Abstract
A considerable number of avian species tend to use bryophytes as nest construction material, but the selectivity of birds in their bryophyte use in nest construction in relation to the availability of particular mosses in individual territories remains largely unexplored. We studied the bryophyte composition of nests in two passerine species: the Blue Tit Cyanistes caeruleus and the Great Tit Parus major in two floristically and structurally contrasting study areas (a mature deciduous forest and an urban parkland). We also studied territories within a radius of 10 m from each occupied nest box, listing all bryophyte species and checking to what extent the moss composition of the nest reflected what was available in the surrounding of the nest box. Both tit species appeared to be selective. The tits used a total of 31 bryophyte species in their nests (27 found in Blue Tits and 19 in Great Tits), whereas 25 additional bryophyte species were found around (10 m radius) the studied nests, but not in the nests. The number of bryophyte species found in individual nests varied in the urban parkland between 1 and 6 and in the forest between 1 and 11. The average number of bryophytes in the nest ranged from 2.31 in Great Tit from the urban parkland to 3.52 in Blue Tit in the urban parkland. Only a few species were used in a higher proportion than 5% of the volume within a single nest and, considering only these most abundant bryophyte species, the mean number ranged from 1.28 in Great Tits in the forest to 1.57 in Blue Tits in the urban parkland. Our study also showed that, in general, the bryophyte species composition of the nests seems to be affected by the site (the urban park vs the forest) rather than the parid species (Blue Tit vs Great Tit).
Introduction
In birds, nesting requires some time-consuming undertakings, including nest construction, egg laying, incubation and chick rearing. Constructing some sort of nest is common among most bird species (Hansell Citation2000; Heenan Citation2013; Mainwaring Citation2015). A bird’s nest is a structure which exhibits several functions. It provides a specific environment for eggs, nestlings and parents (e.g. protects against excessive moisture and low temperatures); it may contribute to protection from predators and fix the position of eggs during incubation (Collias & Collias Citation1984; Deeming Citation2011; Deeming et al. Citation2012; Lambrechts et al. Citation2016a, Citation2016b; Akresh et al. Citation2017; Deeming & Campion Citation2018). In some passerine species, like tits, some nest materials may also serve as parasite repellents and/or antimicrobial agent (Petit et al. Citation2002; Pires et al. Citation2012; Dubiec, Góźdź & Mazgajski Citation2013; Zabłotni et al. Citation2020; Glądalski et al. Citation2020a).
Nest construction is a plastic behaviour, which is characterized by considerable interspecific and intraspecific variation (Collias & Collias Citation1984; Mainwaring et al. Citation2008, Citation2014; Mainwaring & Hartley Citation2009, Citation2013; Deeming et al. Citation2019). A nest of a tit usually consists of two layers. The lining part is considered to be associated with thermoregulation and it mainly consists of animal-derived material, including silk, wool, fur, hair and feathers (Alabrudzińska et al. Citation2003; Hanmer et al. Citation2017; Stenning Citation2018). The bryophyte part is considered as a sanitary layer that may also absorb excessive amounts of moisture or increase the humidity during harsh conditions, increase thermal insulation and ensure a structural support for parents and offspring (Álvarez & Barba Citation2008, Citation2011; Álvarez, Belda, Verdejo & Barba Citation2013; Britt & Deeming Citation2011; Lambrechts et al. Citation2012, Citation2014, Citation2015; Glądalski et al. Citation2016, Citation2018; Biddle et al. Citation2018, Citation2019). Some authors also distinguish an outer, decorative/functional layer in tit nests (e.g. large bird feathers brought by a male; delicate, movable lining-like cover for eggs or fresh, aromatic plants; Sanz & García-Navas Citation2011; Reynolds et al. Citation2019; Pires et al. Citation2020; Glądalski et al. Citation2020a). Different studies show that nest composition in Great Tits Parus major and Blue Tits Cyanistes caeruleus is affected by a set of factors, including amounts of rainfall, spring temperatures, latitude, parents and territory quality, availability of construction materials or risk of predation (Mainwaring et al. Citation2012, Citation2014; Heenan Citation2013; Kaliński et al. Citation2014; Reynolds et al. Citation2016; Biddle et al. Citation2018; Deeming et al. Citation2019; Alambiaga et al. Citation2020).
A considerable number of species from small to medium-sized birds (including tits Paridae, with an exception of the Willow Tit Poecile montanus) use bryophytes as important nest material (Hřibek Citation1985; Glime Citation2017). Mosses and liverworts may constitute about 73–76% of the dry mass of Blue Tit and about 65–80% of Great Tit nests (Mainwaring Citation2015; Glądalski et al. Citation2016). In the ornithological literature bryophyte species are collectively referred to as “green mosses” and species identification is typically not conducted. Moreover, issues of bird selectivity in their bryophyte use and the presence/absence of available mosses in the nest in individual territories are usually not pursued (Wesołowski & Wierzcholska Citation2018).
