Abstract
From the BIOPEARL 1 and 2 Programs to the Amundsen and Scotia Seas a large series of Tanaidacea was collected using an epibenthic sled. We carried out a thorough morphological analysis of 169 individuals and provide a description of 14 pseudotanaid species distributed in three genera. Twelve species of the genus Pseudotanais are represented by three morphogroups: “affinis+longisetosus”, “denticulatus+abathagastor”, and “forcipatus”. We provide a redescription of Akanthinotanais gaussi, supplement the definitions of the genera Akanthinotanais and Beksitanais, and transfer Pseudotanais abyssi to the genus Beksitanais. Based on the literature and new data, we summarize knowledge of the Antarctic Pseudotanaidae and discuss this family’s spatial and bathymetric distribution.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:1A8F69F7-454D-4CB1-B745-3660AFD3BB82
Introduction
Since 2000 there has been considerable progress in understanding the Antarctic benthos (Brandt et al. Citation2007b, Citation2012; De Broyer & Danis Citation2011; Kaiser et al. Citation2013; Błażewicz-Paszkowycz Citation2014). The inventory of the marine fauna of the Southern Ocean accounts for over 1300 species of Peracarida alone (De Broyer & Danis Citation2011). These crustacean brooders were dominated by mobile, morphologically diverse, and often big forms like Amphipoda or Isopoda, while the Tanaidacea, the smaller and more immobile congeners, were represented by only 160 species among 20 families (Błażewicz-Paszkowycz Citation2014). Three recent publications have supplemented the list of Southern Ocean tanaidaceans by another five species (Esquete et al. Citation2012; Błażewicz-Paszkowycz Citation2014; Segadilha & Araújo-Silva Citation2015; Segadilha et al. Citation2017). These 165 species represent just 0.3–0.7% of a predicted number of tanaidacean species (Appeltans et al. Citation2012; Błażewicz-Paszkowycz et al. Citation2012) and is undoubtedly far from complete, with the continental slope and oceanic floor likely hiding more diverse habitats and remaining hardly explored (Pabis et al. Citation2014). A real measure of tanaidacean species richness and diversity, especially diverse shelf depths (McCallum et al. Citation2015; Poore et al. Citation2015) has not yet been properly addressed, so the discovery of new species in each new survey is quite expected.
The paratanaoidean family Pseudotanaidae (Sieg, 1976) often dominates deep-sea tanaidacean communities (Golovan et al. Citation2013; Błażewicz et al. Citation2019; Stępień et al. Citation2019; Jóźwiak et al. Citation2020). Recently, the family has received more taxonomical attention, resulting in a total of 74 formally described species. They are characterised by a three-articulated antennule and by a poorly calcified, short, and robust body (except Parapseudotanais Bird & Holdich, Citation1989a). The first three body segments (pereonites 1–3), are always greatly shortened (concurrently with the marsupium formed only from pereonite-4), positioning the first three pairs of pereopods at short intervals. These long, slender, and closely situated pereopods are often entangled and make morphological observation of them on whole specimens a great challenge. The three features combined length allow confident identification of pseudotanaids from the paratanaoideans (e.g. Larsen & Wilson Citation2002; Błażewicz-Paszkowycz Citation2007; Bird & Larsen Citation2009). Furthermore, the brood pouch (marsupium) in pseudotanaids is composed of one pair of oostegites and is perhaps the sole apomorphy expressed by the family. The long pereopods and unique character of the marsupium is considered an advantage for effective protection of fertilized eggs and juveniles during active walking.
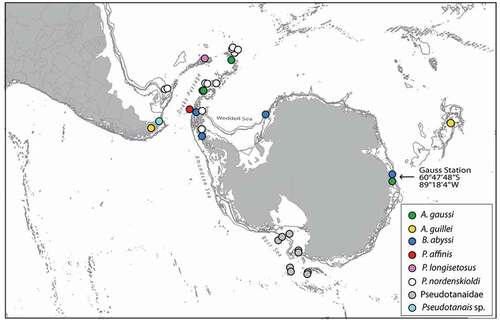
Until now, the Pseudotanaidae in the Antarctic have been known from six species in two genera (Błażewicz-Paszkowycz Citation2014). Four of them, Akanthinotanais gaussi Vanhöffen, Citation1914; A. guillei Shiino, Citation1978; Pseudotanais nordenskioldi Sieg, Citation1977; and P. longisetosus Sieg, Citation1977, were originally described from the Southern Ocean (Vanhöffen Citation1914; Sieg Citation1977; Shiino Citation1978). Two other species, P. abyssi Hansen, Citation1913 and P. affinis Hansen, Citation1887, have been recorded in the Antarctic, although they were originally described from the Arctic (Hansen Citation1887, Citation1913; Kudinova-Pasternak Citation1990). The distribution of the Pseudotanaidae found in the Southern Ocean is visualized in . (Appendix 1).
Figure 1. Spatial distribution of Antarctic Pseudotanaidae based on Vanhöffen (Citation1914); Shiino (Citation1978); Sieg (Citation1986a, Citation1986b); Kudinova-Pasternak (Citation1993); Błażewicz-Paszkowycz and Siciński (Citation2014); and Pabis et al. (Citation2015). The details of the records are in Appendix 1
During two cruises under the acronym BIOPEARL 1 and 2 (Biodiversity dynamics: Phylogeography, Evolution And Radiation of Life in Antarctica) in 2006 and 2008 to the Amundsen and Scotia Seas respectively, a rich series of the Tanaidacea using epibenthic sled was collected (Brandt & Barthel Citation1995); The 549 tanaidacean individuals collected belonged to 85 species included in 26 genera (Pabis et al. Citation2014). In that study, the Pseudotanaidae was an abundant and diverse group of 169 individuals. For this paper, we conducted a thorough morphological analysis of the latter group of crustaceans and provide a description of 14 new species together with a supplementary redescription of Akanthinotanais gaussi (Vanhöffen, Citation1914), which complements some morphological features missing in the work by Sieg (Vanhöffen Citation1914; Sieg Citation1977). Finally, we summarize current knowledge on Pseudotanaidae in the Southern Ocean using the published literature and analyse the distribution and bathymetric range for each species.
