Abstract
The choice of suitable nest materials is a key to nest-building process in birds. During the breeding season (March-August) in 2018 and 2019, we discovered that crested mynas (Acridotheres cristatellus) added snake slough (2–18.3 cm in length) to their nests. The addition of snake slough continued throughout the breeding period, with a proportion of 38.9% being during the nest-building period. The use of snake slough in 2018 (38.5%) was significantly less than in 2019 (71.4%). No significant differences were found in clutch size, egg mass, and egg size between nests with snake slough (the slough group) and nests without snake slough (the non-slough group). There were also no significant differences in the number of nestlings hatched for each nest, nestling growing and breeding success between the snake slough and the non-snake slough group. This is the first study to report that crested mynas add snake slough to their nests throughout their breeding period, but snake slough had little impact on most of the breeding parameters of crested mynas. We speculate that snake slough in crested myna nests may serve to reduce nest predation, improve the immunity of nestlings or serve as a post-mating sexual signal eliciting differential reproductive investment in mates; however, these speculations require further investigation.
Introduction
Nests are essential for successful reproduction in most birds, and may protect their eggs and nestlings from bad weather and predation (Collias & Collias Citation1984; Hansell Citation2000; Mainwaring et al. Citation2014; Deeming & Reynolds Citation2015). Nest-building requires considerable investment (Mainwaring & Hartley Citation2013) in energy (Withers Citation1977; Lens et al. Citation1994; Stanley Citation2002) and time (Nores & Nores Citation1994). In birds, nest-building is thought to be a life-history trade-off (Collias & Collias Citation1984). For example, males who build nests trade off the cost of collecting nest materials with benefits acquired from retaining a territory (Bailey et al. Citation2016). Therefore, during the nest-building process, birds carefully adjust their nesting behavior to optimize the balance between cost and benefit, while selecting a more appropriate material combination to maximize fitness (Collias & Collias Citation1984; Jose et al. Citation1998).
Many factors may affect birds’ choice of nest materials. Choosing the appropriate nest materials is the basis of successful breeding (Hansell Citation2000; Mainwaring et al. Citation2014). The utilization of green plants as nest materials has received special attention in studies of evolutionary ecology (Dubiec et al. Citation2013). Besides reinforcing the nest structure, green plants also serve other functions in bird nests; these include propagating sexual signals (Fauth et al. Citation1991; Gwinner Citation1997; Brouwer & Komdeur Citation2004; Polo et al. Citation2004; Moreno Citation2012; Tomás et al. Citation2013), ensuring nest protection (Trnka & Prokop Citation2011; Liu & Liang Citation2021a), reducing the abundance of parasites and pathogens (Clark & Mason Citation1985; Lafuma et al. Citation2001; Shutler & Campbell Citation2007; Mennerat et al. Citation2009; Scott-Baumann & Morgan Citation2015; Ruiz-Castellano et al. Citation2017), and improving the immunity of nestlings and promoting their growth (Gwinner et al. Citation2000; Tomás et al. Citation2012). Adding green leaves to the nest is thought to be a form of self-medication (de Roode et al. Citation2013). While a number of studies focus on the effects of the use of green plants, so far there has been little work on the effects of the use of snake slough as nest materials (Strecker Citation1926; Medlin & Risch Citation2006; Trnka & Prokop Citation2011).
During the breeding season (March-August) in 2018 and 2019, we observed that crested mynas adding snake slough to their nests (ESM Video S1). In order to explore the effect of this behavior, we compared the breeding parameters and nestling growth between the slough group and the non-slough group.
Materials and methods
Study area and study species
Our study was conducted in a closed orchard in northern Yangheng village of Huangzhu, Ding’an county, Hainan, south China (19°28′56.96″ N, 110°24′47.81″ E). This location is in the central northeast part of Hainan island and is characterized by a tropical marine climate with an average annual temperature being 24°C. The orchard covered an area being around 19 ha. The trees in the orchard consisted mainly of lychee (Litchi chinensis), jackfruit (Artocarpus heterophyllus), wax apple (Syzygium samarangense), mango (Mangifera indica), areca palm (Areca catechu), and Chinese quince (Chaenomeles sinensis) (Liu & Liang Citation2021b).
