This genus is known from the south-western Palaearctic, and comprises Beronium coiffaiti (Beron, Citation1973), Beronium veronicae Haitlinger, Citation1994 and B. laemostenis (Beron Citation1973; Haitlinger Citation1994; Mayoral & Barranco Citation2005b; Mayoral Citation2013). The monotypic genera Hoplothrombium Ewing, Citation1925, and Alhamitrombium Mayoral & Barranco, 2005 are known from the Nearctic and Palaearctic regions, respectively (Ewing Citation1925; Vercammen-Grandjean Citation1967; Mayoral & Barranco Citation2005a); they include the species Ho. quinquescutatum and Alhamitrombium tetraseta Mayoral & Barranco, 2005.
Welbourn (Citation1983) listed Ho. quinquescutatum and Ho. coiffaiti under Hoplothrombium, in addition to the species Trombidium cicindelae Floch & Abonnenc, Citation1941 and He. spatuliferum. The generic affiliation of “coiffaiti”, “cicindelae” and “spatuliferum” was not followed by subsequent authors since those specimens do not comply with the generic diagnosis of the genus and Hoplothrombium has therefore remained monotypic.
Here, we redescribe Hexathrombium abirami from new specimens collected in Peru, parasitizing Tetracha fulgida (Klug, Citation1834) (Carabidae). The load of parasites reported here for T. fulgida is the highest infestation record of terrestrial Parasitengona mites associated with arthropods. The diagnostic traits for the four genera in Hexathrombiini were reviewed and representatives of the different genera were studied (except Hoplothrombium that we relied on published descriptions). An updated list of relevant characters, a key to the genera in Hexathrombiini and a provisional key to Hexathrombium species are provided.
Material and methods
A specimen of T. fulgida infested by larvae was collected in Peru (dept. Huánuco, Rio Yuyapichis, ACP Panguana, 9º37’S, 74º56ʹW, 230 m.a.s.l.), 01.05–21.05.2015, by S. Friedrich, F. Wachtel and M. Steinherr. The material was preserved in 80% Ethanol.
Larvae were detached from the host with an entomological pin and mounted on microscopic slides in Hoyer’s medium. The overall number of larvae attached to the host was counted and the measurements were taken using NIS-Elements Br software, under a Nikon Eclipse E600 microscope coupled with DS-Fi1 camera system. Photos were taken with a DS-Fi3 camera attached to a Nikon Eclipse 80i microscope and the images were stacked using Helicon Focus software (© Helicon Soft Ltd., 2000). All measurements are given in micrometers. The terminology follows Southcott (Citation1993), Vercammen-Grandjean (Citation1967) and Wohltmann et al. (Citation2007). The material (slide-mounted and alcohol preserved larvae; host specimen) is deposited in the Department of Invertebrate Systematics and Ecology, Wrocław University of Environmental and Life Sciences, Poland (DISE WUELS). Five larvae (slide-mounted) are deposited at the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM).
For the purpose of comparison and re-appraisal of characters used in the diagnosis of Hexathrombiini, the type material of He. abirami, He. lubomirae, He. marittae, He. mamerti, B. veronicae, B. laemostenis and A. tetraseta was studied. The references to other members of the tribe are based on original descriptions.
Results
Hexathrombiini Fain & Drugmand, Citation1993Hexathrombiini Fain & Drugmand, Citation1993: 123.Hexathrombiini: Southcott Citation1993: 943.
Diagnosis
(after Fain & Drugmand Citation1993; Southcott Citation1993, verified).
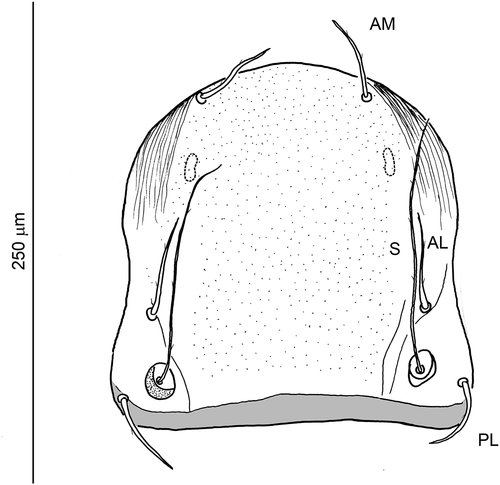
Larva. Idiosoma constricted behind the level of coxae III, covered with scutum and four median shields, encompassing the bases of setae c1, d1, e1 and h1; the posterior-most shield may be divided into two separate sclerites. Eyes sessile, each composed of two lenses (Hexathrombium), one lens (Hoplothrombium and Alhamithrombium) or eyes reduced to ocular plates (Beronium). Scutum bearing non-sensillary setae (AM, AL, PL) and a pair of sensilla (S). fCx = 2-1-1 or 2-2-2. Medial coxala I (1a) simple. Setae on coxae II and III modified (short, thickened) or simple. Tarsi I and II terminated with two claws and empodium. Tarsus III highly modified, terminated with two claws and lophotrix (“penicala” sensu Southcott Citation1993). Inner (posterior) claw short and robust, median claw long, falciform. Lophotrix shifted to dorsodistal part of the segment. Gnathosoma with stephanostome. Hypostomalae thick, short.
Active postlarval forms. Not known.
Type genus. Hexathrombium Cooreman, Citation1944
Genera included
Hexathrombium Cooreman, Citation1944; Hoplothrombium Ewing, Citation1925; Beronium Southcott, Citation1986; Alhamitrombium Mayoral & Barranco, 2005.
Remarks
The position of seta 1a may indistinctly vary in all genera in question, as the seta is inserted close to the coxal plate margin and its actual location in the mounted specimens may be considered dubious. Vercammen-Grandjean (Citation1967) considered 1a, observed within the coxal plate in Ho. quinquescutatum, a “migrated sternal seta”.
The chaetotaxy of terminal leg segments (Ge – Ta I, Ge – Ta II, Ge – Ta III), with special reference to specialized setae, has been variously interpreted due to the difficulties in their visualization, specially their actual nature and presence. The more detailed close-up to the setae, should be carried out to clarify their actual state in all members of the genus.