Material and methods
Stations and collection
The tanaidaceans were collected during the Austral summers 2005/06 and 2007/08 by the RRS James Clark Ross in the Amundsen and Scotia Seas using an epibenthic sled (EBS) (Brandt & Barthel Citation1995). This gear is considered an efficient way for collecting the light fraction of epi-macrobenthic communities (Frutos et al. Citation2016). In the Amundsen Sea, the samples were collected in two localities, i.e., the inner part of the sea (stations BIO4 and BIO5), and outer region (stations BIO3 and BIO6); in the Scotia Sea, the samples were collected in seven areas along the western side of the Antarctic Peninsula extending to the Scotia Arch, i.e., at Livingston Island (LI), Elephant Island (EI), South Orkney Islands (PB, Palmer Bay), South Sandwich Islands (ST, Southern Thule), South Georgia (SG), and Shag Rocks (SR). The depth range of the studied material is from 200 to 1500 m (, ).
Table I. Details on the sampling stations collected with an epibenthic sledge during the BIOPEARL 1 and 2 Expeditions
Figure 2. Distribution of the sampling stations collected with an epibenthic sledge during the BIOPEARL 1 and 2 Expeditions. For details of the station see
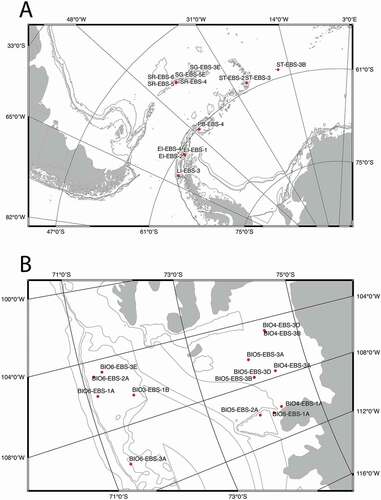
The EBS was deployed in 17 stations of the Amundsen Sea at depths 500–1500 m and in 19 stations of the Scotia Sea from 200 to 1600 m. During these surveys pseudotanaids were recovered in 14 stations of the former and 14 stations of the latter. The samples were sieved on a 500 μm mesh and fixed in 96% un-denatured pre-cooled ethanol and frozen for at least 48 hours at −20°C.
Morphological analyses and Linnean taxonomy
Specimens were dissected with chemically sharpened tungsten needles, and the dissected appendages were then mounted on slides with glycerine as a medium and a paraffin-wax sealant ring (). Drawings were prepared using a light microscope (Nikon Eclipse 50i) equipped with a camera lucida. Digital drawings were inked and arranged to plate in Photoshop.
Morphological terminology is largely as in Jakiel et al. (Jakiel et al. Citation2019, Citation2020);
the unique blade-like spine characteristic of most pseudotanaids is categorized as “long” when is at least 0.6x propodus, “intermediate” when it is 0.5x propodus and “short” when it is at most 0.3x the propodus;
setae types are recognized as (1) simple setae (=without ornamentation), (2) serrate – with serration or denticulation, (3) plumose – with any type of plumose or delicate setulae distributed along the main axis, (4) penicillate – with a tuft of setules located distally and with a small knob on which a seta is fixed to the tegument and (5) rod setae – slightly inflated distally and with a pore;
the dorsodistal seta occurring on the carpus of pereopod 4–6 has a chemosensory function – (“rod seta” Jakiel et al. Citation2019); it is categorized as “long” when it is at least 0.8x propodus, “intermediate” when it is 0.5x propodus and “short” when it is at most 0.25x propodus.
Splitting of Pseudotanais into different morphogroups (“affinis+longisetosus”, “denticulatus+abathagastor” and “forcipatus”) follows Bird & Holdich (Citation1989a) and Jakiel et al. (Citation2019).
Type material has been lodged at the Natural History Museum (NHM) in London (UK).
Measurements and developmental stage identification
Total body length (BL) was measured along the main axis of symmetry from the rostrum to the end of the telson. Body width (BW) was measured at the widest point along the main axis of symmetry. The length was measured along the axis of symmetry, and the width perpendicular to the axis of symmetry at the widest spot. To simplify species descriptions, the expression “Nx” replaces “N times longer than/as long as” and “N L:W” replaces “N times longer than wide”. The measurements were made with a camera connected to the microscope (Nikon Eclipse Ci-L) and NIS-Elements View software (www.nikoninstruments.com). The body width and the length of the cephalothorax, pereonites, pleonites, and pleotelson were measured on whole specimens.
For all individuals, developmental stages were identified. In particular, we refer to the following stages:
two stages of manca, i.e., “manca-2” and “manca-3” which refer to specimens without or with buds of pereopod-6, respectively.
two stages of females, i.e., juvenile female with oostegites buds (equivalent to “preparatory female” sensu Bird & Holdich Citation1989a), and brooding female with developed marsupium).
neuter – a stage that is morphologically similar to juvenile female, but lacking oostegite buds.
“juvenile male” that shows incompletely developed sexual dimorphic characters, i.e., resembling the neuter but has thicker antennules (equivalent to “preparatory male” sensu Bird & Holdich Citation1989a).
In our collection no sexually mature male (“swimming male”) was recovered.