The crested myna (Acridotheres cristatellus) is a member of the Sturnidae family in the order Passeriformes. It is a common resident bird widely distributed in southern China, of which the Hainan crested myna (A. c. brevipennis) is a subspecies (Feare & Craig Citation1998; Zheng Citation2017). However, studies on breeding ecology of crested mynas are relatively rare and limited to a few cases of observations (Yu & Xi Citation1992; Han et al. Citation1995; Ren et al. Citation2015; Liu & Liang Citation2021b). Additionally, the effects of snake sloughs in crested mynas’ nests have not been explored.
Field data collection
At the beginning of March, the wooden nest boxes (length × width × height: 15 cm × 15 cm × 30 cm; sample sizes: 2018, n = 94; 2019, n = 110) in the research area were numbered and put up (Zhang et al. Citation2019). Then, they were monitored at least once a week. If crested myna nest materials were observed in a nest box, the frequency of monitoring was adjusted to once every three days. A breeding nest referred to a nest where it was observed that at least one egg was produced.
The crested mynas’ nests are divided into the slough group (birds that added snake slough to the nest) and the non-slough group (birds that did not add snake slough to the nest) during the breeding period. The two groups’ parameters were recorded and measured during nest monitoring: (1) the time the snake slough appeared in the nest box; (2) the length of the snake slough (cm); (3) clutch size; (4) mean egg parameters per nest (egg length and width were measured by the vernier caliper that had an accuracy of 0.01 mm; egg mass was measured by an electronic scale that had an accuracy of 0.1 g); (5) nestling mass, accurate to 0.1 g (a nestling was regarded as 0 days old the day it hatched); (6) the number of nestlings hatched in each nest; (7) the number of nestlings that successfully fledged from each nest; (8) the utilization rate of snake slough in the nest; this was calculated as the percentage of observed nests with snake slough out of the total observed breeding nests; (9) breeding success; this referred to the proportion of successful nests (at least one nestling survived and fledged from the nest) out of the total breeding nests.
Data processing and statistical analysis
In 2018 and 2019, 62 and 28 crested myna breeding nests were observed, respectively. Ten nests observed in 2018 were used for other experiments during the incubation period and were excluded from data analysis for this study. All data analysis was performed on IBM SPSS 22.0 for Windows (IBM Corp., Armonk, NY, USA). The one-sample Kolmogorov-Smirnov (K-S) test was used to assess whether the data in both groups followed a normal distribution. If the assumption of normality was satisfied, a T-test was used to compare the mean values; otherwise, a Mann-Whitney U test was used. Differences in utilization rate of snake slough was analyzed either by a Fisher’s exact test or a Chi-square test. ANOVA was used to analyse the effects of year and snake slough on the clutch size, egg parameters and the number of nestlings hatched. Logistic regression was used to analyse the effects of year (2018 or 2019) and snake slough (slough group or non-slough group) on breeding success, the relative level refers to the slough group in 2018. Linear mixed model including year and snake slough as categorical variable and age (days) as continuous variable was used to analyse the effects of year and snake slough on nestling mass (Zuur et al. Citation2009). In this model, we use the mass of each nestling, so the nest was used as a random factor. All tests were two-tailed with a significance level of P < 0.05.