The highly modified termination of tarsus III is similar in all Hexathrombiini and differs to those observed in Eutrombidiini. Southcott (Citation1993) described in Hexathrombiini the presence of a branched seta (“penicala” sensu Southcott Citation1993) on the dorsodistal projection of tarsus III, and also two claws located ventrodistally. From those, the median claw is long, falciform and simple, whereas the posterior claw (i.e. smilum) (anterior claw sensu Southcott Citation1993) is in the form of a short and thick hook with a dorsal spur-like process. In Eutrombidiini, there is a moustache-shaped seta about 3/4 along the dorsum of tarsus III (“cultala” sensu Southcott Citation1993), a multipronged seta with several large setules arising dorsally at the distal end of tarsus (“dumala” sensu Southcott Citation1993), and a thickened setae with setules in the ventrodistal portion of the tarsus (“calcanala” sensu Southcott Citation1993) (Southcott Citation1993). Husband and Wohltmann (Citation2011) observed in Eutrombidium the presence of a scopa on tarsus III (“cultala” sensu Southcott, Citation1993) and lophotrix (“dumala” sensu Southcott Citation1993). Despite the different position of branched, pectinate seta in Hexathrombiini (seta shifted to a dorsodistal position), its structure is congruent with the lophotrix observed in Eutrombidiini and also in some other microtrombidiid mites. The short and thick inner claw located ventrodistal and covered with few bristles should be considered a smilum. Another modified seta, a scopa, is absent in Hexathrombiini.
Hexathrombium Cooreman, Citation1944Hexathrombium Cooreman, Citation1944: 1.Hexathrombium: Southcott Citation1993: 945.
Diagnosis
(after Southcott, Citation1993, verified).
Larva. The posterior-most shield on dorsal idiosoma (pygidial shield, Q5) entire (He. marittae, He. lubomirae, He. willisi) or divided into two separate sclerites (He. abirami, He. cicindelae, He. fageli, He. mamerti, He. sorayae, He. southcotti, He. spatuliferum). Eyes composed of two lenses inserted in ocular plates. Odontus bifid. fCx = 2-1-1. Medial coxala I (1a) simple (spike-like or slender, tapering). Lateral coxala I (1b), coxalae II (2b) and III (3b) bilobed, with diverged processes (in He. willisi – 1b, 2b, 3b - bilobed, with indistinctly diverged, rounded apically processes). Seta 3a shifted to intercoxal position or absent. Pre-anal tubercle present. fV = 12–16 (18–20 in He. southcotti); for comparison of character state in South American species see . Hypostomala reniform.
Type species
Hexathrombium spatuliferum Cooreman, Citation1944
Species included and country records
He. abirami, Brazil, Peru; He. cicindelae, French Guiana; He. fageli, Ethiopia, Ivory Coast; He. lubomirae, Madagascar, Sumatra; He. mamerti, Australia; He. marittae, Chile; He. sorayae, China; He. southcotti, China; He. spatuliferum, Zaire; He. willisi, USA. Additional records: He. cf. cicindelae: Almada & Cédola (Citation2017), Argentina; He. cf. marittae: Pérez-Espinoza & Moreno Salas (Citation2016), Chile. Welbourn (Citation1983) reported two presumably new species of Hoplothrombium from Ecuador; they were never formally described, however, they both should be placed in Hexathrombium (Cal Welbourn, pers. comm.).
Hosts
Coleoptera: Carabidae, Erotylidae, Staphylini- dae; Hymenoptera: Ichneumonidae (see also ).
Redescription of Hexathrombium abirami Haitlinger, Citation1997Hexathrombium abirami Haitlinger, Citation1997: 81.
Diagnosis
Larva. Pygidial shield (Q5) divided. Coxalae 1a shifted to the margin of coxal plates, slender, tapering. Coxalae 1b and 2b bilobed, with horizontally diverged processes.
Description
Metric data provided in (except for few dimensions given below).
Table I. Metric data for Hexathrombium spp. Species with divided pygidial shield Q5: He. abirami, He. cicindelae, He. fageli, He. mamerti, He. sorayae, He. southcotti, He. spatuliferum. Species with undivided pygidial shield: He. marittae, He. lubomirae. For He. willisi see Southcott (Citation1993)
Gnathosoma ()) compact, with well-sclerotized frames of subcapitulum, chelicerae and palps. Stephanostome present. Internal horseshoe-like sclerite inserted between inner and outer cuticular sheath. One pair of nude adoral setae (or). Subcapitular setae reniform. Cheliceral claws distinctly curved. Palps relatively small and robust. fPp = 0-N-N-NNB2-BNNNNNωζ. Seta on palp femur and palp genu short, thorn-like. Two setae on palp tibia short, thorn-like, the third seta long, with 1–2 fine barbs. Odontus bifid. The longest, most proximal seta on palp tarsus with few (2–3) indistinct barbs only. Palpal supracoxalae (elcp) not detectable.
Figure 1. Hexathrombium abirami, larva. a) Gnathosoma (complete chaetotaxy of palp tibia and palp tarsus not shown). b) Details of palp tibia and palp tarsus
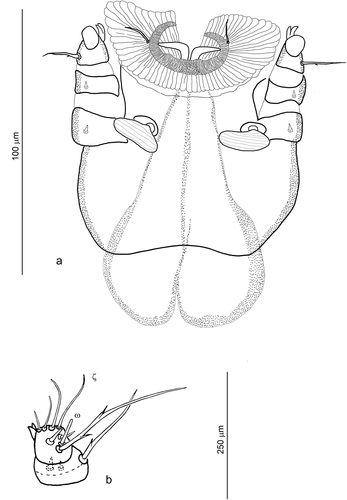
Supplementary measurements, not included in the (format: mean (range)), for 10 specimens from Peru: GL = 101 (90–111), PaFe = 13 (11–15), PaGe = 9 (7–10), PaTi = 9 (8–11), PaTa = 7 (6–8), Odo = 6 (5–7), Ch base = 87 (77–104), Ch claw = 18 (13–24), bs = 10 (8–12).