Distribution analysis
All 28 samples obtained with the EBS were included in the analysis in order to investigate the similarity/dissimilarity among pseudotanaid communities. The hierarchical agglomerative clustering analysis was performed by using PRIMER 7 (Clarke & Gorley Citation2015) on presence-absence data, applying the Bray-Curtis formula and the group average method. A SIMPROF test with 5% significance level was performed to assess the multivariate structure within groups.
Divergences in pseudotanaid composition between the areas were statistically tested using Analysis of Similarities (ANOSIM), where an R value closer to 1 signifies those similarities are greater within the areas while a value of zero signifies uniform similarities between and within the areas (Clarke & Gorley Citation2015). The similarity/dissimilarity between the samples were visualized with Heatmap.
Systematics
Order Tanaidacea Dana, 1849Suborder Tanaidomorpha Sieg, 1980Superfamily Paratanaoidea Lang, 1949Family Pseudotanaidae Sieg, 1976Genus Akanthinotanais Sieg, Citation1977
Diagnosis
Amended after Sieg (Citation1977): Eyes present or absent. Antenna article 2–3 with seta. Molar acuminate. Carpus pereopods 2–6 without blade-like spine, but with slender or bayonet-like spines.
Species included
Akanthinotanais breviaquas Larsen, 2012; A. cabrali Bamber, 2014; A. gaussi Vanhöffen, Citation1914; A. gerlachi Sieg, Citation1977; A. guillei Shiino, Citation1978; A. longipes Hansen, Citation1913; A. makrothrix Dojiri & Sieg, 1997; A. malayensis Sieg, Citation1977; A. mortenseni Sieg, Citation1977; A. scrappi Bamber, 2005; A. siegi Kudinova-Pasternak, 1985; A. similis Sieg, Citation1977; A. rossi sp. nov.
Akanthinotanais gaussi (Vanhöffen, Citation1914)()
Figure 4. Akanthinotanais gaussi Vanhöffen, Citation1914, female, (a, c), dorsal; (b, d), lateral. Scale line = 1 mm
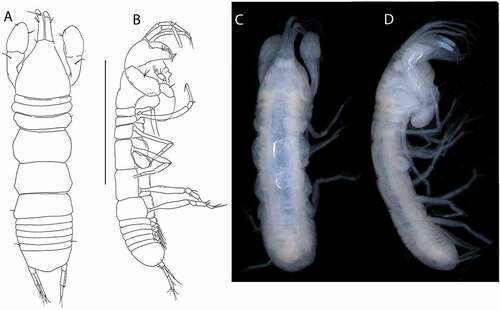
Figure 5. Akanthinotanais gaussi Vanhöffen, Citation1914, female, (a), antennule; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible; (f), maxillule, with (f’), detail of distal end of endite; (g), maxilliped; (h), epignath; Scale lines = 0.1 mm
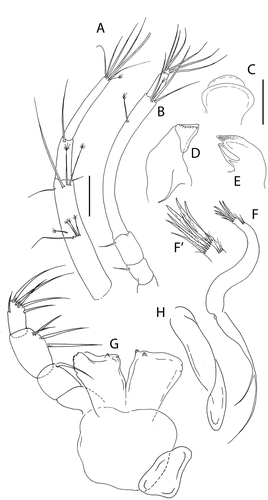
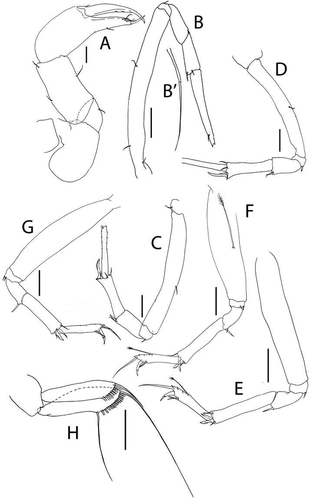
Figure 6. Akanthinotanais gaussi Vanhöffen, Citation1914, female, (a), cheliped; (b to d), pereopods 1 to 3; (e), pereopod-5, with (e’), detail of distal articles; (f), pleopod; (g), uropod. Scale lines = 0.1 mm
Synonymy. Pseudotanais gaussi: Vanhöffen (Citation1914): 483–484, ; Kudinova-Pasternak (Citation1975): 225; Sieg (Citation1984b): 102; Bird & Holdich (Citation1989a): 293; Błażewicz-Paszkowycz (Citation2014): 483.
Pseudotanais (=Akanthinotanais) gaussi: Sieg (Citation1977): 50–54, figs: 34–38. Larsen et al. (Citation2012): 42; Sieg and Heard (Citation1988): 41.
Material examined
Brooding female 1.9 mm, neuter 1.3 mm, manca-2 1.1 mm, ICUL8821, BIO3-EBS-1B (NHM UK 2021.84); neuter 1.3 mm, ICUL8890, BIO4-EBS-1A (NHM UK 2021.85); two juvenile females 1.5–2.1 mm, two neuters 1.3–1.5 mm, ICUL8820, BIO4-EBS-3A (NHM UK 2021.86); juvenile female 1.4 mm, ICUL8825, BIO4-EBS-3A (NHM UK. 2021.87); dissected neuter 1.4 mm, ICUL8913, BIO4-EBS-3A (NHM UK 2021.88); three neuters 1.4 mm, ICUL8822, BIO4-EBS-3B (NHM UK 2021.89); neuter 1.3 mm, ICUL8823, BIO4-EBS-3D (NHM UK 2021.90); juvenile female 2.0 mm, ICUL8824, BIO6-EBS-2A (NHM UK 2021.91)..
Diagnosis
Eyes absent. Antennule article-3 without thick-rod seta. Mandible molar acuminate. Cheliped carpus 2.6 L:W; palm 1.3 L:W; fixed finger 0.8x palm. Pereopod-1 carpus 0.5x propodus; propodus 0.7x dactylus and unguis combined length. Uropod exopod 0.7x endopod, two-articled; uropod endopod two-articled.
