Abstract
The present contribution is the first record of ornamental freshwater shrimp Caridina babaulti from European open waters and the first evidence of introduction outside its native range. Among 120 non-native freshwater shrimps collected in Miskolctapolca, Hungary, seven individuals (5.8%) were identified as C. babaulti and included one ovigerous female, while the others (94.2%) were Neocaridina davidi. Morphological analysis and the Standard DNA Barcoding Procedure confirmed the taxonomic identification of the two species. The presence of C. babaulti in Miskolctapolca is most likely due to intentional or unintentional releases by aquarium owners. The low number of captured individuals and this species’ absence in a previous survey suggest that the shrimp is of recent introduction. C. babaulti and the well-established N. davidi have similar environmental tolerance; hence, this species might potentially form a viable population if not removed.
1. Introduction
Releases of non-indigenous aquarium pets into new locations have been observed worldwide (Patoka et al. Citation2016, Citation2020; Harris et al. Citation2017; Hossain et al. Citation2018). In many cases, introduced fish and aquatic invertebrates quickly became invasive, damaging native organisms and natural ecosystems (Capps & Flecker Citation2013; Chucholl Citation2014). For instance, crayfish from the Cambaridae family are superior competitors and vectors for the crayfish plague agent, Aphanomyces astaci, responsible for the mass destruction of European crayfish populations (Lodge et al. Citation2012; Svoboda et al. Citation2017; Lo Parrino et al. Citation2020). With increasing global temperature and the thriving pet trade, other crustaceans, such as warm-adapted ornamental freshwater shrimps, when released into the natural environment, may in the near future have the potential of becoming invasive species. The recent discovery of stable populations of ornamental shrimps outside their native range seems to point in this direction (Mitsugi & Suzuki Citation2018; Schoolmann & Arndt Citation2018; Levitt-Barmats et al. Citation2019).
The most common genera of freshwater shrimp kept in aquaria are Caridina and Neocaridina of the Atyidae family (De Grave et al. Citation2008). These shrimps are characterized by a wide variety of colourations, which make them particularly valuable for both private and professional aquarists (Maciaszek et al. Citation2018). However, shrimps with poor coloration are often disregarded and might consequently be used as live food in aquaculture or released in natural environments (Hung et al. Citation1993). Established populations of aquarium shrimps have been reported in Hawaii (Englund & Cai Citation1999), Japan (Mitsugi & Suzuki Citation2018), Israel (Levitt-Barmats et al. Citation2019), Germany (Klotz et al. Citation2013), Poland (Jabłońska et al. Citation2018a), and Hungary (Weiperth et al. Citation2019). To date, the only nonindigenous freshwater aquarium shrimp occurring in European waters belong to the genus Neocaridina and are primarily found in thermally polluted reservoirs and watercourses (Klotz et al. Citation2013; Jabłońska et al. Citation2018a; Weiperth et al. Citation2019).
In temperate regions, thermally altered water plays a key role in the establishment of exotic warm adapted species (Piazzini et al. Citation2010; Emde et al. Citation2016). For instance, in Miskolctapolca, Hungary (our study area), Neocaridina davidi is not only well established in heated water but has also been observed in adjacent non-thermal water subject to seasonal fluctuations in temperature (Weiperth et al. Citation2019).
In the present study, we report the occurrence of Caridina babaulti in European waters for the first time. This is also the first evidence of human-mediated introduction of this crustacean outside its native range. C. babaulti is native to inland waters of central, western, and southern India (Pandya & Richard Citation2019). This shrimp is sold as a pet in the aquarium trade, most commonly in its green variety. Until recently, it was the only green-colored freshwater shrimp available in the pet trade; however, with the mass production of new and more attractive varieties of N. davidi, including a dark green one, C. babaulti gradually lost its appeal among hobbyists. Hence, its chances of being released in natural environments increased.