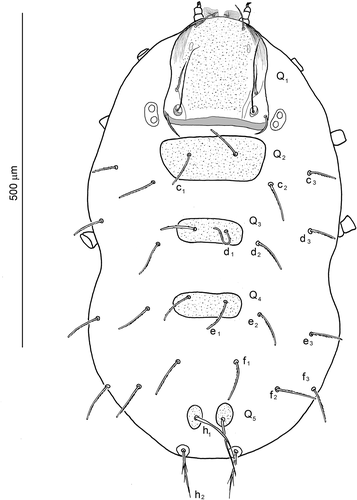
Dorsal idiosoma () oval, slightly constricted behind the level of coxae III, rounded at anterior and posterior termination. Integument, except for sclerites, folded in lines. Scutum (Q1) () pentagonal in outline, rounded anteriorly, bearing paired, non-sensillary setae AM, AL, PL, and a pair of sensilla (S). Antero-lateral parts of scutum with linear pattern, medial part of the sclerite porous. Posterior margin almost straight, bordered with delicate lamellar band. Additionally, small octal-shaped or semi-oval mark, probably representing the less sclerotized part of the sclerite, present antero-laterally in lower layer of scutum (or [?] immediately under the scutum surface), on each side of symmetry axis. Bases of sensilla located between AL and PL bases, closer to PL and slightly shifted to medial position. AM smooth or with 1–2 barbs, AL, PL and S with few barbs along entire stem length. Paired eye lenses (), each pair on a common, weakly sclerotized plate located close to the postero-lateral margins of the prodorsal sclerite (Q1). Scutellum (Q2) trapezoidal in shape, with a pair of c1 setae. Second and third scutellum (Q3, Q4) with a pair of d1 and e1 setae, respectively (in one specimen (7802/13) with duplicated seta e1 on Q4 sclerite); both sclerites rectangular in shape, with rounded corners. Setae e1 located close to the anterior margin of Q4. The fifth shield (Q5) divided into two separated, oval plates, each bearing a h1 seta. fD: (2)4-(2)4-(2)4-6-(1 + 1)2 = 28. Setae in rows C, D, E, F, except for c1, d1, e1, located on small platelets; stems of setae in C-F rows only slightly narrowed at termination and covered with short barbs along the entire length. Setae c3, d3, e3 slightly shorter than other setae in the respective rows C-E. Setae h1 and h2 longer than the preceding setae, distinctly narrowed apically; h2 inserted in roundish plates (of diameter similar to the width of h1 plates, i.e. Q5/2); shafts of h2 slenderer than those of h1 and with distinct setules.
Ventral idiosoma ()
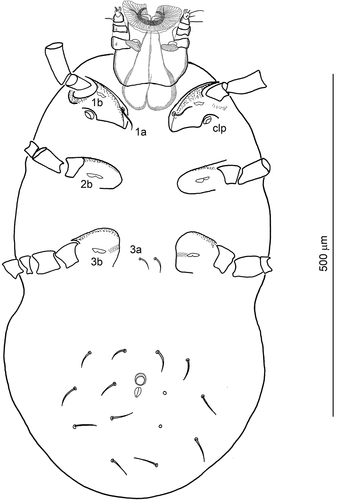
Coxal plates (I – triangular in outline, II – rectangular, rounded at base, III – square-shaped in outline) well sclerotized along the anterior border; the posterior border weakly marked or discontinuous. Anterolateral part of coxa I frame as well as its distal portion strongly sclerotized (); sclerotization of the respective parts of coxae II and III less pronounced. Claparède’s organs (clp) present at posterolateral corner of coxa I. Coxa I with two setae, medial coxala (1a) normal, nude, tapering, placed on cuticular band forming the medial extension of the most sclerotized part of coxal frame; lateral coxala (1b) modified, bilobed ()); coxa II and coxa III with one modified, bilobed seta each (2b and 3b, respectively); all modified coxalae (1b, 2b, 3b) with horizontally diverged processes (lobes); lobes indistinctly narrowing at termination and slightly extending beyond the setal base (widely diverging in 2b, making the seta the widest in comparison with 1b and 3b). Setae 3a (20 long) simple, slender, nude, and located between coxae III. Supracoxalae of coxae I (elc I) not detectable. fV: 4-4u-4-2 = 14. Ventral setae tapering, with thinner shafts than dorsal setae. Anal opening surrounded with membraneous valves, anal sclerites absent. Pre-anal protuberance (tubercle) present, circle-like, similar in diameter to the length of anus, and located anterior to excretory slit. The tubercle, slightly elevated above the idiosoma surface, surrounded with more sclerotized, porous sides.
Legs ())
Segmentation formula 6-6-6. For leg chaetotaxy see . Normal setae on legs setulated to smooth. Robust, fan-like seta present in distal part of tarsus II. Tarsi I and II terminated with two claws and empodium. Claws similar in length, covered with onychotrichs. Empodium claw-like, slightly spatulate distally. Tarsus III highly modified at termination, with lophotrix in dorsodistal position and two claws located ventrodistal. Lophotrix composed of one branch, with one long, proximal, secondary branch on one side and with several, gradually shortening, secondary and tertiary branches on the other ()). Inner claw short, robust, with small spurs; medial claw long, falciform.
Figure 5. Hexathrombium abirami, larva. Legs (trochanter – tarsus). a) leg I. b) leg II. c) leg III. Abbreviations: lo – lophotrix, sm – smilum
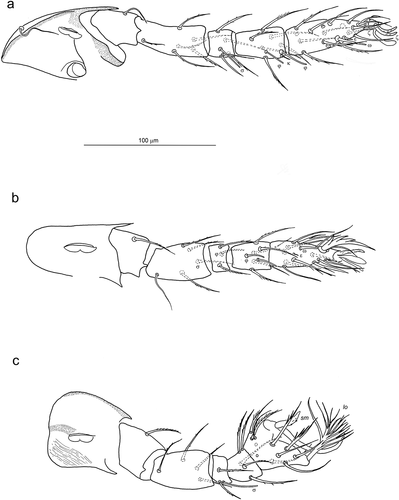
Figure 6. Lateral coxalae I (1b), II (2b), III (3b) in larvae of Hexathrombiini. a) Hexathrombium abirami, holotype. b) Beronium veronicae, holotype. c) Alhamitrombium tetraseta, holotype. Not to scale
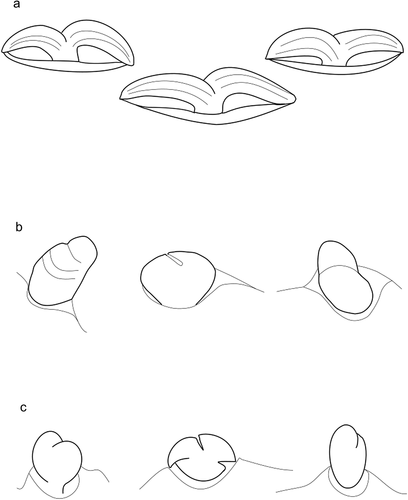
Figure 7. Lophotrix in larvae of Hexathrombiini. a) Hexathrombium abirami. b) Hexathrombium lubomirae, holotype. c) Hexathrombium mamerti. d) Beronium veronicae, holotype. e) Alhamitrombium tetraseta, holotype. Not to scale
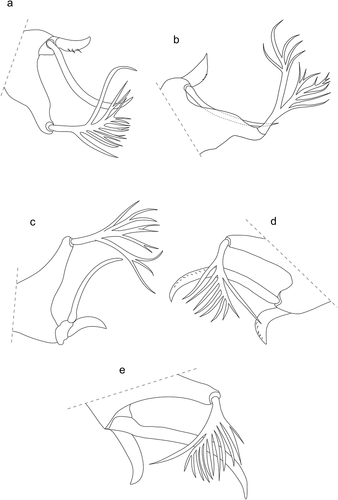
Table II. Leg chaetotaxy of Hexathrombium spp.1 Species with divided pygidial shield Q5: He. abirami, He. fageli, He. mamerti, He. sorayae, He. southcotti. Species with undivided pygidial shield: He. marittae, He. lubomirae, He. willisi.