Etymology
The species was named in honour of Johann Friedrich Carl Gauss, German mathematician and physicist. The type locality of Pseudotanais gaussi is related to the Gauss Station, located during voyage of the Deutsche Südpolar-Expedition during 1901–1903.
Redescription of female with oostegites. BL = 1.8 mm. Body robust ()) 3.8 L:W. Cephalothorax 1.1 L:W, 1.9x pereonites 1–3, 0.3x BL. Pereonites 0.5x BL, pereonites 1–6: 0.13, 0.17, 0.2, 0.5, 0.5 and 0.4 L:W, respectively. Pleon short, 0.2x BL. Pleonites 0.6 L:W. Pleotelson 2.8x pleonite-5.
Antennule ()) elongate, slender; article-1 4.9 L:W, 3.3x article-2, with one simple and four penicillate mid-length setae, and three simple (one long, two short) and two penicillate distal setae; article-2 2.6 L:W, 0.5x article-3, with dorsodistal seta longer than article-2; article-3 8 L:W, with five simple and one penicillate setae, and one aesthetasc.
Antenna ()) just longer than antennule; article-1 fused with body, naked; article-2 1.1 L:W, 0.7x article-2, with fine dorsodistal seta; article-3 1.5 L:W, 0.1x article-4, with fine dorsodistal seta; article-4 11.1 L:W 5.0x article-5, with subdistal penicillate seta, and three simple and one penicillate setae distally; article-5 3.0 L:W, with distal seta; article-6 minute with five distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor distally pointed, margin finely serrate, lacinia mobilis well developed, distally serrate; molar acuminate, naked.
Right mandible ()) incisor unequally bifid, margin serrate, molar as in left mandible.
Maxillule ()) endite with seven distal spines (’)), setulate distally and subdistally; palp with two distal setae. Maxilla simple, semi-rounded.
Labium not seen.
Maxilliped ()) basis wide proximally, subrectangular; palp article-1 naked; article-2 with inner setae (outer seta not seen); article-3 with four inner setae; article-4 with five distal and one subdistal setae. Maxilliped endites ()) separated, with one gustatory cusp.
Epignath ()) ribbon-shaped, flexible, distally rounded.
Cheliped ()) basis large, 1.25 L:W, with round posterior lobe; merus shorter than carpus ventral margin, with midventral seta; carpus 2.6 L:W, 1.4x palm with dorsodistal seta, dorsoproximal seta broken; chela robust, non-forcipate, 2.3 L:W, 1.6x carpus L, palm 1.3 L:W, with seta near dactylus insertion; fixed finger 2.1 L:W, 0.8x palm, with ventral seta, cutting edge extended-convex, margin serrate with three setae, distal spine pointed; dactylus slender, dactylus 1.3x unguis, proximal seta present, unguis 4.2 L:W.
Pereopod-1 ()) overall very slender; coxa present, seta not seen; basis slender, 12.6 L:W, 4.2x merus, with middorsal penicillate seta; ischium naked; merus 3.8 L:W, 0.7x carpus, naked; carpus anaxially articulated to merus, 5.5 L:W, 0.5x propodus, naked; propodus 8.0 L:W, 0.7x dactylus and unguis combined length, with one dorsal and one ventral subdistal setae; dactylus 0.5x unguis, with dorsoproximal long seta.
Pereopod-2 ()) shorter and stouter than pereopod-1; coxa present, seta not seen; basis 6.0 L:W, 4.3x merus, naked; ischium with small ventral seta; merus 1.8 L:W, 0.5x carpus, with ventrodistal seta; carpus 4.0 L:W, 1.3x propodus, with small dorsodistal seta, long spine and seta ventrodistally; propodus 4.2 L:W, 0.8x dactylus and unguis combined length, with distal spine (1.6x dactylus); dactylus 0.7x unguis.
Pereopod-3 ()) coxa present, seta not seen; basis 9.3 L:W, 5.0x merus, with middorsal penicillate seta; ischium with small ventral seta; merus 2.2 L:W, 0.6x carpus, with two simple and one penicillate distal setae; carpus 3.5 L:W, 1.1x propodus, with short dorsodistal spine, long spine, and seta ventrodistally; propodus 4.0 L:W, with distal spine (1.5x dactylus); unguis broken.
Pereopod-4 broken.
Pereopod-5 () basis 5.2 L:W, 5.2x merus, with two long penicillate ventral setae; ischium with ventrodistal seta; merus 1.7 L:W, 0.5x carpus, with one longer and one fine ventrodistal setae; carpus 3.6 L:W, 1.0x propodus, with four distal setae; propodus 4.2 L:W, 4.0x dactylus and unguis combined length, with two ventrodistal and one dorsodistal spine; dactylus fused with unguis, unequally bifurcate.
Pleopod ()) peduncle 1.6 L:W; endopod distinctly shorter than exopod, 4.2 L:W, with five plumose setae; exopod 3.5 L:W, with eight plumose setae.
Uropod ()) peduncle 1.2 L:W; exopod and endopod two-articled; exopod 0.7x endopod, article-1 4.8 L:W, with seta, article-2 5.0 L:W with two setae; endopod article-1 5.0 L:W, 1.8x article-2, with two penicillate distal setae, article-2 4.45 L:W, with six simple and one penicillate setae.
Distribution
Amundsen Sea, 489–1414 m.
Remarks
We redescribe much of the morphology of Akanthinotanais gaussi, as Sieg’s (Citation1977) description and figures were based on a subadult (“neuter”), and were somewhat stylized.