Investigations on the effects of urbanization on avian populations have focused mainly on breeding parameters expressed after the moment of building the nest (Lambrechts et al. Citation2017; Seress et al. Citation2020). Therefore, urban studies have mostly ignored that some nest characteristics may be associated with the degree of urbanization. Reynolds et al. (Citation2019) in their review on urbanization and nest building by birds focus on differences of anthropogenic (e.g. cigarette butts, plastic twine, fishing line, knitwear fragments) and natural nesting material, but they did not mention variation in moss or any plant species in bird nests. Lambrechts et al. (Citation2017) suggest that urban-related alterations in vegetation characteristics might not be a limiting factor for nest size, because the Great Tit is an opportunistic species in the selection of nest material, but nest composition may be affected by habitat type and tree species.
In the last decade, the number of studies on tit nests has increased (Stenning Citation2018). Most of them consider the relationship between nest parameters and breeding characteristics of Blue and Great Tits. Studies concerning the moss composition of the nest and the relationship between the nest moss composition and the territory characteristics are very scarce (Wesołowski & Wierzcholska Citation2018). The aim of this study is to investigate the use of bryophytes in the nest construction of two tit species (the Blue Tit and the Great Tit) breeding in two floristically and structurally contrasting study areas (a mature deciduous forest and an urban parkland).
Methods
This study was carried out in 2016 and 2017 in two structurally and floristically contrasting habitats: an urban park (51°45′N, 19°24′E) and a rich deciduous forest (51°50′N, 19°29′E), located 10 km apart. This is part of a long-term project of research into the breeding biology of secondary cavity nesters around Łódź, Central Poland (Wawrzyniak et al. Citation2020; Glądalski et al. Citation2020b). Both these study areas were supplied with standard wooden nest boxes with a removable front wall and with internal dimensions of 11.5 × 11 cm (floor) × 30 cm (height) and a 30-mm diameter entrance located 20 cm from the bottom of the nest box (Lambrechts et al. Citation2010). All nest boxes were placed on trees at a height of about 3 m. In both study areas, distances between neighbouring nest boxes were about 50 m.
The forest study site was about 130 ha in area and contained 300 nest boxes in the center of a mature mixed deciduous forest (1250 ha in total), with oaks (Quercus) as the predominating tree species. Among native deciduous trees, the most numerous were pedunculate oak Quercus robur, sessile oak Q. petraea, silver birch Betula pendula, aspen poplar Populus tremula, small-leaved limes Tilia cordata, sycamore maples Acer pseudoplatanus, black alders Alnus glutinosae and many others, usually much more dispersed (Kurowski Citation2001). The urban park study site (including the zoological garden of 16 ha and the botanical garden of 64 ha) was about 80 ha, with 200 nest boxes distributed in an artificial, fragmented tree cover. Tree patches are a mosaic of different stands of deciduous and coniferous trees, in some part exotic species, usually dispersed among native species (Wawrzyniak et al. Citation2020; Glądalski et al. Citation2020b).
The most frequent bird species occupying the boxes in both the study areas were Great Tits and Blue Tits. A smaller number of Pied Flycatchers (Ficedula hypoleuca) usually arrive at the forest area later (up to 15 pairs) and occupy free nest boxes. During the breeding season, the nest boxes were checked at least once a week to monitor the breeding performance of tits. After the breeding season, a sample of nests (only first clutches were studied) was randomly chosen. One hundred nests (from 100 territories) were randomly sampled (n = 100) from the urban parkland (21 nests of Blue Tits and 35 nests of Great Tits) and the forest study areas (15 nests of Blue Tits and 29 nests of Great Tits).
All samples of the nests were collected from the nest boxes after the end of the breeding season. Sampled nests were placed in tightly sealed, labeled plastic bags and then placed in a freezer (–18°C) for 24 hours in order to efficiently kill parasitic and non-parasitic arthropods by freezing. After the freezing procedure, the nests were dried for 24 h in an oven at 60°C until reaching constant dry mass (by M.G., Glądalski et al. Citation2016) and then the bryophyte species were identified, and the percentage (by weight) of each moss species in the nest was estimated (by G.J.W.). The nests were dismantled manually, with bryophytes being separated from the rest of the nest material and identified in the laboratory, usually after a few weeks after their collection (by M.G and G.J.W.).
The tits frequently gather bryophytes in the immediate surroundings of the nest boxes, but they may also bring moss from distances of up to 80 m (Wesołowski & Wierzcholska Citation2018). Following these authors, we limited the area studied to the radius of 10 m around the breeding nest box. Within each radius, the mosses and liverworts growing on all substrates available in forests were searched, that is on epiphytic (on tree trunks up to 2 m), epigeic (soil, litter) and epixylic substrates (stumps, branches, logs). In several cases, the moss was found on a concrete fence (only in the urban parkland area). Almost all bryophyte species were identified in the field after the breeding season, only in several cases, samples were collected (placed in tightly sealed, labeled bags) and identified later in the lab using a microscope (by G.J.W.).