2. Material and methods
2.1. Locality
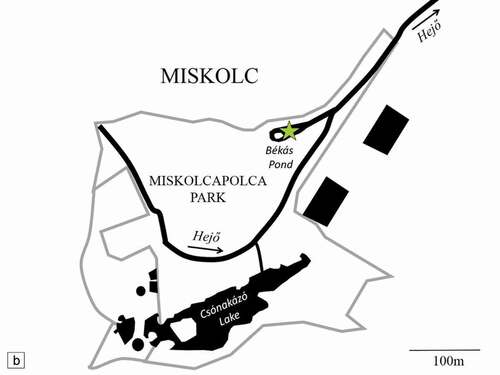
Miskolctapolca Park, located in Miskolc city, north-east Hungary, belongs to one of the most popular country recreational destinations due to its thermal spring and related public spa (). Heated waters are present in open water bodies of the park such as the Békás Pond (,)), which is known to host several non-native species such as the turtle Trachemys scripta (Schoepf, 1792), the fishesCyprinus carpio (Linnaeus, and Lepomis gibbosus (Linnaeus, 1758), and the shrimp N. davidi (Weiperth et al. Citation2019); however, the presence of the crayfish Procambarus virginalis (Lyko, 2017) was also detected during our studies. The pond waters are subject to temperature fluctuations; however, they remain over 20°C all year round (Weiperth et al. Citation2019). The rocky walls of the reservoir and numerous aquatic plants are covered with algae providing food and shelter for crustaceans (,)). The thermal waters of Békás Pond flow directly into the regulated Hejő brook, where the water gradually dissipates heat before flowing into the Tisza river. At least 32 native and non-native fish species and the shrimp N. davidi have been observed in Hejő brook (Harka & Szepesi Citation2007; Weiperth et al. Citation2019).
2.2. Shrimp detection and identification
A total of 120 adult shrimps (>0.5 cm) were collected from the pond using a hydrobiological net on the 10 April 2019. As initially similarly coloured shrimps immediately changed their colouration, they were separated into two groups differing in colour. Collected individuals were then measured and photographed under a stereo microscope for morphological identification. The morphological examination was carried out based on the available literature (Klotz et al. Citation2013; Pandya & Richard Citation2019).
2.3. Genetic analysis
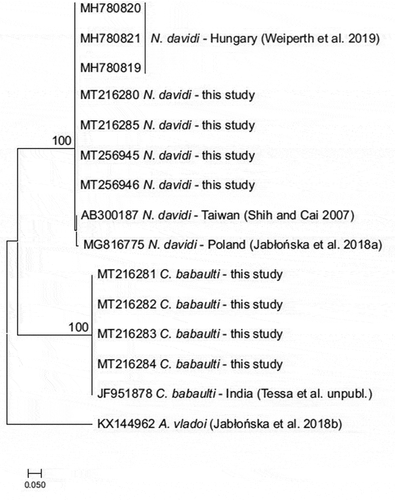
A sample of four individuals identified as C. babaulti and two individuals recognised as N. davidi were used for molecular identification using the standard DNA barcoding procedure with the cytochrome C oxidase subunit I (COI) marker. Pleopod muscle tissue was taken for DNA extraction using the Chelex method (Casquet et al. Citation2012). The polymerase chain reaction (PCR) with the primer pair HCOJJ/LCOJJ (Astrin & Stüben Citation2008) was applied according to the protocol by Hou et al. (Citation2007). PCR products were purified with Exonuclease I and FastAP alkaline phosphatase (Werle et al. Citation1994) and then sent to Macrogen Inc., Europe for the BigDye terminator technology sequencing. Obtained sequences were aligned with the pairwise alignment based on Smith and Waterman (Citation1981) algorithm and trimmed to the same length (590 bp) in Geneious (Kearse et al. Citation2012). The sequences were subsequently deposited in BOLD Systems (Ratnasingham & Hebert Citation2007) and in the GenBank database (Benson et al. Citation2005) with accession numbers MT216280, MT216285, MT256945, and MT256946 for N. davidi, as well as from MT216281 to MT216284 for C. babaulti. Additional sequences of C. babaulti and N. davidi were retrieved from GenBank. The accession number was JF951878 for C. babaulti (Tessa et al., unpublished), while for N. davidi were AB300187 (Shih & Cai Citation2007), MG816775 (Jabłońska et al. Citation2018a), and MH780819-21 (Weiperth et al. Citation2019). The Maximum Likelihood phylogenetic tree was constructed with the HKY substitution model, in which the validity of nodes was estimated by the bootstrap test of 1000 replicates (Hasegawa et al. Citation1985; Felsenstein Citation1985). The analysis was conducted in MEGA 7.0 (Kumar et al. Citation2016). As an outgroup, the sequence of Atyaephyra vladoi, deposited in GenBank (accession number KX144962) by Jabłońska et al. (Citation2018b), was used. Haplotypes were detected in the DnaSP software (Librado & Rozas Citation2009).