Akanthinotanais gaussi is one of three blind species in the genus. The two-articled uropod exopod distinguishes A. gaussi from A. longipes and A. similis, where it is one-articled. Additionally, a two-articled uropod endopod differentiates A. gaussi from A. similis with its one-articled endopod. Finally, the absence of thick rod seta on antennule article-3 differentiates A. gaussi from A. longipes, that has this seta.
McLelland (Citation2008) pointed out that the only species of Akanthinotanais with two distal setae on the propodus of pereopod-1 was A. guillei, originally recorded from the Kerguelen Islands at depths of 10 and 32 m (Shiino Citation1978). In fact, although not described or figured by Sieg (Citation1977) in his redescription of the lectotype, A. gaussi shares this feature. Akanthinotanais guillei is one of few Antarctic species known to have eyes.
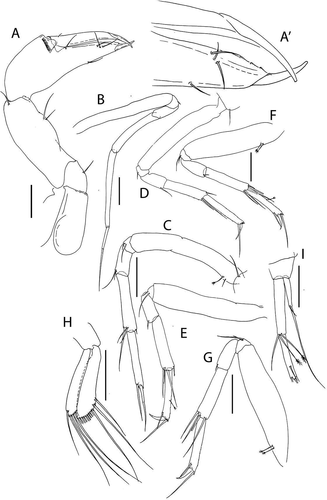
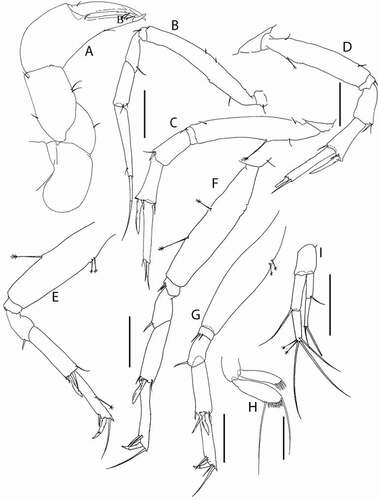
Akanthinotanais rossi sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:38AE8A73-31FB-40FB-A2C5-E9E5C16321C5
Figure 7. Akanthinotanais rossi sp. nov., holotype female, (a, b), dorsal; (c), lateral. Scale line = 1 mm
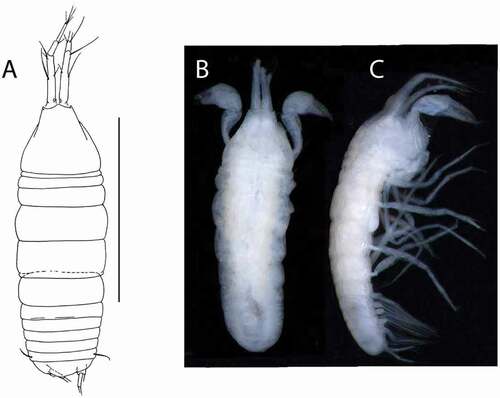
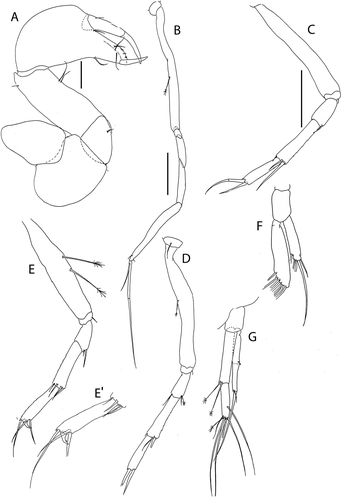
Figure 8. Akanthinotanais rossi sp. nov., (a), antennule; (b), antenna; (c), left mandible; (d), right mandible; (e), maxillule; (f), epignath; (g), labium; (h), maxilliped endites; (h’), maxilliped palp. Scale lines = 0.1 mm
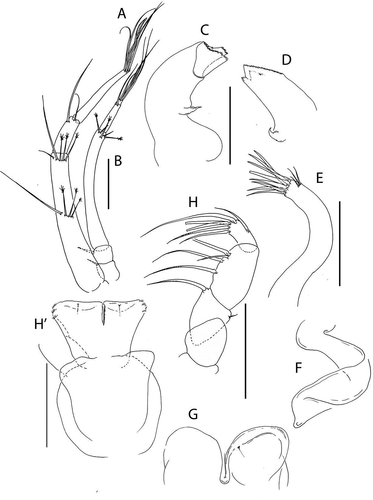
Figure 9. Akanthinotanais rossi sp. nov., (a), cheliped; A’, detailed of chela; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
Material examined
Holotype, juvenile female 1.6 mm, ICUL8847, SR-EBS-5 (NHM UK. 2021.92). Paratypes: neuter 1.1 mm, ICUL8885, SG-5E (NHM UK. 2021.93); neuter 1.5 mm, ICUL8849, SR-EBS-4 (NHM UK.2021.94); dissected neuter 1.5 mm, ICUL8918, SR-EBS-4 (NHM UK.2021.95); dissected 2 microscope slides (NOT LISTED IN ORIGINAL MS -LEH:), two neuters 1.4–1.5 mm, ICUL8848, SR-EBS-5 (NHM UK.2021.96); dissected neuter 1.4 mm, ICUL8902, SR-EBS-5 (NHM UK.2021.97).
Diagnosis
Eyes absent. Antennule article-3 without thick rod seta. Mandible molar acuminate, short. Cheliped carpus 2.5 L:W; palm 1.4 L:W; fixed finger 1.1x palm. Pereopod-1 carpus 0.8x propodus; propodus 1.2x dactylus and unguis combined length. Uropod exopod one-articled, 0.3x endopod; endopod two-articled.
Etymology
Species name in honour of Sir James Clark Ross, a British Royal Navy officer and explorer of the Antarctic.