Results
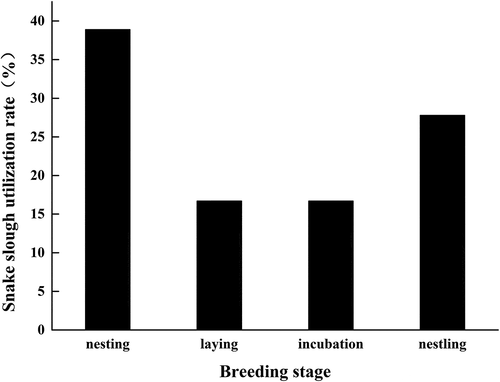
Snake sloughs were mainly from the Natrix genus in the Colubridae family. The average length of snake slough added to the nest in 2018 was significantly shorter (4.11 ± 1.25 cm, n = 12) than in 2019 (8.35 ± 6.04 cm, n = 13) (t = 2.476, P = 0.028); the shortest snake slough measured was 2 cm (2018) and the longest 18.3 cm (2019). The utilization rate of snake slough in 2018 (38.5%) was significantly less than in 2019 (71.4%) (Chi-square test, χ2 = 7.912, df = 1, P = 0.005). This may be due to a shortage of snakeskin in 2018. Furthermore, up to 38.9% of snake slough nests were added during the nest-building period in 2019 ().
In 2018 and 2019, our results showed that years had no effect on the clutch size, egg parameters and the number of nestlings hatched of crested mynas (). There were also no significant differences in the clutch size (3.95 ± 0.69, n = 39 vs. 3.92 ± 0.59, n = 38), egg mass (6.77 ± 0.48 g, n = 94 vs. 6.74 ± 0.49 g, n = 129), egg length (28.77 ± 1.26 mm, n = 94 vs. 28.78 ± 1.01 mm, n = 129), egg width (21.13 ± 0.41 mm, n = 94 vs. 21.10 ± 0.58 mm, n = 129) and number of nestlings hatched (2.36 ± 1.72, n = 39 vs. 2.47 ± 1.83, n = 38) between the snake slough and the non-snake slough groups (all, P > 0.05). And our results did not show any effects of year and snake slough on breeding success and nestlings mass of crested mynas (all, P > 0.05) (). In addition, there was also no significant difference between breeding success of the snake slough (52.5%, n = 40 nests) and the non-snake slough group (45%, n = 40 nests).
Table I. Results of multi-way ANOVA assessing effects of snake slough, year on the clutch size, egg parameters and the number of nestlings hatched of crested mynas. In the table, group refers to the slough group and non-slough group
Table II. Results of logistic regression assessing the effects of year and snake slough on breeding success of crested mynas. In the table, the years include 2018 and 2019, the groups include slough group and non-slough group
Table III. Results of linear mixed model assessing effects of snake slough and age (days) on nestling mass of crested mynas. In the table, group refers to the slough group and non-slough group
Discussion
In this study, we compared clutch size, egg parameters, hatching success, breeding success and nestling mass during the breeding period between the snake slough and the non-snake slough groups. In general, there were no significant differences in clutch size, egg parameters, hatching success, or nestling mass between the two groups. To the best of our knowledge, this is the first study to report that crested mynas add snake slough to their nests throughout their breeding period, although snake slough had little impact on most of the breeding parameters of crested mynas.
Our results showed that, in Hainan, the utilization rate of snake slough in the nest was significantly lower in 2018 than in 2019 and the utilization rates of both years were lower than those observed in a study in Shanxi (100%) (Yu & Xi Citation1992). We speculate that utilization of snake slough in breeding crested mynas may be affected by the geographical region and related to the number of snake slough around the study area. However, the presence of snakes or snake sloughs in the study area needs to be confirmed in future investigation.
Trnka and Prokop (Citation2011) found that great reed warblers (Acrocephalus arundinaceus) incorporated snake slough with other materials in the early stage of nest-building and assumed that snake slough was added to consolidate the structure of the nest. Besides this function, Trnka and Prokop (Citation2011) also suggested that sloughs in great reed warbler nests may serve as a post-pairing signal revealing female parental quality. And in another study, Sanz and Garcianavas (Citation2011) found that in blue tits (Cyanistes caeruleus), males carry feathers to the nest and place them outside the nest cup; male feather-carrying ability constitutes an honest signal of their parental quality, ornamented nests resulted in more fledged young with better body condition than those without feathers carried by males (Sanz & Garcianavas Citation2011). In our study, we did not find snake slough correlate with most breeding parameters of crested mynas. Therefore, this study may not support the above functions.