Material examined
Holotype and two paratypes, deposited in the Museum of Natural History, University of Wroclaw, Poland. Ten larvae collected on T. fulgida from Peru in 2015 (present study) and randomly selected from a sample of 361 specimens parasitizing one host (for details – see Material and methods).
Remarks
The new specimens collected in Peru and studied in this work were compared with the type material of He. abirami, and also with other species of Hexathrombium, with an emphasis on the South American species in the genus. A comparison of the metric data of the Peruvian specimens with the type series is included in . The differences observed in metric data between the type series of He. abirami and specimens collected in Peru () are attributed to intraspecific variations. These variations may be a result of the differences in geographic locations, ecological factors, and the mounting quality of the type material that may have affected the reliability of some measurements.
Based on the reexamination of the holotype and two paratypes of He. abirami Haitlinger, Citation1997, some characters redescribed here differ from those reported in the original description. A pair of eyes (each composed of two lenses placed on a common sclerite) are present at each side of scutum (original description, , p. 82: one lens present at posterolateral margin of scutum, on each side of symmetry axis). Setae AL = 54 (original description: AL broken in all specimens); fn Fe = 6-5-4 (original description: fn Fe = 5-5-4); fn Ge = 5-2-2 (original description: fn Ge = 5-5-3); fn Ti = 6-5-5 (original description: fn Ti = 8-7-5); fsol Ti = 2-2-0 (original description: 2-1-0); vestigiala on tibia I, 2 eupathidia on tarsus I, famulus on tarsus II present. Preanal tubercle present.
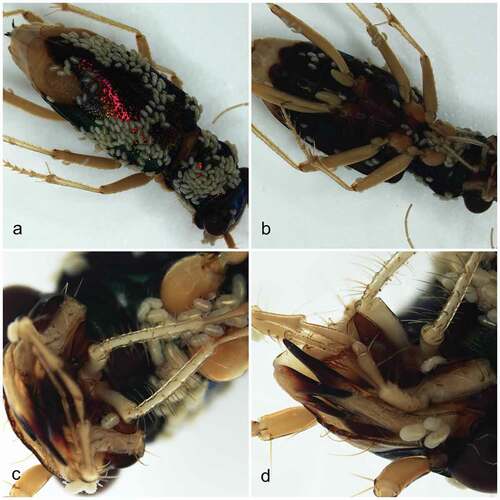
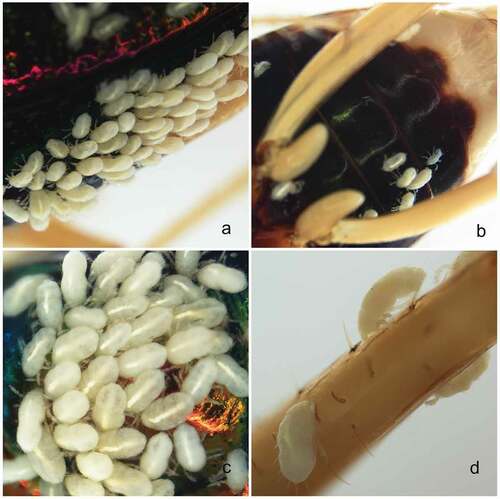
Parasitism of specimens collected in Peru
Altogether, 361 larvae were found parasitizing a single carabid host, T. fulgida ( and 9(a–d)). Eight were attached to the head (including labrum) (), 86 to the pronotum ()), 183 to the elytra (22 under elytra) ( and 9(a),(c)), 50 to the thorax and venter of abdomen ( and 9(b)), and 26 were distributed among various leg segments ()); for the remaining eight larvae the attachment sites were not recorded. Parasites seem to show a preference for the dorsal parts of the host and there was no preference for softer cuticle areas (including the dorsal abdomen), and instead the preference was for externally exposed areas that seem to be out of the reach of the host legs. Larvae were relatively firmly attached, and except for eight specimens, the detachment had to be performed with an entomological pin and forceps.
Distribution
Neotropical (Brazil - original description, Peru - present data).
Supplementary data to the original descriptions of He. lubomirae, He. mamerti and He. marittae
Hexathrombium lubomirae (Haitlinger, Citation1994)Beronium lubomirae Haitlinger, Citation1994: 50.Hexathrombium lubomirae: Haitlinger Citation1997: 81.
Material examined
Holotype and six paratypes from Sumatra, mounted on one slide. For leg chaetotaxy see .
Pygidial shield (Q5) undivided, with indistinct median incision at the posterior margin. Setae 1a shifted beyond the margin of coxa. Setae 3a absent. Setae 1b, 2b, 3b as in He. abirami. Preanal tubercle present. fn Fe = 6-5-4 (original description: fn Fe = 5-5-4); fn Ge = 5-2-2 (original description: fn Ge = 5-3-3); fsol Ge = 1-1-1 (original description fsol Ge = 0-0-0); fκ Ti = 1-0-0 (original description: fκ Ti = 0-0-0); fζ Ta = 2-0-0 (original description: 0-0-0); fε Ta = 1-1-0 (original description: 1-0-0). Lophotrix two-branched, each branch with several bristles ()).