Description of juvenile female (holotype). BL = 1.6 mm. Body robust ()) 2.9 L:W. Cephalothorax 0.9 L:W, 2.1x pereonites 1–3, 0.3x BL. Pereonites 0.5x BL, pereonites 1–3 progressively longer, pereonites 1–6: 0.01, 0.12, 0.2, 0.4, 0.4 and 0.4 L:W, respectively. Pleon short, 0.3x BL. Pleonites 0.2 L:W, pleonite-5 with conspicuous lateral seta (one on each side). Pleotelson 1.8x pleonite-5.
Antennule ()) article-1 5.1 L:W, 3.5x article-2, with one simple and three penicillate mid-length setae, and two simple and three penicillate distal setae; article-2 2.7 L:W, 0.6x article-3, with two (short and long) dorsodistal setae; article-3 7.4 L:W, with five simple setae and one aesthetasc distally.
Antenna ()) shorter than antennule; article-2 1.1 L:W; 1.1x article-3, with seta (0.6x article-2); article-3 1.0 L:W, 0.15x article-4, with robust seta (1.0x article-3); article-4 8.3 L:W, 3.9x article-5, with two simple and three penicillate setae, distally; article-5 3.4 L:W, 5.7x article-6, with simple seta; article-6 1.0 L:W, with four distal setae.
Labrum not seen.
Left mandible ()) incisor with distal margin truncate, with three blunt teeth, margin irregularly serrate; lacinia mobilis well developed, distal margin serrate; molar acuminate, small.
Right mandible ()) incisor unequally bifid, outer margin serrate, molar as in left mandible.
Maxillule ()) endite with eight distal spines and outer subdistal tuft of setules.
Maxilla not seen.
Labium ()) simple smooth, with cusps in the middle of inner margin.
Epignath ()) ribbon-shaped, flexible, distally truncate.
Maxilliped ()) bases about as long as broad, naked; palp article-1 naked, article-2 with one fine outer and one long inner setae, article-3 with four (three long and one short) inner setae, article-4 with one subdistal and five distal setae, the distalmost seta very long; all inner/distal setae with distal half of inner margin finely denticulate; endites partially fused, with distal cleft about 0.3x total length, with one small gustatory cusp.
Cheliped ()) slender; basis 2.3 L:W; merus 0.4x carpus ventral margin, with ventral seta; carpus 2.5 L:W, 1.4x palm, with two midventral setae and dorsodistal simple seta (dorso-proximal seta not seen); chela non-forcipate, 3.0 L:W, 1.4x carpus L, palm 1.4 L:W, with comb of 15 (14 small and one longer setae) on inner side, and with seta near dactylus insertion; fixed finger 3.0 L:W, 1.1x palm, with ventral seta; cutting edge almost simple but with few small teeth distally, with three setae; dactylus 5.0 L:W, cutting edge smooth, proximal seta not seen; unguis 0.4x dactylus.
Pereopod-1 ()) very slender and elongate; basis 9.3 L:W, 5.1x merus, naked; ischium with ventral seta; merus 2.2 L:W, 0.3x carpus, naked; carpus 6.4 L:W, 0.8x propodus, naked; propodus 10.5 L:W, 1.2x dactylus and unguis combined length, naked; dactylus 0.5x unguis.
Pereopod-2 ()) coxa with seta; basis 6.3 L:W, 4.5x merus, with one penicillate and one simple dorsoproximal setae; ischium with ventral seta; merus 2.0 L:W, 2.5x carpus, with two (short and long) ventrodistal setae; carpus 5.0 L:W, 1.1x propodus, with small dorsodistal seta, two setae and slender spine ventrodistally; propodus 6.4 L:W, 0.9x dactylus and unguis combined length, with long distal spine (1.9x dactylus); dactylus 0.4x unguis.
Pereopod-3 ()), coxa with seta; basis 6.4 L:W, 5.3x merus, naked; ischium with ventral seta; merus 1.6 L:W, 0.5x carpus, with ventrodistal seta; carpus 3.8 L:W, 0.9x propodus, with one dorsodistal and two ventrodistal setae; propodus 6.3 L:W, 2.5x dactylus and unguis combined length, with one distal slender spine (4.8x dactylus); dactylus 0.7x unguis.
Pereopod-4 ()) basis 5.0 L:W, 4.3x merus, naked; ischium with long and short setae; merus 2.1 L:W, 0.5x carpus, with ventrodistal seta; carpus 4.3 L:W, 1.0x propodus, with three (two long and one short) ventrodistal setae and dorsodistal seta; propodus 7.3 L:W, 2.9x dactylus and unguiscombined length, with penicillate setae on dorsal margin, one ventrodistal seta and one long, dorsodistal seta (2.1x dactylus and unguis combined length); dactylus 4.0x unguis.
Pereopod-5 ()) basis 4.0 L:W, with two dorsoproximal penicillate setae, 4.3x merus; ischium with long and short setae; merus 1.7 L:W, 0.5x carpus, with ventrodistal seta; carpus 5.2 L:W, 1.2x propodus, with four simple setae and one seta distally; propodus 7.3 L:W, 2.4x dactylus and unguis combined length, with penicillate seta on dorsal margin, ventrodistal serrate seta and disto dorsal serrate seta (2.0x dactylus and unguis combined length); dactylus 3.2x unguis.
Pereopod-6 ()) basis 4.4 L:W, 4.7x merus, with two dorsoproximal penicillate setae; ischium with long and short seta; merus 2.1 L:W, 0.5x carpus, with ventrodistal seta; carpus 4.3 L:W, 1.0x propodus, with four distal setae; propodus 6.0 L:W, 3.0x dactylus and unguiscombined length, with one ventral and one dorsal serrate setae (2.1x dactylus and unguis combined length); dactylus 4.0x unguis.