In our study, we found that the addition of snake slough in crested myna nests lasted throughout the entire breeding period and snake slough were mainly discovered in the nest cup and rarely incorporated with other nest materials. European starling (Sturnus vulgaris) and spotless starling (Sturnus unicolor) males bring green leaves into the nest in the early stage of nest-building to attract females (the courtship hypothesis) (Feare Citation1984; Gwinner Citation1997; Brouwer & Komdeur Citation2004; Dubiec et al. Citation2013). However, we found that crested mynas continuously added snake slough to the nest throughout the breeding period and the proportion of the added snake slough increased up to 27.8% during the incubation period, which is contrary to the courtship hypothesis.
Bird parasites acquire resources directly from birds and this has negative effects on bird reproduction (Møller Citation1990; Møller et al. Citation2004; Asghar et al. Citation2015). We found that there were no significant differences in breeding success between the snake slough and non-snake slough groups. In addition, we did not find that nestlings in the snake slough group grew faster than nestlings in the non-snake slough group. We could not propose that snake slough in crested myna nests may serve to improve the immunity of nestlings or may act as an anti-parasite disinfectant that could promote their growth. Hannam (Citation2006) found that no significant differences in nestling mass or survival rates were found among nests of eastern bluebirds (Sialia sialis) containing different amounts of parasites; however, nestlings from nests with fewer parasites have shorter nestling periods. As our sample size was relatively small, we could not draw a conclusion that the nestling period in the snake slough group was significantly shorter than that of the non-snake slough group. Medlin and Risch (Citation2006) found that by adding snake slough to nests of great crested flycatchers (Myiarchus crinitus), they were effective in deterring mammalian predators, especially the southern flying squirrel (Glaucomys volans). Although our results showed that the breeding success of the snake slough group was not significantly higher than that of the non-snake slough group, we could not rule out the possibility that the snake slough can function as anti-predation, because these boxes’ location (hanged from smooth iron bars) can help reduce predation, especially for reptiles and small mammals. Therefore, the function of snake slough in nests of the crested myna and the evolution of snake slough-adding behavior in breeding crested mynas need to be investigated.
In conclusion, we showed that there were no significant differences in clutch size, egg parameters, and breeding success between the snake slough and the non-snake slough groups in breeding crested mynas. We speculate that snake slough in crested myna nests may function as serve a post-mating sexual signal, reduce nest predation, or improve the immunity of nestlings; however, these speculations require further investigation. To the best of our knowledge, this is the first study to report that crested mynas add snake slough to their nests throughout their breeding period, although snake slough had little impact on most of the breeding parameters of crested mynas.
Authors’ contributions
Wei Liang designed the study; Jinmei Liu carried out field experiments; Jianping Liu performed laboratory and statistical analyses; Jinmei Liu wrote the draft manuscript. Wei Liang revised and improved the manuscript. All authors approved the final submission.
Ethical approval
The experiments reported here comply with the current laws of China. Fieldwork was carried out without specific permit. Experimental procedures were in agreement with the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University (permit no. HNECEE-2016-004).
Supplemental Material
Download MP4 Video (1.9 MB)Acknowledgements
We would like to thank Guangyi Lu for helpful comments on the manuscript, Bo Zhou and Yameng Jin for their help with fieldwork.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Asghar M, Hasselquist D, Hansson B, Hansson B, Zehtindjiev P. 2015. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347:436–438. DOI: 10.1126/science.1261121.
- Bailey IE, Morgan KV, Oschadleus HD, DeRuiter SL, Meddle SL, Healy SD. 2016. Nest-building males trade off material collection costs with territory value. Emu 116:1–8. DOI: 10.1071/MU15022.
- Brouwer L, Komdeur J. 2004. Green nesting material has a function in mate attraction in the European starling. Animal Behaviour 67:539–548. DOI: 10.1016/j.anbehav.2003.07.005.