3. Results
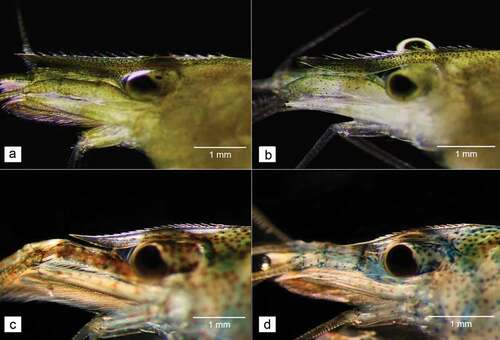
Among the 120 collected shrimps, seven individuals (5.8%) were morphologically identified as C. babaulti, while the remaining individuals (94.2%) were identified as N. davidi. During harvesting, shrimps were characterised by numerous small chocolate pigment spots present all over the body. The only exception were females, whose whole body was covered by chocolate pigment. After they were caught, all specimens were transparent, and turned into two different colours. All shrimp that turned their colouration to greenish yellow were then identified as members of C. babaulti, while most of the specimens, which turned blue, were recognised as representatives of N. davidi. With over 70% of the individuals being females, the sex ratio of both C. babaulti and N. davidi was heavily skewed toward this gender. An ovigerous female was detected among the captured C. babaulti. The average length of C. babaulti was 20.6 mm ± 6.8 SD, while N. davidi was characterised by 16.5 mm ± 8.3 SD (). The average ratio between the carapax length and total length was 1:2.86 for C. babaulti and 1:3.05 for N. davidi. The rostrum of C. babaulti had three to seven postorbital teeth, while that of N. davidi had 2–3 teeth ().
Table I. Characteristics of Caridina babaulti and Neocaridina davidi collected in Békás Pond, Miskolc, Hungary
Figure 3. Rostrum differences: (a) female and (b) male C. babaulti, (c) female, and (d) male N. davidi.
DNA barcodes confirmed morphological identification. As shown by the phylogenetic tree, the collected shrimps are grouped within two, well-distinguished clades (). Obtained sequences of C. babaulti belonged to one haplotype, which was also shared with a specimen sampled from India by Tessa et al. (unpublished). Sequences of N. davidi were ascribed to three haplotypes. Individuals collected during this study and those reported by Weiperth et al. (Citation2019) belong to the same haplotype (). In BOLD systems, the sequences of C. babaulti were ascribed to the non-ubique BIN, ACH2908, together with three specimens mined from the GenBank collection (acc. no. JF951876-8), while the sequences of N. davidi were ascribed to the non-unique BIN, AAD9045, which brings together a total of 129 members deposited by various authors.
4. Discussion
To our knowledge, Caridina babaulti has never been previously reported from natural environments outside its native range. We here provide the first evidence of its introduction in European waters. The presence of an ovigerous female and smaller and immature individuals in Békás Pond suggests that this thermally polluted water provides a suitable environment for its survival and reproduction. However, given the limited number of individuals collected and the sampling carried out only once, we cannot confirm that C. babaulti is currently well established in the waterbody. The low number of captured individuals and the absence of this species in a previous survey (Weiperth et al. Citation2019) suggests that the shrimp are an effect of recent introduction. The presence of individuals of various sizes might result from human-mediated introduction and may not necessarily represent a different generation of shrimp born in the pond. However, the sex ratio in both shrimps species was similarly skewed toward females. Temperature severely affects the sex ratio in N. davidi, with females being more prevalent at lower temperatures (80% at 20°C) and males at higher temperatures (Serezli et al. Citation2017). The water temperature of Békás Pond fluctuates over time, with the lowest temperature being 21.9°C near its outlet (Weiperth et al. Citation2019). If a similar mechanism occurs in C. babaulti, the observed inequality among sexes might result from several reproductions within the pond. C. babaulti has similar environmental requirements to the already well-established N. davidi and might potentially generate a viable population. However, predation by fish and possible intraspecific competition with the more numerous N. davidi might limit the establishment of this species. Unfortunately, due to the snapshot nature of this study, the long-term population dynamics of C. babaulti in Békás Pond are not known.
Although both caught species were different in body and rostrum shape, observations confirmed less numerous C. babaulti resembling its colouration to predominating N. davidi. As all the shrimps were characterized with chocolate pigments, they may be identical in camouflage. This ability may cause potential problems in the identification of shrimp within their natural environments, but also at new sites, where the occurrence of several species may not be initially expected. As colour change being shrimp response to stress (Bauer Citation2004) was the first observed distinction between the species, we suggest that it can be used as an initial, but not sole, feature to determine possible differences within the collected individuals.