Pleopods ()) peduncle 1.0 L:W; endopod lightly shorter than exopod, 6.2 L:W, with five setae; exopod 4.3 L:W, with eleven setae.
Uropod ()) peduncle 0.7 L:W; exopod one-articled, 5.9 L:W, 0.3x endopod, with one short and one long setae; endopod two-articled, article-1 5.3 L:W, with one simple and two penicillate distal setae; article-2 8.3 L:W, with one subdistal simple seta and three distal setae.
Distribution
Shag Rocks, South Georgia, Southern Thule (South Sandwich Is), 201–505 m.
Remarks
Akanthinotanais rossi sp. nov., along with A. gaussi, A longipes, A. similis, forms a group of blind species in the genus. It also has a short uropod exopod (0.3x endopod), which is usually 0.7x in the known blind Akanthinotanais species. It has an unusual short and simple molar, which is often longer, e.g., A. similis (Sieg Citation1977: 48, ) and occasionally serrate, e.g., A. longipes (Sieg Citation1977: 33, ).
A one-articled uropod exopod is an apomorphy that separates A. rossi from the other blind Antarctic species, A. gaussi. Furthermore, the two-articled uropodal endopod distinguishes A. rossi from A. similis (known from Bloscon-Roscoff off Brittany, depth 20 m) that has a one-articled endopod. Finally, the absence of a thick rod seta on antennule article-3 separates A. rossi from A. longipes (Sieg Citation1977: ), which occurs off Iceland (depth 1438 m).
Genus: Beksitanais Jakiel, Palero & Błażewicz, Citation2019
Diagnosis
After Jakiel et al. Citation2019, amended. Antennule article-3 with one or two long thickened rod setae. Antenna article 2 and 3 with seta; article-6 with or without thickened rod seta. Maxilliped palp article-4 without thickened rod seta; endites almost completely fused, with two slender (palpate) gustatory cusps. Chela forcipate with serrate incisive margins, propodus (palm) without small folds in dorsodistal corner, proximal seta on fixed finger. Pereopods 4–6 dactylus and unguis fused with a small hook at tip. Uropod exopod one or two-articled, 0.5x endopod; endopod with pseudo-articulation (weakly two-articled).
Species included. Beksitanais abyssi (Hansen, Citation1913); B. apocalyptica Jakiel, Palero & Błażewicz, Citation2019; B. vanhoeffeni sp. nov.
Beksitanais abyssi (Vanhöffen, Citation1914), new combination
Synonymy. Pseudotanais abyssi: Vanhöffen (Citation1914): 449, 456, 483; Sieg (Citation1977): 30, 50–55; Sieg (Citation1984b): 102; Sieg (Citation1986a): 4, 9, 152; Sieg (Citation1986b): 4, 5, 8, 17; Sieg & Heard (Citation1988): 41; Błażewicz-Paszkowycz (Citation2014): 483
non Pseudotanais abyssi: Hansen (Citation1913): 25, 128, ; Holdich and Bird (Citation1985): 445.
Remarks
Pseudotanais abyssi, redescribed by Sieg (Citation1977), bears a thick rod seta on the antennule and has the characteristic shape of the forcipate type of chela but with the serrate margins and lack of small folds in dorsodistal corner apparently representative of the genus Beksitanais.
The only Antarctic record of B. abyssi is that of Vanhöffen (Citation1914) at its type locality, the Gauss Station (60°47ʹ48ʹʹ S; 89°18ʹ4ʹʹ, depth 385 m) and gave little descriptive detail, merely recognizing his material as being of Hansen’s species. Sieg (Citation1977) did not specify the origin of the material on which he based his redescription. We presume that the material described by Vanhöffen (Citation1914) is actually a sister species to B. vanhoeffeni, and that B. abyssi is not bipolar in its distribution. Consequently, we move this species from Pseudotanais into Beksitanais.
Beksitanais vanhoeffeni sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:4F5F543C-0856-4E1E-8865-CB9DD96A0263
Figure 10. Beksitanais vanhoeffeni sp. nov., holotype female, (a, d), dorsal; (b, e,) lateral; ©, juvenile male. Scale line = 1 mm
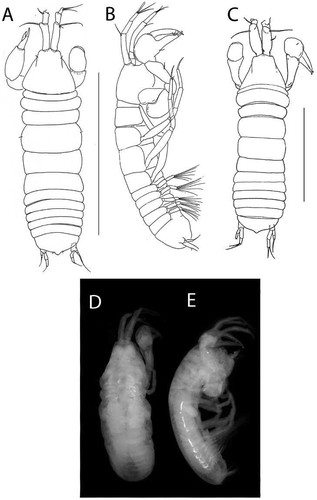
Figure 11. Beksitanais vanhoeffeni sp. nov., (a), female antennule; (b), male antennule; (b’) detail of male antennule; (c), female antenna; (d), labrum; (f), left mandible; (e), right mandible; (g), maxillule; (g’), maxillule endite, distal; (h), maxilla; (i), labium; (j), maxilliped. Scale lines = 0.1 mm
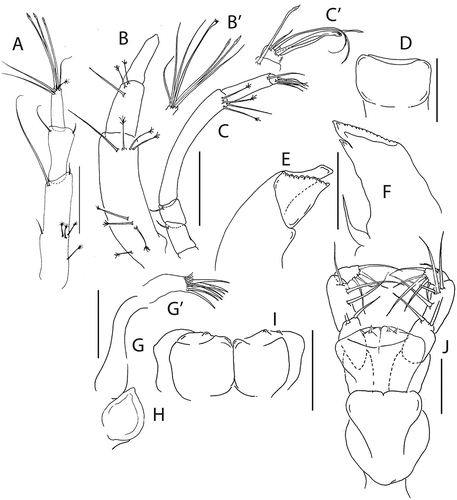
Figure 12. Beksitanais vanhoeffeni sp. nov., (a), cheliped; A’, detail of chela; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
Material examined
Holotype juvenile female 1.2 mm, (1 microscope slide NOT IN ORIGINAL MS_ LEH;), ICUL8839, BIO4-EBS-3A (NHM UK.2021.98). Paratypes: neuter 0.9 mm, ICUL8842, BIO3-EBS-1B (NHM UK. 2021.99); neuter 1.9 mm, ICUL8840, BIO4-EBS-3A (NHM UK.2021.100); female 1.1 mm, juvenile female 1.1 mm, juvenile male 1.1 mm, neuter 0.8 mm, manca 1.0 mm, ICUL8843, BIO4-EBS-3B (NHM UK.2021.101); dissected neuter 1.2 mm, ICUL8907, BIO4-EBS-3B (NHM UK.2021.102); dissected neuter 1.2 mm, ICUL8928, BIO4-EBS-3B (NHM UK.2021.103); neuter 1.2 mm, ICUL8841, BIO4-EBS-3D (NHM UK.2021.104); dissected male, ICUL8906, ST-EBS-3B (NHM UK.2021.105); one individual, ICUL8844, BIO5-EBS-3B (NHM UK.2021.106); one individual, ICUL8893, BIO5-EBS-3B (NHM UK.2021.107).