Based on the collected data, we calculated a selectivity indicator (Ti) (Lechowicz Citation1982; Meffe & Berra Citation1988), which refers to the proportion of bryophyte species available within the 10 m radius (from the nest) which is present in the nest to all the bryophyte species that are present in the nest. It might range from 0 – no bryophyte species in the nest is available within the 10 m radius, to value 1 – all bryophyte species from the nest are available within the 10 m radius (after Wesołowski & Wierzcholska Citation2018).
Bryophytes can be generally divided into two categories: pleurocarpous and acrocarpous species. This division concerns to, among other things, how a species grows, with some species being less and some more branched, wherein acrocarpous mosses are more unbranched (Ingrouille Citation1992). Given the form of growth, it was examined which of them (branched or unbranched) were more often selected for nesting.
If the data met the assumption of normality, t-test was used (for unequal variances Cochran–Cox test was used). If the data did not meet the assumption of normality, Mann–Whitney and Kruskal–Wallis ANOVA tests were used. χ2 test was used to examine the selectivity between two bryophyte categories (pleurocarpous and acrocarpous) (Stanisz Citation2006). To assess nest similarity in terms of bryophyte species we used the Gower distance measure and UPGMA joining method, and the results are presented in the form of dendrograms (Sneath & Sokal Citation1973; Pavoine et al. Citation2009).
Results
A total of 56 species of bryophytes have been identified, considering the two habitats, in pooled tit nests and within the plots of the 10 m radius from the tree with the nest, while in all the nests themselves we have found 31 species of bryophytes (see Electronic Supplementary Material for the list of all bryophytes species). The number of bryophytes was variable and depended on the territory and bird species.
The number of bryophyte species found in individual nests varied in the urban parkland between 1 and 6 (Blue Tit: 2–6; Great Tit 1–6) and in the forest between 1 and 11 (Blue Tit: 1–10; Great Tit 1–11). The mean number of bryophyte species in a nest ranged from 2.31 in Great Tit from the urban parkland to 3.52 in Blue Tit in the urban parkland (). Only a few species were used in larger quantities (more than 5% of volume). Considering only these abundant species, the mean number of species ranged from 1.28 in Great Tits in the forest to 1.57 in Blue Tits in the urban parkland (). Blue Tits used significantly more bryophyte species than Great Tits in the urban parkland (U-MW, Z = 2.84, p < 0.01, n1 = 21, n2 = 35), but there was no significant difference in the forest (Mann–Whitney U test, Z = 0.42, p = 0.67, n1 = 15, n2 = 29). There were more bryophyte species in the forest than in the urban parkland within the 10 m radius from the tree with the nest (t-test, t = 5.96, df = 98, p < 0.001, n1 = 56, n2 = 44) (see Electronic Supplementary Material for the list of all bryophyte species).
Table I. Mean number (± SD) of all bryophyte species used in nest construction, and also considering only those amounting to more than 5% of the nest volume, and species available in the environment for individual bird species
Table II. The use of different bryophytes as nest construction material by Blue Tits and Great Tits
The tits used in their nests a total of 31 bryophyte species (27 found in Blue Tits and 19 in Great Tits), whereas 25 bryophyte species were found within the 10 m radius from the tree with the nest, but not in the nests (see Electronic Supplementary Material for the list of all bryophyte species). Only three moss species were the most frequently used by both tit species, constituting more than 80% volume in a single nest: Hypnum cupressiforme (>80% vol. in 18 out of 36 studied nests of Blue Tits and >80% vol. in 18 out of 64 studied nests of Great Tits), Brachythecium rutabulum (>80% vol. in 6 out of 36 studied nests of Blue Tits and >80% vol. in 12 out of 64 studied nests of Great Tits) and Rhytidiadelphus squarrosus (>80% vol. in 4 out of 36 studied nests of Blue Tits and >80% vol. in 14 out of 64 studied nests of Great Tits) (). These three bryophyte species are taxa with a wide ecological amplitude in relation to habitats and substrates on which they grow. Nevertheless, in our study sites, they were most often recorded in epigeic habitats, less often epiphytic and epixylic habitats (see Electronic Supplementary Material for the list of all bryophyte species).
The Blue Tit preferred pleurocarpous mosses in its nests (χ2 = 9.52, p < 0.01) – more such species were present in their nest, whereas there was no significant difference between those two bryophyte categories in Great Tit nests (χ2 = 1.90, p = 0.17). Selectivity indicator (Ti, ) differed between the four species/site groups (the nests of Great Tits in the urban parkland differed from the other groups, Kruskal–Wallis ANOVA, H = 12.19, p < 0.01).