Hexathrombium mamerti Haitlinger, Citation1999Hexathrombium mamerti Haitlinger, Citation1999: 58.
Material examined
Holotype and eight paratypes mounted on one slide. For leg chaetotaxy see .
Setae 1b, 2b, 3b as in He. abirami. fn Fe = 6-5-4 (original description: fn Fe = 5-5-4); fκ Ti = 1-0-0 (original description: fκ Ti = 0-0-0); fζ Ta = 2-0-0 (original description: 0-0-0); fε Ta = 1-1-0 (original description: 0-0-0). Tarsus I, tarsus II and femur III bearing a distinct seta, smooth or with few short barbs, much longer than the other setae in the segment. Lophotrix composed of two branches; the most proximal branch bifurcate at termination. The distal branch of the stem with several (c. 5) secondary branches (at least three of those as well as the main stem are bifurcate at termination ()).
This species is similar to He. abirami, however it shows smaller measurements for most of the characters studied (see ).
Hexathrombium marittae (Haitlinger, Citation1994)Beronium marittae Haitlinger, Citation1994: 48.Hexathrombium marittae: Haitlinger Citation1997: 81.
Material examined
Holotype and four paratypes mounted on one slide and collected from Ceroglossus sybarita (now Ceroglossus buqueti sybarita); two paratypes mounted on another slide and collected from Ceroglossus darwini. Metric data are shown in . For leg chaetotaxy see .
Pygidial shield (Q5) undivided. fV = 14 (original description: fV = 20). Preanal tubercle present. Distal part of tarsus II with a fan-like seta, similar to one observed in He. abirami, but less robust.
Hoplothrombium Ewing, Citation1925Hoplothrombium Ewing, Citation1925: 263.Hoplothrombium: Vercammen-Grandjean Citation1967: 2.
Diagnosis
(after Vercammen-Grandjean Citation1967).
Larva. The posterior-most shield on idiosoma dorsum (pygidial shield, Q5) oblong, undivided. Eye composed of one lens inserted in an elongate ocular plate. Odontus simple. fCx = 2-2-2. Medial coxala I (1a) simple, elongate, smooth. Lateral coxala I (1b), medial and lateral coxala II (2a, 2b) and lateral coxala III (3b) spike-like. Medial coxala III (3a) barbed. fV = 18. Hypostomala in the form of a thick, short spine.
Type species
Hoplothrombium quinquescutatum Ewing, Citation1925
Species included and country record. Ho. quinquescutatum, Canada.
Hosts
[?] Oribatida (Ewing Citation1925).
Beronium Southcott, Citation1986Beronium Southcott, Citation1986: 62.
Diagnosis
(after Southcott Citation1986; Haitlinger Citation1994; Mayoral & Barranco Citation2005b, verified).
Larva. The posterior-most shield on idiosoma dorsum (pygidial shield, Q5) oblong, undivided. Eye lenses absent, ocular plate elongate. Odontus bifid. fCx = 2-1-1. Medial coxala I (1a) simple, thickened in anterior half, then acuminating or short, thin, setulose. Lateral coxala I (1b) short, stout either with blunt end or indistinctly bifid at termination, sometimes amorphic in anterior part. Lateral coxala II (2b) short, stout with rounded ends, slightly bifid. Coxala III (3b) short, peg-like, unilobed or indistinctly bifid. Setae 3a absent. Pre-anal tubercle present. fV 18–23. Setae around anal slit short, slightly thickened or thick, spine-like. Hypostomala reniform.
Type species
Hoplothrombium coiffaiti Beron, Citation1973
Species included and country records. B. coiffaiti, Morocco; B. veronicae, Spain (Canary Islands); B. laemostenis, Spain.
Hosts
Coleoptera: Carabidae (Platyninae: Sphodrini: Sphodrina).
Remarks
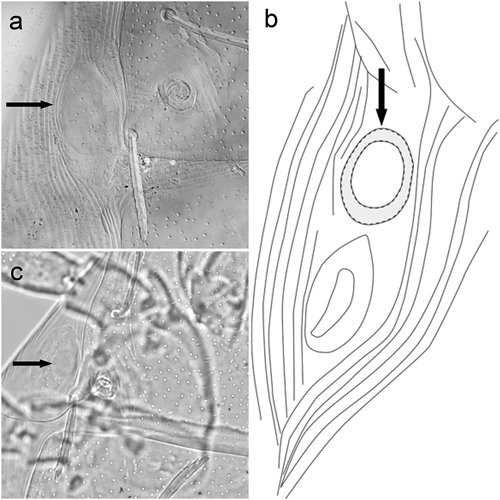
The absence of eyes, hitherto reported for Beronium, constitutes one of the main differences which allow to distinguish between Beronium spp., Alhamitrombium, spp. and Hexathrombium spp. In B. laemostenis and B. veronicae, ocular sclerites are present and they are elongated in shape, narrowing anteriorly and truncated posteriorly ()); they are located adjacent to the posterolateral margins of the scutum, and in a similar location to the ocular plates observed in other trombidioid genera (including Hexathrombium). The plates are punctated over the entire surface and surrounded by folded in lines cuticle. The shape of these sclerotized structures resembles the plates surrounding the eye lenses in Hoplothrombium (Vercammen-Grandjean Citation1967: 5).
Figure 10. Beronium veronicae, holotype, larva. a) ocular sclerite. b) preanal tubercle. Alhamitrombium tetraseta, holotype, larva. c) ocular sclerite. Not to scale
Beronium veronicae Haitlinger, Citation1994Beronium veronicae Haitlinger, Citation1994: 50.
Material examined
Holotype and two paratypes mounted on one slide. For leg chaetotaxy see .
Supplementary data to the original description of B. veronicae: pre-anal tubercle present ()); coxalae 1b and 2b short, thick, indistinctly bifid, rounded lobes; coxalae 3b unilobed, peg-like and rounded terminally; fsol Ge = 1-1-1 (original description fsol Ge = 1-1-0). Setae on legs distinctly setulated. Lophotrix composed of two branches diverging from the main stem; one branch nude, the other one with several (c. 7) secondary branches ()).