- Clark L, Mason JR. 1985. Use of nest material as insecticidal and anti-pathogenic agents by the European starling. Oecologia 67:169–176. DOI: 10.1007/BF00384280.
- Collias NE, Collias EC. 1984. Nest building and bird behavior. Princeton: Princeton University Press.
- de Roode JC, Lefèvre T, Hunter MD. 2013. Self-medication in animals. Science 340:150–151. DOI: 10.1126/science.1235824.
- Deeming DC, Reynolds SJ. 2015. Nests, eggs, and incubation: New ideas about avian reproduction. New Yok: Oxford University Press.
- Dubiec A, Góźdź I, Mazgajski TD. 2013. Green plant material in avian nests. Avian Biology Research 6:133–146. DOI: 10.3184/175815513X13615363233558.
- Fauth PT, Krementz DG, Hines JE. 1991. Ectoparasitism and the role of green nesting material in the European starling. Oecologia 88:22–29. DOI: 10.1007/BF00328399.
- Feare C. 1984. The starling. Oxford: Oxford University Press.
- Feare C, Craig A. 1998. Starlings and mynas. London: Christopher Helm.
- Gwinner H. 1997. The function of green plants in nests of European starlings (Sturnus vulgaris). Behaviour 134:337–351. DOI: 10.1163/156853997X00575.
- Gwinner H, Oltrogge M, Trost L, Nienaber U. 2000. Green plants in starling nests: Effects on nestlings. Animal Behaviour 59:301–309. DOI: 10.1006/anbe.1999.1306.
- Han S, Tan M, Song X. 1995. Breeding ecology of crested mynas in captivity in Dalian, Liaoning. Chinese Wildlife 6:13–14.
- Hannam K. 2006. Ectoparasitic blow flies (Protocalliphora sp.) and nestling eastern bluebirds (Sialia sialis): Direct effects and compensatory strategies. Canadian Journal of Zoology 84:921–930. DOI: 10.1139/z06-079.
- Hansell M. 2000. Bird nests and construction behaviour. Cambridge: Cambridge University Press.
- Jose J, Møller AP, Soler M. 1998. Nest building, sexual selection and parental investment. Evolutionary Ecology 12:427–441. DOI: 10.1023/A:1006520821219.
- Lafuma L, Lambrechts MM, Raymond M. 2001. Aromatic plants in bird nests as a protection against blood-sucking flying insects? Behavioural Processes 56:113–120. DOI: 10.1016/S0376-6357(01)00191-7.
- Lens L, Wauters LA, Dhondt AA. 1994. Nest-building by crested tit Parus cristatus males: An analysis of costs and benefits. Behavioral Ecology and Sociobiology 35:431–436. DOI: 10.1007/BF00165846.
- Liu J, Liang W. 2021a. Snake slough in birds’ nests acts as a nest predator deterrent. Ethology Ecology & Evolution 1–12. DOI: 10.1080/03949370.2021.1871965.
- Liu J, Liang W. 2021b. The breeding ecology of the crested myna Acridotheres cristatellus on tropical Hainan Island. Ornithological Science 20:83–92.
- Mainwaring MC, Hartley IR. 2013. The energetic costs of nest building in birds. Avian Biology Research 6:12–17. DOI: 10.3184/175815512X13528994072997.
- Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds’ nests. Ecology and Evolution 4:3909–3928. DOI: 10.1002/ece3.1054.
- Medlin EC, Risch TS. 2006. An experimental test of snake slough use to deter nest predation. Condor 108:963–965. DOI: 10.1093/condor/108.4.963.
- Mennerat A, Mirleau P, Blondel J, Perret P, Lambrechts MM, Heeb P. 2009. Aromatic plants in nests of the blue tit Cyanistes caeruleus protect chicks from bacteria. Oecologia 161:849–855. DOI: 10.1007/s00442-009-1418-6.