Table III. Statistical characteristics of selectivity indicator in four study groups (refers to the proportion bryophyte species available within 10 m radius (from the nest) that are present in the nest to all the bryophyte species that are present in the nest)
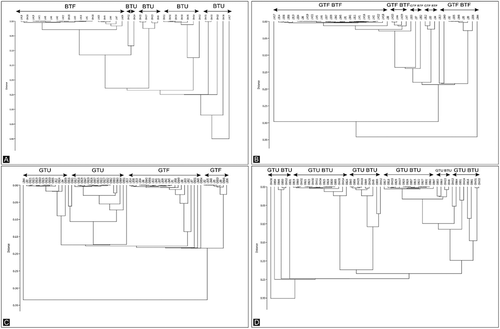
Nest similarity analysis performed for the nests from different habitats (urban parkland and forest) indicates that nests built by Great Tits and Blue Tits differed from each other in terms of the species of mosses used. Great and Blue Tits () in the forest and the urban parkland built nests differing in bryophyte composition. However, the nest similarity analysis for both species from the same area together indicates that the nests of Blue Tits and Great Tits are quite similar. Neither in the forest nor in the urban park were clear differences found in the composition of moss species in individual nests (). In general, both species used the same moss species within each habitat, but there were differences in the moss species used between habitats.
Discussion
Both Great Tits and Blue Tits appeared to be selective in their bryophyte use during the nest construction period. Forty-five percent of 56 species of available mosses were not recorded in the nests analyzed, with both the tit species regularly using 2–6 moss species and, in the vast majority of nests, the bulk (>80%) of the total bryophyte volume of the nest consisted of just 3 species. The bryophyte species most used in the nests were in some cases those present in the vicinity of the nest, but in other cases they were collected far away. In general, all the species of moss used in nests were found within the 10 m radius of some nests, so that they are listed amongst the 56 moss species identified in this study. Many other bryophyte species that were present at study plots were practically unused. It is difficult to be sure that species composition does not reflect bryophyte abundance around the nest (at the scale relevant to the birds). Tits might concentrate searching in those places where mosses are more abundant and ignore the species located in substrates or microhabitats where they are scarcer or more difficult for the birds to reach. Our study shows that common, polysubstrate mosses (mainly epigeic species), with a broad ecological spectrum (Dierβen Citation2001; Wolski Citation2013) are most often used for the construction of nests. This is confirmed by some results from the Czech Republic (Hřibek Citation1985) and Japan (Hamao et al. Citation2016). But this is in contrast to results from the Białowieża National Park in Poland (Wesołowski & Wierzcholska Citation2018), where birds used more epiphytic mosses. These differences are most likely related to the availability of appropriate species of epiphytes, which only confirms the uniqueness of the Białowieża Forest in this respect.
In the study from Białowieża forest Wesołowski and Wierzcholska (Citation2018) indicate that tits from this area did not used near 40% of 54 species of available mosses. These authors also state that the studied birds used mainly only three bryophyte species (the bulk of 80–89% of the total bryophyte volume). Hamao et al. (Citation2016), studying nests of Japanese Tits Parus minor, found 21 bryophyte species in nests, but in 91% of nests the total bryophyte volume consisted of only 3 species. We agree with the authors cited above that such patterns suggest that only some of moss species are characterized by appropriate functional parameters to be used as proper material for nest construction. Following the methods proposed by Wesołowski and Wierzcholska (Citation2018) we used the 10 m radius circle plots surrounding the tree with a nest box to study the occurrence of moss species and, similarly, we recorded some bryophytes in tit nests that were not found in the vicinity of the nest boxes. It suggests that the tits are not constrained by the 10 meter distance from their nests and may collect moss species characterized by proper functional parameters from much larger distances – up to 80 m (suggested by Wesołowski & Wierzcholska Citation2018).
Blue Tits and Great Tits belong to the few passerines that incorporate fresh green, aromatic plant material into their nests (Bańbura et al. Citation1995; Lambrechts & Dos Santos Citation2000; Mainwaring Citation2017). The drug hypothesis suggests that aromatic plants present in nests have positive effects on nestlings’ development and body condition and may act as natural repellents (against blood parasite arthropods) and antimicrobial agents (Pires et al. Citation2012). There is an increasing number of experimental evidence that morphological and physiological condition of tit nestlings gains when this aromatic plant material is present in the nest (Mennerat et al. Citation2009a, Citation2009b; Glądalski et al. Citation2020a; Pires et al. Citation2020). It was also shown that Blue Tits are very choosy when collecting those aromatic plants (Petit et al. Citation2002). Betts (Citation1955) showed that the Blue Tit during winter consumed large numbers of capsules from the moss Dicranoweisia cirrata and ignored the capsules of all other bryophyte species. Therefore, it is possible that some particular bryophyte species are preferred by both tit species not only because they are common, relatively abundant and have water absorbing capabilities. Wesołowski and Wierzcholska (Citation2018) showed that moss species selectivity related to water-absorbing function was not the case as the water-uptake ratios did not differ between used and not-used moss species. On the other hand, many bryophyte species are known for their medical properties (antimicrobial, antifungal, antiseptic; Asakawa Citation2007; Bukvički et al. Citation2012; Sevim et al. Citation2017), and this may be the next reason why those birds are quite choosy when collecting bryophyte, but unfortunately there are practically no studies that examine those properties in tit nests and more studies on this subject would be welcome.
In a study where tit nests were vacuum-packed and tested for insulation properties, Deeming and Biddle (Citation2015) suggested tremble-thrusting in tits as a way to add air gaps in the moss-made nest wall. It may suggest that differences in air-trapping capabilities of different moss species could also be a reason for their selective use.