Mayoral & Barranco (Citation2005b) in their key to Beronium spp., and followed by Mayoral (Citation2013), referred to the presence of one solenidion on genu I and the lack of solenidia on genu III as characters to differentiate B. veronicae from the other two species in the genus. This was based on the original description of B. veronicae, in which Haitlinger (Citation1994) described one solenidion on genu I and no solenidia on genu III. The present reexamination of the type material of B. veronicae allowed to confirm the presence of one solenidion on Ge I, but there is also a solenidion present on Ge III. Therefore, the presence of one solenidion on genu III should be treated as character shared by all three species assigned to Beronium. It is still possible to separate B. laemostenis from B. veronicae based on the number of solenidia on Ge I (2 vs. 1) (see ). Future reexamination of B. coiffaiti should help to verify the taxonomic status of this species.
Table III. Meristic traits related to idiosoma of Hexathrombium spp. Species with divided pygidial shield Q5: He. abirami, He. cicindelae, He. fageli, He. mamerti, He. sorayae, He. southcotti. Species with undivided pygidial shield: He. marittae, He. lubomirae. For He. willisi see Southcott (Citation1993)
Table IV. Host and distribution data on Hexathrombium spp. recorded from South America
Table V. Leg chaetotaxy of Beronium spp
The leg chaetotaxy of all three nominal species assigned to the genus is provided in . The differences between the members of Beronium may pertain also to the number of setae in fV formula (B. coiffaiti – 19, B. laemostenis – 18–23, B. veronicae – 22(23)).
Alhamitrombium Mayoral & Barranco, 2005Alhamitrombium Mayoral & Barranco, Citation2005a: 111.
Diagnosis
(after Mayoral & Barranco Citation2005a, verified).
Larva. The posterior-most shield on idiosoma dorsum (pygidial shield, Q5) entire, oblong. Eye composed of one lens inserted in elongate ocular plate ()). Odontus bifid. fCx = 2-1-1. Medial coxala I (1a) long, simple, slender. Lateral coxala I (1b) short, stout, distinctly bilobed, coxala II (2b) bilobed, with diverged rounded processes (fan-like), and coxala III (3b) short, peg-like, unilobed ()). Setae 3a absent. fV = 21–23. Setae around anal slit long, robust, tapering and similar to 1a setae. Pre-anal tubercle present. Hypostomala reniform. Lophotrix composed of a main branch with 9 smaller secondary branches that shorten gradually towards distal end; a secondary long branch without ramifications is located anteriorly ()).
Type species
Alhamitrombium tetraseta Mayoral & Barranco, 2005
Species included and country record
A. tetraseta, Spain.
Hosts
Coleoptera: Carabidae (Harpalinae: Lebiini).
Key to Hexathrombiini genera
[incl. Hoplothrombium - monotypic, Alhamitrombium - monotypic, Beronium, Hexathrombium]
Modified from Southcott Citation1993; Haitlinger Citation1994; Citation1997; Mayoral & Barranco Citation2005a, Citation2005b and based on the type material and material examined in this study (see: Material and methods)
1. fCx 2-2-2; lateral coxala I (1b) not modified, simple Hoplothrombium [Ho. quinquescutatum]
- fCx 2-1-1; lateral coxala I (1b) modified, stout, peg-like or bifid 2
2. seta 3a absent; coxala III (3b) not bifid, with rounded or only indistinctly incised termination 3
- seta 3a present or absent; coxala III (3b) bifid, with diverging or adjacent to each other processesHexathrombium [for the selection of species see provisional key below]
3. one eye on each side of prodorsum; ocular plate present; medial coxala (1a) slender, setulose, lateral coxala I (1b) stout, bilobedAlhamitrombium [A. tetraseta]
- eyes absent; ocular plate present; medial coxala (1a) simple, lateral coxala I (1b) stout, indistinctly bifid or bluntBeronium [B. coiffaiti, B. laemostenis, B. veronicae]
Provisional key to Hexathrombium species
1. setae 3a present between coxal plates III [He. abirami, He cicindelae, He. fageli, He. mamerti, He. southcotti, He. spatuliferum, He. willisi] 2
-. setae 3a absent [He. lubomirae, He. marittae, He. sorayae] 6
2. coxalae 1b, 2b, 3b with pointed, widely divergent lobes; pygidial shield (Q5) divided into two separate plates, each encompassing the base of one h1 seta [He. abirami, He. cicindelae, He. fageli, He. mamerti, He. southcotti, He. spatuliferum] 3
-. coxalae 1b, 2b, 3b with rounded, not widely divergent lobes; pygidial shield (Q5) undivided, encompassing the bases of paired h1 setaeHe. willisi [USA, Oklahoma]
3. elongate, needle-shaped seta, much longer than other leg setae, present on tarsus I, tarsus II and femur IIIHe. mamerti [Australia]
-. elongate, needle-shaped seta absent[He. abirami, He. cicindelae, He. fageli, He. southcotti, He. spatuliferum] 4
4. leg setae setulated He. fageli [Ethiopia, Ivory Coast]
- leg setae barbed or nude[He. abirami, [?]He. cicindelae, [?]He. southcotti, He. spatuliferum] 5
5. setae PL levelled with SHe. southcotti [China]
-. setae PL posterior of S [He. abirami [Brazil, Peru], [?]He. cicindelae*, He. spatuliferum*]
6. pygidial shield (Q5) undivided, at most with medial incision at posterior border[He. lubomirae, He. marittae]10
pygidial shield (Q5) dividedHe. sorayae [China]
7. pygidial shield oblong; AP > 50; QL5 < 100He. marittae [Chile]
-. pygidial shield incised posteriorly; AP < 35; QL5 > 100He. lubomirae [Madagascar, Sumatra]
*He. cicindelae [French Guiana] excluded from further key identification due to the insufficiency of data provided in the description; He. spatuliferum [Zaire]– re-examination of type necessary.
Discussion
Fain and Drugmand (Citation1993) and Southcott (Citation1993) erected, independently and within the same year (Citation1993), the tribe Hexathrombiini within Eutrombidiinae to accommodate Beronium, Hexathrombium and Hoplothrombium. Due to the time coincidence, the authorship of the tribe has been assigned by different authors to either Fain and Drugmand (Fain & Jocqué Citation1996) or Southcott (Mayoral & Barranco Citation2005a). Fain and Drugmand published their paper on September 10th, 1993, and Southcott’s paper was published on October 1st, 1993. In accordance with the Article 24 and 50.6 of the International Code of Zoological Nomenclature (Citation1999), we attribute the authorship of the tribe to Fain and Drugmand (Citation1993); thus, Hexathrombiini Southcott, Citation1993 become an objective synonym of Hexathrombiini Fain & Drugmand, Citation1993.