The occurrence of several warm-adapted non-native species in temperate European inland waters can be traced to releases of exotic species in thermally polluted waters (Bonk et al. Citation2018; Labecka & Domagala Citation2018; Weiperth et al. Citation2019). Exotic species often have a detrimental impact on native species and the whole ecosystem (Zavaleta et al. Citation2001; Gallardo et al. Citation2016; Kiruba-Sankar et al. Citation2018). Biodiversity loss is commonly associated with predation and competition for resources with native species (Mooney & Cleland Citation2001; Doherty et al. Citation2016). However, non-native species are also potential vectors for pests and diseases, which might have a devastating effect on the local fauna (Svoboda et al. Citation2017). Shrimps of the Atyidae family have been proposed as a vector of the crayfish plague (Mrugała et al. Citation2019) and are a known host of unwanted parasites (Patoka et al. Citation2016; Maciaszek et al. Citation2018, Citation2020). In the presence of such unwanted organisms, the co-occurrence of several non-native shrimps and crayfish in Békás Pond might potentially result in cross contamination. Even if C. babaulti might not establish in the water surrounding the thermal pond, possible cross-infection with N. davidi might spread pests and diseases to the local fauna. We, therefore, suggest the removal of all non-native species from the site. Increased awareness through education and the implementation of more restrictive measures to counteract the uncontrolled release of ornamental pets in situ is needed.
Geolocation information
Békás Pond: 48.9625°N; 20.7478°E
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Astrin JJ, Stüben PE. 2008. Phylogeny in cryptic weevils: Molecules, morphology and new genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae). Invertebrate Systematics 22(5):503–522. DOI:10.1071/IS07057.
- Bauer RT. 2004. Remarkable shrimps: Adaptations and natural history of the carideans. Vol. 7. University of Oklahoma Press. pp 95–102.
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL. 2005. GenBank. Nucleic Acids Research 33(Suppl 1):D34–D38. DOI:10.1093/nar/gki063.
- Bonk M, Zając K, Lipińska AM. 2018. Rapid expansion of the Asian clam Corbicula fluminea (O. F. Müller, 1774): A new alien species in the mollusk community of the Vistula. Oceanological and Hydrobiological Studies 47(1):75–86. DOI:10.1515/ohs-2018-0009.
- Capps KA, Flecker AS. 2013. Invasive aquarium fish transform ecosystem nutrient dynamics. Proceedings of the Royal Society B: Biological Sciences 280(1769):20131520. DOI:10.1098/rspb.2013.1520.
- Casquet J, Thebaud C, Gillespie RG. 2012. Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Molecular Ecology Resources 12(1):136–141. DOI:10.1111/j.1755-0998.2011.03073.x.
- Chucholl C. 2014. Predicting the risk of introduction and establishment of an exotic aquarium animal in Europe: Insights from one decade of Marmorkrebs (Crustacea: Astacida: Cambaridae) releases. Management of Biological Invasions 5(4):309–318. DOI:10.3391/mbi.2014.5.4.01.
- De Grave S, Cai Y, Anker A. 2008. Global diversity of shrimps (Crustacea: Decapoda: Caridea) in freshwater. Hydrobiologia 595(1):287–293. DOI:10.1007/s10750-007-9024-2.
- Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences 113(40):11261–11265. DOI:10.1073/pnas.1602480113.
- Emde S, Kochmann J, Kuhn T, Dörge DD, Plath M, Miesen FW, Klimpel S. 2016. Cooling water of power plant creates “hot spots” for tropical fishes and parasites. Parasitology Research 115(1):85–98. DOI:10.1007/s00436-015-4724-4.
- Englund RA, Cai Y. 1999. The occurrence and description of Neocaridina denticulata sinensis (Kemp, 1918) (Crustacea: Decapoda: Atyidae), a new introduction to the Hawaiian Islands. Bishop Museum Occasional Paper 58:58–65.
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39(4):783–791. DOI:10.1111/j.1558-5646.1985.tb00420.x.
- Gallardo B, Clavero M, Sánchez MI, Vilà M. 2016. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology 22(1):151–163. DOI:10.1111/gcb.13004.