The next reason for the tit bryophyte species selectivity may be the efficient plucking. Wesołowski and Wierzcholska (Citation2018) conducted plucking simulations of various moss species (although those simulations mimicked reality only roughly as they stated) and partly showed that birds tended to pick the species that were characterized by larger (heavier) and longer bundles of moss. Our study also shows that in general the bryophyte species composition of the nest is affected by the site (the urban park vs the forest) rather than by the species of tit (Blue Tit vs Great Tit). We found that in our samples there were more bryophyte species in the forest than in the city park. Both areas differ in species composition of vascular plants (Kurowski Citation2001; Marciniak et al. Citation2007) and an analogous difference also occurs for bryophyte species diversity (Filipiak Citation1996; Wolski et al. Citation2012; Wolski Citation2013). When comparing the nests of both tit species in the same site, it seems that they are generally quite similar. Therefore, in the same area, both the tit species tend to choose similar bryophytes to build nests, while the Blue Tit tends also to pick larger amounts of pleurocarpous rather than acrocarpous moss species. Wesołowski and Wierzcholska (Citation2018) showed that in Białowieża Blue Tits and Great Tits tended to use pleurocarpous bryophyte species. Bryophyte species diversity differs between the forest and the urban parkland and both tit species use similar groups of bryophytes at each site. So differences in moss diversity between habitats are probably the main cause explaining their differential use by tit species. Wesołowski and Wierzcholska (Citation2018) suggested that tits may select moss species partially as a function of the size of the tree cavity they use for nesting. These differences are not relevant in the present study, where both the tit species use nest boxes of the same size.
We agree with Wesołowski and Wierzcholska (Citation2018) that the topic of bird selectivity in their bryophyte use is clearly understudied. Therefore, we would suggest that further investigations should focus on expanding the studied sites beyond the territory of Poland.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures using animals were authorized under permits from the University of Łódź.
Supplemental Material
Download MS Excel (77.5 KB)Acknowledgements
We thank A. Jaksa, M. Winsche, D. Mańkowska, and J. Białek for their help and consent to conducting research in the areas under their administration. We would like to thank M. Przybylski for statistical suggestions. We are obliged to P. Procter for linguistic consultation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Akresh ME, Ardia DR, King DI. 2017. Effect of nest characteristics on thermal properties, clutch size, and reproductive performance for an open-cup nesting songbird. Avian Biology Research 10(2):107–118. DOI:10.3184/175815617X14878495604724.
- Alabrudzińska J, Kaliński A, Słomczyński R, Wawrzyniak J, Zieliński P, Bańbura J. 2003. Effects of nest characteristics on breeding success of great tits Parus major. Acta Ornithologica 38(2):151–154. DOI:10.3161/068.038.0202.
- Alambiaga I, Álvarez E, Diez-Mendez D, Verdejo J, Barba E. 2020. “The tale of the three little tits”: Different nest building solutions under the same environmental pressures. Avian Biology Research 13(3):49–56. DOI:10.1177/1758155920943116.
- Álvarez E, Barba E. 2008. Nest quality in relation to adult bird condition and its impact on reproduction in great tits Parus major. Acta Ornithologica 43(1):3–9. DOI:10.3161/000164508X345275.
- Álvarez E, Barba E. 2011. Nest characteristics and reproductive performance in great tits Parus major. Ardeola 58(1):125–136. DOI:10.13157/arla.58.1.2011.125.
- Álvarez E, Belda EJ, Verdejo J, Barba E. 2013. Variation in Great Tit nest mass and composition and its breeding consequences: A comparative study in four Mediterranean habitats. Avian Biology Research 6(1):39–46. DOI:10.3184/175815513X13609517587237.
- Asakawa Y. 2007. Biologically active compounds from bryophytes. Pure and Applied Chemistry 79(4):557–580. DOI:10.1351/pac200779040557.
- Bańbura J, Blondel J, de Wilde-Lambrechts H, Perret P. 1995. Why do female blue tits (Parus careuleus) bring fresh plants to their nests? Journal of Ornithology 136(2):217–221. DOI:10.1007/BF01651244.
- Betts MM. 1955. The food of titmice in oak woodland. Journal of Animal Ecology 24(2):282–323. DOI:10.2307/1715.
- Biddle LE, Deeming DC, Goodman AM. 2018. Birds use structural properties when selecting materials for different parts of their nests. Journal of Ornithology 159(4):999–1008. DOI:10.1007/s10336-018-1571-y.
- Biddle LE, Dickinson A, Broughton RE, Gray LA, Bennett SL, Goodman AM, Deeming DC. 2019. Nest materials affect the hydrological properties of bird nests. Journal of Zoology 309:161–171. DOI:10.1111/jzo.12713.
- Britt J, Deeming DC. 2011. First-egg date and air temperature affect nest construction in blue tits Cyanistes caeruleus, but not in great tits Parus major. Bird Study 58(1):78–89. DOI:10.1080/00063657.2010.524916.