Members of Hexathrombiini display relatively high consistency of character states, however the few differences observed at intratribal level seem to justify the independent status of genera hitherto assigned to this tribe. Among them, the presence/absence of eyes and the shape of coxal setae 1b, 2b, 3b (see also )) are particularly important. On the other hand, a limited number of differences between nominal species assigned to Hexathrombium and Beronium poses the question of the actual species borders. This is accentuated by the limited number of known species for each of these two genera. The answer to the question should be reconsidered in the future when more species are described, taking in consideration larvae, active postlarval forms and molecular results when they are available. The status of the species assigned to Hexathrombium, with special reference to South American and African members, should be revised in the future when the redescriptions of He. cicindelae and He. spatuliferum become available.
The functional significance of pre-anal tubercle in Hexathrombiini should be elucidated. The presence of this structure, referred by Southcott (Citation1993) as a “small near-circular structure”, was confirmed in the species He. willisi, He. cf. marittae and He. cf. cicindelae in Hexathrombium (Southcott Citation1993; Pérez-Espinoza & Moreno Salas Citation2016; Almada & Cédola Citation2017) and also in the species A. tetraseta, B. laemostenis and B. veronicae (see also )) during our reexamination of these type specimens. The tubercle, due to its overall small size, may have been overlooked by some authors and thus omitted in their descriptions.
In Beronium Southcott, Citation1986, the shape of coxal setae, especially 1b, has been variously reported and there seems to be some variability in between specimens and species. Mayoral & Barranco (Citation2005b) reported the presence of blunt and conical lateral coxala I in Beronium. In the current revision of the type material of B. laemostenis, it was possible to observe that there is some variation in the shape of this character among specimens. In this species, 1b seta is mostly thick and conical, but some specimens show a deformation of the seta and in some others, it looks slightly bifid. Haitlinger (Citation1994) did not describe the seta 1b in B. veronicae, (except for referring to it as “thick seta”) but in the present re-examination of the holotype we can confirm the presence of 1b which is indistinctly bifid at termination ()). This character (seta 1b) should be verified in B. coiffaiti, that according to the description of Beron (Citation1973) is dilated.
There are very little data available about the biology and ecology of Hexathrombiini. The infestation of the bright metallic tiger beetle (T. fulgida) by the parasitic mite larvae He. abirami may have taken place on the ground – in wet sand or mud – with sparse or no vegetation. This is the natural habitat of adults of T. fulgida. Remarkably, this beetle has been proposed as a natural predator of the pest mole cricket in golf courses (T. fulgida in GBIF Citation2019). The number of mite larvae recorded (361) on a single host specimen reported in this study is the highest number of parasites recorded in arthropod-associated terrestrial Parasitengona mites. The next two highest values reported were also recorded for Eutrombidiinae larvae. Severin (Citation1944) reported the presence of 175 larvae of Eutrombidium locustarum on Dissosteira carolina (Orthoptera), and Pérez-Espinoza & Moreno Salas (Citation2016) reported 186 larvae of He. cf. marittae infesting Ceroglossus buqueti (Coleoptera: Carabidae). The preferred attachment site of Hexathrombium spp. larvae varied across different hosts, despite the similarities in the hosts’ body (they are closely related species). Around 50% of larvae collected in this study were attached to elytra. No larvae were attached to dorsal abdomen, which is consistent with the observations of Pérez-Espinoza and Moreno Salas (Citation2016). Almada and Cédola (Citation2017) reported that most larvae displayed a preference for the ventral thorax; in our study, only around 14% of larvae were attached to the thorax and venter abdomen.
Several morphological traits (presence of stephanostome facilitating a firm attachment to the host, relatively short legs), behavioral (preference to attachment sites with lower accessibility to hosts’ legs) and ecological (relatively narrow host spectrum) are of significant adaptive advantage and may reflect the relatively long co-evolution of Hexathrombium and other Hexathrombiini with their hosts.
Authors’ contributions
JMąkol and JMayoral contributed to the study design. SF performed the collection of T. fulgida with parasitic mites as well as the morphological identification of the host species. JMąkol performed the morphological analyses of new material of Hexathrombium collected from Peru and type material of Hexathrombium spp. and B. veronicae. JMayoral examined the type material of B. laemostenis and Alhamithrombium. JMąkol and JMayoral wrote the manuscript. All authors reviewed, read and approved the manuscript.
Acknowledgements
We thank Andreas Wohltmann (Ritterhude, Germany) for the discussion on homologies related to the modified termination of tarsus III in microtrombidiid mites. We thank Ryszard Haitlinger (Wrocław University of Environmental and Life Sciences, Poland) who made available to this study the type material of He. abirami, He. lubomirae, He. mamerti, He. marittae and B. veronicae. We thank Franz Wachtel (Grünwald, Germany) and Miriam Steinherr (Augsburg, Germany) for their assistance in collecting T. fulgida in Panguana, Peru.
Furthermore, we thank Diana Silva Dávila (Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru) for her cooperation and the Peruvian authority Servicio Nacional Forestal y de Fauna Silvestre (SERFOR) for issuing the collecting (# 007-2014-SERFOR-DGGSPFFS) and exporting (# 0001757-SERFOR) permits. We are thankful to Maciej Skoracki (Adam Mickiewicz University, Poznań, Poland) for bringing to Poland the Peruvian Hexathrombium from the collection in Germany. Finally, we thank Piotr Daszkiewicz (Muséum national d’Histoire naturelle, Paris, France), Jenny Foster (CSIRO Publishing, Australia) and Camille Locatelli (Royal Belgian Institute of Natural Sciences, Brussels, Belgium) for their help in determining the exact dates of publication of the journal issues containing the descriptions of Hexathrombiini.
Availability of data and material
All data generated or analysed during this study are included in this published article. The type material examined has been loaned from museum institutions. The non-type material of Hexathrombium abirami collected from Peru along with host specimen is stored in the collection of the Department of Invertebrate Systematic and Ecology, Wrocław University of Environmental and Life Sciences, Poland.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Almada MS, Cédola C. 2017. First record of Hexathrombium (Acari: Microtrombidiidae) on Tetracha (Tetracha) brasiliensis brasiliensis (Coleoptera: Cicindelidae) in Argentina. Systematic and Applied Acarology 22(7):1087–1090. DOI:10.11158/saa.22.7.15.