- Møller AP. 1990. Effects of parasitism by a haematophagous mite on reproduction in the barn swallow. Ecology 71:2345–2357. DOI: 10.2307/1938645.
- Møller AP, Christe P, Garamszegi LZ. 2004. Coevolutionary arms races: Increased host immune defense promotes specialization by avian fleas. Journal of Evolutionary Biology 18:46–59. DOI: 10.1111/j.1420-9101.2004.00774.x.
- Moreno J. 2012. Avian nests and nest-building as signals. Avian Biology Research 5:238–251. DOI: 10.3184/175815512X13534385822786.
- Nores AI, Nores M. 1994. Nest building and nesting behavior of the brown cacholote. Wilson Bulletin 106:106–120.
- Polo V, Veiga JP, Cordero PJ, Vinuela J, Monaghan P. 2004. Female starlings adjust primary sex ratio in response to aromatic plants in the nest. Proceedings of the Royal Society of London B: Biological Sciences 271:1929–1933. DOI: 10.1098/rspb.2004.2801.
- Ren Y, Song D, Yu W, Li Z. 2015. Study on the behavioral rhythms of Acridotheres cristatellus during the breeding period. Heilongjiang Animal Science and Veterinary Medicine 58:25–27.
- Ruiz-Castellano C, Tomás G, Ruiz-Rodríguez M, Soler JJ. 2017. Nest material preferences by spotless starlings. Behavioral Ecology 29:137–144. DOI: 10.1093/beheco/arx139.
- Sanz JJ, Garcianavas V. 2011. Nest ornamentation in blue tits: Is feather carrying ability a male status signal? Behavioral Ecology 22:240–247. DOI: 10.1093/beheco/arq199.
- Scott-Baumann JF, Morgan ER. 2015. A review of the nest protection hypothesis: Does inclusion of fresh green plant material in birds’ nests reduce parasite infestation? Parasitology 142:1016–1023. DOI: 10.1017/S0031182015000189.
- Shutler D, Campbell AA. 2007. Experimental addition of greenery reduces flea loads in nests of a non‐greenery using species, the tree swallow Tachycineta bicolor. Journal of Avian Biology 38:7–12. DOI: 10.1111/j.2007.0908-8857.04015.x.
- Stanley TR. 2002. How many kilojoules does a black-billed magpie nest cost? Journal of Field Ornithology 73:292–298. DOI: 10.1648/0273-8570-73.3.292.
- Strecker JK. 1926. On the use, by birds, of snakes’ slough as nesting material. Auk 43:501–507. DOI: 10.2307/4075138.
- Tomás G, Merino S, Martínez-de la Puente J, Merino S, Moreno J. 2012. Interacting effects of aromatic plants and female age on nest-dwelling ectoparasites and blood-sucking flies in avian nests. Behavioral Processes 90:246–253. DOI: 10.1016/j.beproc.2012.02.003.
- Tomás G, Merino S, Martínez-de la Puente J, Moreno J, Morales J, Rivero-de Aguilar J. 2013. Nest size and aromatic plants in the nest as sexually selected female traits in blue tits. Behavioral Ecology 24:926–934. DOI: 10.1093/beheco/art015.
- Trnka A, Prokop P. 2011. The use and function of snake slough in the nests of great reed warblers Acrocephalus arundinaceus. Ibis 153:627–630. DOI: 10.1111/j.1474-919X.2011.01124.x.
- Withers PC. 1977. Energetic aspects of reproduction by the cliff swallow. Auk 94:718–725. DOI: 10.2307/4085268.
- Yu X, Xi Y. 1992. Observations on breeding of crested myna. Chinese Journal of Zoology 37:30–32.
- Zhang J, Wang J, Liu J, Liang W. 2019. Latitudinal variation in use of artificial nestbox by cavity-nesting birds in China. Chinese Journal of Zoology 54:465–470.
- Zheng G. 2017. A checklist on the classification and distribution of the birds of China. 3rd ed. Beijing: Science Press.
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer-Verlag.