- Bukvički D, Veljič M, Soković M, Grujić S, Marin PD. 2012. Antimicrobial activity of methanol extracts of Abietinella abietina, Neckera crispa, Plathypnidium riparoides, Cratoneuron filicinum and Campylium protensum mosses. Archives of Biological Science Belgrade 64(3):911–916. DOI:10.2298/ABS1203911B.
- Collias NE, Collias EC. 1984. Nest building and bird behaviour. Princeton: Princeton University Press.
- Deeming DC. 2011. Importance of nest type on the regulation of humidity in bird nests. Avian Biology Research 4(1):23–31. DOI:10.3184/175815511X13013963263739.
- Deeming DC, Biddle LE. 2015. Thermal properties of bird nests depend on air-gaps between the materials. Acta Ornithologica 50(1):121–125. DOI:10.3161/00016454AO2015.50.1.011.
- Deeming DC, Campion E. 2018. Simulated rainfall reduces the insulative properties of bird nests. Acta Ornithologica 53(1):91–97. DOI:10.3161/00016454AO2018.53.1.009.
- Deeming DC, Mainwaring MC, Hartley IR, Reynolds SJ. 2012. Local temperature and not latitude determines the design of blue tit and great tit nests. Avian Biology Research 5(4):203–208. DOI:10.3184/175815512X13528874959581.
- Deeming DC, Morton FEM, Laverack KL. 2019. Nestbox size affects mass and proportions of materials used in blue tit Cyanistes caeruleus nests. Bird Study 66(1):130–135. DOI:10.1080/00063657.2019.1618243.
- Dierβen K. 2001. Distribution, ecological amplitude and phytosociological characterization of European bryophytes. Bryophytorum Bibliotheca 56:1–289.
- Dubiec A, Góźdź I, Mazgajski TD. 2013. Green plant material in avian nests. Avian Biology Research 6(2):133–146. DOI:10.3184/175815513X13615363233558.
- Filipiak E. 1996. Brioflora Ogrodu Botanicznego w Łodzi. Łódź: Przyroda Ogrodu Botanicznego w Łodzi. pp 91–96. in polish.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2020a. Consequences of experimental addition of fresh, aromatic plants into nests of blue tits (Cyanistes caeruleus) on the physiological condition of nestlings. Behavioral Ecology and Sociobiology 74(3):29. DOI:10.1007/s00265-020-2812-7.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2016. Effects of nest characteristics on reproductive performance in blue tits Cyanistes caeruleus and great tits Parus major. Avian Biology Research 9(1):37–43. DOI:10.3184/175815516X14447556559088.
- Glądalski M, Kaliński A, Wawrzyniak J, Bańbura M, Markowski M, Skwarska J, Bańbura J. 2018. Physiological condition of nestling great tits Parus major in response to experimental reduction in nest micro- and macro-parasites. Conservation Physiology 6(1). DOI: 10.1093/conphys/coy062.
- Glądalski M, Mainwaring MC, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Bańbura J, Hartley IR. 2020b. Consequences of hatching deviations for breeding success: A long-term study on blue tits Cyanistes caeruleus. The European Zoological Journal 87(1):385–394. DOI:10.1080/24750263.2020.1787532.
- Glime JM. 2017. Birds and bryophytes intersect. In: Glime JM, editor. Bryophyte ecology, vol 2. Bryological interaction. EBook. Chaps 16-1. Sponsored by Michigan Technological University and the International Association of Bryologists. pp. 2–31. Available: http://digit.alcom.mons.mtu.edu/bryop/hyte-ecolo/gy2/.
- Hamao S, Higuchi M, Jinbo U, Maeto K, Furuki K. 2016. Interaction among birds, mosses and insects in bird nests. Japanese Journal of Ornithology 65(1):37–42. DOI:10.3838/jjo.65.37.
- Hanmer HJ, Thomas RL, Beswick GJF, Collins BP, Fellowes MDE. 2017. Use of anthropogenic material affects bird nest arthropod community structure: Influence of urbanisation, and consequences for ectoparasites and fledging success. Journal of Ornithology 158(4):1045–1059. DOI:10.1007/s10336-017-1462-7.
- Hansell M. 2000. Bird nest construction behavior. Cambridge: Cambridge University Press.
- Heenan CB. 2013. An overview of the factors influencing the morphology and thermal properties of avian nests. Avian Biology Research 6(2):104–118. DOI:10.3184/003685013X13614670646299.
- Hřibek M. 1985. The use species of moss (Bryophyta sp.) in the building of nests the Rits (Parus major L., 1758) and Blue Tit (Parus caeruleus L., 1758). Zprávy Moravského Ornitologického Sdruženi 43:39–45.
- Ingrouille M. 1992. Diversity and evolution of land plants. London: Chapman & Hall.