- Beron P. 1973. Une nouvelle larve d’Acarien (Hoplothrombium coiffaiti sp.n., Trombidiidae) parasite d’un Coleoptere cavernicole du Maroc. Annales de Spéléologie 3:413–416.
- Cooreman J. 1944. Notes et observations sur les Acariens III. Bulletin du Musée royal d’histoire naturelle de Belgique 20:1–16.
- Ewing HE. 1925. A contribution to our knowledge of the taxonomy of chiggers including the descriptions of a new genus, six new species and a new variety. The American Journal of Tropical Medicine 5:251–265. DOI:10.4269/ajtmh.1925.s1-5.251.
- Fain A, Drugmand D. 1993. Notes on the genus Hexathrombium Cooreman 1944 (Acari, Trombidiidae) with description of a new tribe and species from afrotropical Staphylinidae (Coleoptera). Bulletin et annales de la Société royale belge d’entomologie 129:121–128.
- Fain A, Jocqué R. 1996. A new genus and species of larval Eutrombidiinae Thor, 1935 (Acari: Microtrombidiidae) from an afrotropical spider. International Journal of Acarology 1:11–16. DOI: 10.1080/01647959608684075.
- Felska M, Wohltmann A, Mąkol J. 2018. A synopsis of host-parasite associations between Trombidioidea (Trombidiformes: Prostigmata, Parasitengona) and arthropod hosts. Systematic and Applied Acarology 23(7):1375–1479. DOI:10.11158/saa.23.7.14.
- Floch H, Abonnenc E. 1941. Trombidides de la Guyana Francaise. Le “pou d’agouti”. Archives de l’Institut Pasteur de la Guyane Française et du Territoire de l'Inini 20:1–22.
- Haitlinger R. 1994. Four new larval mites (Acari: Trombidiidae: Eutrombidiinae) ectoparasitic on carabids (Insecta: Coleoptera: Carabidae). Revista Chilena Entomologia 21:47–56.
- Haitlinger R. 1997. A new species of Hexathrombium Cooreman 1944 based on parasitic larva on erotylids from Brazil (Acari: Eutrombidiidae). Memórias Do Instituto Oswaldo Cruz 92:81–83. DOI:10.1590/S0074-02761997000100015.
- Haitlinger R. 1999. Three new larval mites (Acari: Prostigmata: Eutrombidiidae, Erythraeidae, Trombellidae) from Australia, Turkey and Thailand. Zeszyty Naukowe Akademii Rolniczej We Wrocławiu, Zootechnika XLV 362:57–73.
- Husband RW, Wohltmann A. 2011. A redescription of Eutrombidium locustarum (Walsh) (Acari: Microtrombidiidae) and a new North American Podapolipoides (Acari: Podapolipidae), parasites of Schistocerca piceifrons (Walker) (Orthoptera: Acrididae) from Yucatan, Mexico. International Journal of Acarology 37:260–292. DOI: 10.1080/01647954.2011.564592.
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature. Fourth. London: International Trust for Zoological Nomenclature. pp. i–xxix + 1–306.
- Mayoral JG. 2013. Notes on the genus Beronium (Acari, Eutrombidiinae) enlightened by new captures of Beronium laemostenis in Spain. Acarologia 53:425–427. DOI: 10.1051/acarologia/20122106.
- Mayoral JG, Barranco P. 2005a. A new genus of the Eutrombidiinae Thor 1935 (Acari: Eutrombidiidae) parasitic on an endemic beetle from the south of Spain. Systematic Parasitology 62:111–115. DOI: 10.1007/s11230-005-5485-8.
- Mayoral JG, Barranco P. 2005b. Beronium laemostenis sp. n. (Acari, Eutrombidiinae), a new larval mite on a troglobius beetle from Spain. Biologia - Section Zoology 60:125–128.
- Pérez-Espinoza SA, Moreno Salas L. 2016. Parasitism of Ceroglossus buqueti (Coleoptera: Carabidae) by Hexathrombium mites (Acari: Microtrombidiidae): Body distribution, prevalence, intensity and attachment preferences in relation to body size and sex. International Journal of Acarology 42(5):247–251. DOI:10.1080/01647954.2016.1178329.
- Severin HC. 1944. The grasshopper mite Eutrombidium trigonum (Hermann) an important enemy of grasshoppers. South Dakota Experiment Station Technical Bulletin 3:1–36.
- Southcott RV. 1986. Studies on the taxonomy and biology of the subfamily Trombidiinae (Acarina: Trombidiidae) with a critical revision of the genera. Australian Journal of Zoology, Suppl. Ser 123:1–116. DOI: 10.1071/AJZS123.
- Southcott RV. 1993. Revision of the taxonomy of the larvae of the subfamily Eutrombidiinae (Acarina: Microtrombidiidae). Invertebrate Taxonomy 7:885–959. DOI: 10.1071/IT9930885.
- Tetracha fulgida (Klug, 1834) in GBIF Secretariat. 2019. GBIF backbone taxonomy. Checklist dataset. DOI:10.15468/39omei. accessed via GBIF.org on 2019-12–03.
- Vercammen-Grandjean PH. 1967. Revision of Hoplothrombium quinquescutatum Ewing, 1925 (Trombidiidae: Acarina). Opuscula Zoologica 96:1–7.
- Welbourn WC. 1983. Potential use of trombidioid and erythraeoid mites as biological control agents of insects pests. In: Hoy MA, Cunningham GL, Knutson L, editors. Biological control of pests by mites. Berkeley: Agricultural Experiment Station, Division of Agriculture and Natural Resources, University of California. pp. 103–140. Special Publication 3304.
- Wohltmann A, Gabryś G, Mąkol J. 2007. Acari: Terrestrial Parasitengona inhabiting transient biotopes. In: Gerecke R, editor. Süßwasserfauna von Mitteleuropa 7/2–1, Chelicerata, Araneae, Acari I. München: Spektrum Elsevier. pp. 158–240. [2006].
- Zheng B. 1997. A new species of genus Hexathrombium Cooreman 1944 (Acari: Microtrombidiidae) from China. Entomologia Sinica 4:38–41.