- Harka Á, Szepesi Z. 2007. Study of the Hejő brook watershed fish fauna. Acta Agraria Debreceniensis 25:113–117.
- Harris A, Page TJ, Fotedar S, Duffy R, Snow M. 2017. Molecular identification of the precise geographic origins of an invasive shrimp species in a globally significant Australian biodiversity hotspot. Biological Invasions 19(2):463–468. DOI:10.1007/s10530-016-1311-2.
- Hasegawa M, Kishino H, Yano T. 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22(2):160–174. DOI:10.1007/BF02101694.
- Hossain MS, Patoka J, Kouba A, Buřič M. 2018. Clonal crayfish as biological model: A review on marbled crayfish. Biologia 73(9):841–855. DOI:10.2478/s11756-018-0098-2.
- Hou Z, Fu J, Li S. 2007. A molecular phylogeny of the genus Gammarus (Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences. Molecular Phylogenetics and Evolution 45(2):596–611. DOI:10.1016/j.ympev.2007.06.006.
- Hung M-S, Chan T-Y, Yu H-P. 1993. Atyid Shrimps (Decapoda: Caridea) of Taiwan, with descriptions of three new species. Journal of Crustacean Biology 13(3):481–503. DOI:10.2307/1548789.
- Jabłońska A, Mamos T, Gruszka P, Szlauer-Łukaszewska A, Grabowski M. 2018a. First record and DNA barcodes of the aquarium shrimp, Neocaridina davidi, in Central Europe from thermally polluted River Oder canal, Poland. Knowledge and Management of Aquatic Ecosystems 14.
- Jabłońska A, Mamos T, Zawal A, Grabowski M. 2018b. Morphological and molecular evidence for a new shrimp species, Atyaephyra vladoi sp. nov. (Decapoda: Atyidae) in the ancient Skadar Lake system, Balkan Peninsula – Its evolutionary relationships and demographic history. Zoologischer Anzeiger 275:66–79. DOI:10.1016/j.jcz.2018.05.004.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647–1649. DOI:10.1093/bioinformatics/bts199.
- Kiruba-Sankar R, Praveen Raj J, Saravanan K, Lohith Kumar K, Raymond Jani Angel J, Velmurugan A, Dam RS. 2018. Chapter 9 - Invasive species in freshwater ecosystems – Threats to ecosystem services. In: Sivaperuman C, Velmurugan A, Ak S, Jaisankar I, editors. Biodiversity and Climate Change Adaptation in Tropical Islands. Academic Press. pp. 257–296.
- Klotz W, Miesen FW, Hüllen S, Herder F. 2013. Two Asian fresh water shrimp species found in a thermally polluted stream system in North Rhine-Westphalia, Germany. Aquatic Invasions 8(3):333–339. DOI:10.3391/ai.2013.8.3.09.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI:10.1093/molbev/msw054.
- Labecka AM, Domagala J. 2018. Continuous reproduction of Sinanodonta woodiana (Lea, 1824) females: An invasive mussel species in a female-biased population. Hydrobiologia 810(1):57–76. DOI:10.1007/s10750-016-2835-2.
- Levitt-Barmats Y, Yanai Z, Cohen T, Shenkar N. 2019. Life-history traits and ecological characteristics of the ornamental shrimp Neocaridina denticulata (De Haan, 1844), recently introduced into the freshwater systems of Israel. Aquatic Invasions 14(4):684–702. DOI:10.3391/ai.2019.14.4.08.
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452. DOI:10.1093/bioinformatics/btp187.
- Lo Parrino E, Ficetola GF, Manenti R, Falaschi M. 2020. Thirty years of invasion: The distribution of the invasive crayfish Procambarus clarkii in Italy. Biogeographia – The Journal of Integrative Biogeography 35.
- Lodge DM, Deines A, Gherardi F, Yeo DCJ, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA, Howard GW, Jerde CL, Peters BW, Peters JA, Sargent LW, Turner CR, Wittmann ME, Zeng Y. 2012. Global introductions of crayfishes: Evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology, Evolution, and Systematics 43(1):449–472. DOI:10.1146/annurev-ecolsys-111511-103919.
- Maciaszek R, Jabłońska A, Prati S, Świderek W. 2020. First report of freshwater atyid shrimp, Caridina formosae (Decapoda: Caridea) as a host of ectosymbiotic branchiobdellidan, Holtodrilus truncatus (Annelida: Citellata). Knowledge and Management of Aquatic Ecosystems 33.