- Kaliński A, Wawrzyniak J, Bańbura M, Skwarska J, Zieliński P, Glądalski M, Bańbura J. 2014. Does the threat of European Pine Marten (Martes Martes) predation influence the height of nests built by blue tits (Cyanistes Caeruleus) and Great Tits (Parus Major)? Avian Biology Research 7(2):83–90. DOI:10.3184/175815514X13983550506873.
- Kurowski JK. 2001. Szata roślinna Lasu Łagiewnickiego w Łodzi. Łódź: Urząd Miasta Łodzi. (in polish).
- Lambrechts M, Adriaensen F, Ardia DR, Artemyev AV, et al. 2010. The design of artificial nestboxes for the study of secondary hole-nesting birds: A review of methodological inconsistencies and potential biases. Acta Ornithologica 45(1):1–26. DOI: 10.3161/000164510X516047.
- Lambrechts M, Blondel J, Dubuc-Messier G, Marrot P, De Franceschi C, Perret P, et al. 2015. Great Tits build shallower nests than blue tits in an insular oak-dominated habitat mosaic. Avian Biology Research 8(2):117–121. DOI: 10.3184/175815515X14279770555217.
- Lambrechts MM, Aimé C, Midamegbe A, Galan M-J, Perret P, Grégoire A, Doutrelant C. 2012. Nest size and breeding success in first and replacement clutches: An experimental study in blue tits Cyanistes caeruleus. Journal of Ornithology 153(1):173–179. DOI:10.1007/s10336-011-0722-1.
- Lambrechts MM, Blondel J, Bernard C, Caro SP, Charmantier A, Demeyrier V, Doutrelant C, Dubuc-Messier G, Fargevieille A, De Franceschi C, Giovannini P, Grégoire A, Hurtrez-Boussès S, Lucas A, Mainwaring MC, Marrot P, Mennerat A, Perret S, Perret P. 2016a. Exploring biotic and abiotic determinants of nest size in Mediterranean great tits (Parus major) and blue tits (Cyanistes caeruleus). Ethology 122(6):492–501. DOI:10.1111/eth.12494.
- Lambrechts MM, Marrot P, Fargevieille A, Giovannini P, Lucas A, Demeyrier V, Midamegbe A, Perret P, Grégoire A, Charmantier A, Doutrelant C. 2016b. Nest size is not closely related to breeding success in blue tits: A long-term nest-box study in a Mediterranean oak habitat. The Auk 133(2):198–204. DOI:10.1642/AUK-15-214.1.
- Lambrechts MM, Charmantier A, Demeyrier V, et al. 2017. Nest design in a changing world: Great tit Parus major nests from a Mediterranean city environment as a case study. Urban Ecosystems 20(6):1181–1190. DOI: 10.1007/s11252-017-0670-5.
- Lambrechts MM, Demeyrier V, Fargevieille A, Giovannini P, Lucas A, Marrot P, Midamegbe A, Perret P, Charmantier A, Doutrelant C, Grégoire A. 2014. Great tits build shallower nests than blue tits. Avian Biology Research 7(4):251–254. DOI:10.3184/175815514X14162394225987.
- Lambrechts MM, Dos Santos A. 2000. Aromatic herbs in Corsican blue tit nests: The ‘potpourri’ hypothesis. Acta Oecologica 21(3):175–178. DOI:10.1016/S1146-609X(00)00122-3.
- Lechowicz MJ. 1982. The sampling characteristics of electivity indices. Oecologia 52(1):22–30. DOI:10.1007/BF00349007.
- Mainwaring MC. 2015. Nest construction and incubation in a changing climate. In: Deeming DC, Reynolds SJ, editors. Nests, eggs, and incu- bation: New ideas about avian reproduction. Oxford: Oxford University Press. pp. 65–74.
- Mainwaring MC. 2017. Causes and consequences of intraspecific variation in nesting behaviors: Insights from blue tits and great tits. Frontiers in Ecology and Evolution 5. DOI: 10.3389/fevo.2017.00039.
- Mainwaring MC, Benskin C, Hartley IR. 2008. The weight of female-built nests correlates with female but not male quality in the Blue Tit Cyanistes caeruleus. Acta Ornithologica 43(1):43–48. DOI:10.3161/000164508X345310.
- Mainwaring MC, Hartley IR. 2009. Experimental evidence for state-dependent nest weight in the blue tit, Cyanistes caeruleus. Behavioural Processes 81(1):144–146. DOI:10.1016/j.beproc.2009.02.001.
- Mainwaring MC, Hartley IR. 2013. The energetic costs of nest building in birds. Avian Biology Research 6(1):12–17. DOI:10.3184/175815512X13528994072997.
- Mainwaring MC, Hartley IR, Bearhop S, Brulez K, Du Feu CR, Murphy G, et al. 2012. Latitudinal variation in blue tit and great tit nest characteristics indicates environmental adjustment. Journal of Biogeography 39(9):1669–1677. DOI: 10.1111/j.1365-2699.2012.02724.x.
- Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds‘ nests. Ecology and Evolution 4(20):3909–3928. DOI:10.1002/ece3.1054.
- Marciniak B, Nadolski J, Nowakowska M, Loga B, Bańbura J. 2007. Habitat and annual variation in arthropod abundance affects blue tit Cyanistes caeruleus reproduction. Acta Ornithologica 42(1):53–62. DOI:10.3161/068.042.0113.
- Meffe GK, Berra TM. 1988. Temporal characteristics of fish assemblage structure in an Ohio Stream. Copeia 1988(3):684–690. DOI:10.2307/1445388.
- Mennerat A, Mirleau P, Blondel J, Perret P, Lambrechts MM, Heeb P. 2009a. Aromatic plants in nests of the blue tit Cyanistes caeruleus protect chicks from bacteria. Oecologia 161(4):849–855. DOI:10.1007/s00442-009-1418-6.
- Mennerat A, Perret P, Bourgault P, Blondel J, Gimenez O, Thomas DW, Heeb P, Lambrechts MM. 2009b. Aromatic plants in nests of blue tits: Positive effects on nestlings. Animal Behaviour 77(3):569–574. DOI:10.1016/j.anbehav.2008.11.008.
- Pavoine S, Vallet J, Dufour A-B, Gachet S, Daniel H. 2009. On the challenge of treating various types of variables: Application for improving the measurement of functional diversity. Oikos 118:391–402. DOI: 10.1111/j.1600-0706.2008.1.
- Petit C, Hossaert-McKey M, Perret P, Blondel J, Lambrechts MM. 2002. Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecology Letters 5(4):585–589. DOI:10.1046/j.1461-0248.2002.00361.x.
- Pires BA, Belo AD, Diamantino F, Rabaça JE, Merino S. 2020. Development of nestling blue tits (Cyanistes caeruleus) is affected by experimental addition of aromatic plants. Avian Biology Research 13(3):44–48. DOI:10.1177/1758155920921075.
- Pires BA, Belo AF, Rabaça JE. 2012. Aromatic plants in Eurasian blue tit nests: The ‘nest protection hypothesis’ revisited. The Wilson Journal of Ornithology 65(1):162–165. DOI:10.1676/11-102.1.
- Reynolds SJ, Davies CS, Elwell E, Tasker PJ, Williams A, Sadler P, Hunt D. 2016. Does the urban gradient influence the composition and ectoparasite load of nests of an urban bird species? Avian Biology Research 9(4):224–234. DOI:10.3184/175815516X14725499175665.
- Reynolds SJ, Ibáñez-Álamo JD, Sumasgutner P, et al. 2019. Urbanisation and nest building in birds: A review of threats and opportunities. Journal of Ornithology 160(3):841–860. DOI: 10.1007/s10336-019-01657-8.
- Sanz JJ, García-Navas V. 2011. Nest ornamentation in blue tits: Is feather carrying ability a male status signal? Behavioral Ecology 22(2):240–247. DOI:10.1093/beheco/arq199.
- Seress G, Sándor K, Evans KL, Liker A. 2020. Food availability limits avian reproduction in the city: An experimental study on great tits Parus major. Journal of Animal Ecology 89(7):1570–1580. DOI:10.1111/1365-2656.13211.
- Sevim E, Baş Y, Celik G, Pınarbaş M, Bozdeveci A, Özdemir T, Akpınar R, Yayli N, Karaoğlu ŞA. 2017. Antibacterial activity of bryophyte species against Paenibacillus larvae isolates, Turk. Vet Anim Science 41:521–531.
- Sneath PHA, Sokal RR. 1973. Numerical taxonomy. San Francisco: W. H. Freeman.
- Stanisz A. 2006. Przystępny kurs statystyki. Kraków: StatSoft Polska. (in polish).
- Stenning M. 2018. The Blue Tit. London: T & AD Poyser.
- Wawrzyniak J, Glądalski M, Kaliński A, Bańbura M, Markowski M, Skwarska J, Zieliński P, Bańbura J. 2020. Differences in the breeding performance of great tits Parus major between a forest and an urban area: A long term study on first clutches. The European Zoological Journal 87(1):294–309. DOI:10.1080/24750263.2020.1766125.
- Wesołowski T, Wierzcholska S. 2018. Tits as bryologists: Patterns of bryophyte use in nests of three species cohabiting a primeval forest. Journal of Ornithology 159(3):745–773. DOI:10.1007/s10336-018-1535-2.
- Wolski GJ 2013. Siedliskowe uwarunkowania występowania mszaków w rezerwatach przyrody chroniących jodłę pospolitą w Polsce Środkowej. PhD thesis, Department of Geobotany and Plant Ecology University of Lodz (in polish).
- Wolski GJ, Stefaniak A, Kowalkiewicz B. 2012. Bryophytes of the experimental and teaching garden of the faculty of biology and environmental protection, University of Łódź (Poland). Ukrainian Botanical Journal 69:519–529.
- Zabłotni A, Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Bańbura J. 2020. Experimental nest replacement suggests that the bacterial load of nests may mediate nestling physiological condition in cavity nesting Great Tits (Parus major). Journal of Ornithology 161(3):819–828. DOI:10.1007/s10336-020-01759-8.