- Maciaszek R, Kamaszewski M, Łapa P, Strużyński W. 2018. Epibionts of ornamental freshwater shrimps bred in Taiwan. Annals of Warsaw University of Life Sciences - SGGW - Animal Science 57(2):133–142. DOI:10.22630/AAS.2018.57.2.13.
- Mitsugi M, Suzuki H. 2018. Life history of an invasive freshwater shrimp Neocaridina davidi (Bouvier, 1904), (Decapoda: Caridea: Atyidae) in the Tomoe River, the Boso Peninsula, eastern Japan. Crustacean Research 47:9–16. DOI:10.18353/crustacea.47.0_9.
- Mooney HA, Cleland EE. 2001. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences 98(10):5446–5451. DOI:10.1073/pnas.091093398.
- Mrugała A, Buřič M, Petrusek A, Kouba A. 2019. May atyid shrimps act as potential vectors of crayfish plague? NeoBiota 51:65–80. DOI:10.3897/neobiota.51.37718.
- Pandya PJ, Richard J. 2019. Report of Caridina babaulti Bouvier, 1918 (Crustacea: Decapoda: Caridea: Atyidae) and description of a new species Caridina kutchi sp. nov. from Gujarat, India. Zootaxa 4568(3):470–482. DOI:10.11646/zootaxa.4568.3.3.
- Patoka J, Bláha M, Devetter M, Rylková K, Čadková Z, Kalous L. 2016. Aquarium hitchhikers: Attached commensals imported with freshwater shrimps via the pet trade. Biological Invasions 18(2):457–461. DOI:10.1007/s10530-015-1018-9.
- Patoka J, Takdir M, Yonvitner AH, Jerikho R, Nilawati J, Tantu FY, Bohatá L, Aulia A, Kamal MM, Wardiatno Y, Petrtýl M. 2020. Two species of illegal South American sailfin catfish of the genus Pterygoplichthys well-established in Indonesia. Knowledge and Management of Aquatic Ecosystems. 28.
- Piazzini S, Lori E, Favilli L, Cianfanelli S, Vanni S, Manganelli G. 2010. A tropical fish community in thermal waters of southern Tuscany. Biological Invasions 12(9):2959–2965. DOI:10.1007/s10530-010-9695-x.
- Ratnasingham S, Hebert PDN. 2007. BOLD: The barcode of life data system. Molecular Ecology Notes 7(3):355–364. Available: http://www.barcodinglife.org
- Schoolmann G, Arndt H. 2018. Population dynamics of the invasive freshwater shrimp Neocaridina davidi in the thermally polluted Gillbach stream (North Rhine-Westphalia, Germany). Limnologica 71:1–7. DOI:10.1016/j.limno.2018.05.001.
- Serezli R, Atalar MS, Hamzacebi S, Kurtoglu IZ, Yandi I. 2017. To what extent does temperature affect sex ratio in red cherry shrimp, Neocaridina davidi? The scenario global warming to offspring sex ratio. Fresenius Environmental Bulletin 26:7575–7579.
- Shih H-T, Cai Y. 2007. Two new species of the land-locked freshwater shrimps genus, Neocaridina Kubo, 1938 (Decapoda: Caridea: Atyidae), from Taiwan, with notes on speciation on the island. Zoological Studies 15.
- Smith TF, Waterman MS. 1981. Identification of common molecular subsequences. Journal of Molecular Biology 147(1):195–197. DOI:10.1016/0022-2836(81)90087-5.
- Svoboda J, Mrugała A, Kozubíková-Balcarová E, Petrusek A. 2017. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. Journal of Fish Diseases 40(1):127–140. DOI:10.1111/jfd.12472.
- Weiperth A, Gábris V, Danyik T, Farkas A, Kuříková P, Kouba A, Patoka J. 2019. Occurrence of non-native red cherry shrimp in European temperate waterbodies: A case study from Hungary. Knowledge and Management of Aquatic Ecosystems 9.
- Werle E, Schneider C, Renner M, Völker M, Fiehn W. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22(20):4354–4355. DOI:10.1093/nar/22.20.4354.
- Zavaleta ES, Hobbs RJ, Mooney HA. 2001. Viewing invasive species removal in a whole-ecosystem context. Trends in Ecology & Evolution 16(8):454–459. DOI:10.1016/S0169-5347(01)02194-2.