Diagnosis
Antennule article-3 with two long thick rod setae. Antenna article-6 with long thick rod seta. Uropod exopod two-articled.
Etymology
Named after the German biologist Professor Dr. Ernst Vanhöffen (1858–1918), in recognition of his studies on the Tanaidacea of the Antarctic (and elsewhere), and who first found (but not recognized) this species during the Deutsche Südpolar Expedition in 1902.
Description of neuter
BL = 1.2 mm. Body robust ()) 3.3 L:W. Cephalothorax 0.7 L:W, 0.9x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonites 1–6: 0.1, 0.3, 0.2, 0.4, 0.4, and 0.3 L:W, respectively. Pleon short, 0.3x BL. Pleonites 0.9 L:W. Pleotelson 1.7x pleonite-5.
Antennule ()) article-1 3.3 L:W, 3.0x article-2, four proximal penicillate seta, and one simple and middle setae; article-2 1.5 L:W, 0.9x article-3, with one long and short distal setae; article-3 2.3 L:W, with three simple, one penicillate, and two long rod setae, and with one aesthetasc, distally.
Antenna ()) as long as antennule; article-2 1.2 L:W, 1.1x article-3, with seta (0.5x article-2); article-3 1.1 L:W, 0.2x article-4, with seta (0.5x article-3); article-4 7.1 L:W, 2.8x article-5, with one simple and three penicillate distal setae; article-5 2.8 L:W, 5.0x article-6, with simple seta; article-6 0.8 L:W, with four distal setae and one long thick rod seta.
Labrum ()) rounded, naked.
Left mandible ()) incisor in shape of blunt tooth, margin simple; lacinia mobilis well developed, distal margin serrate; molar not seen.
Right mandible ()) incisor unequally bifid, distal margin serrate; molar acuminate, simple.
Labium ()) simple smooth, with distal cusps.
Maxillule ()) endite with seven distal spines and distal crown of setules.
Maxilla ()) simple, naked.
Maxilliped ()) basis subrectangular, just longer than broad, naked; palp article-1, naked, article-2 with two inner setae (outer seta not observed), article-3 with four inner setae, article-4 with five distal and one subdistal setae; endites almost fused, with small distal cleft, each with two slender gustatory cusps.
Cheliped ()) slender; basis 1.6 L:W; merus about as long as carpus ventral margin, with ventral seta; carpus 2.5 L:W, 1.6x palm, with two (longer and shorter) ventral setae, and with one mediodorsal and one dorsodistal setae; chela 2.6 L:W, 1.6x carpus L, palm 0.8 L:W; fixed finger 5.8 L:W, 1.4x palm, with ventral seta, cutting edge almost simple with three setae, distal spine curved upward; dactylus 4.3 L:W, cutting edge finely serrate, distalmost denticles larger, proximal seta not seen, unguis curved downward.
Pereopod-1 ()) basis broken; ischium seta not seen; merus 1.7 L:W and 0.5x carpus; carpus 3.5 L:W, 0.9x propodus, with distal seta; propodus 4.6 L:W, with ventrodistal seta; dactylus broken.
Pereopod-2 ()) coxa with seta; basis 5.3 L:W, 3.1x merus, with simple mid-length seta; ischium with ventral seta; merus 2.1 L:W, 1.1x carpus, with short ventrodistal spine; carpus 2.1 L:W, 0.5x propodus, with seta and short blade-like spine (0.3x propodus); propodus 6.8 L:W, 3.0x dactylus and unguis combined length, with one small ventrodistal spine (0.6x dactylus); dactylus 2.0x unguis.
Pereopod-3 ()) basis 4.5 L:W, 2.4x merus, with simple dorsal and ventral proximal setae; ischium with ventral seta; merus 1.9 L:W, 1.0x carpus, with short ventrodistal spine; carpus 2.3 L:W, 0.6x propodus, with seta and short blade-like spine (0.3x propodus); propodus 5.6 L:W, 2.3x dactylus and unguis combined length, with distal spine (0.5x dactylus); dactylus 3.0x unguis.
Pereopod-4 ()) basis 4.9 L:W, 4.5x merus; ischium with ventral seta; merus 1.3 L:W, 0.5x carpus, with slender ventrodistal spine; carpus 3.9 L:W, 1.0x propodus, with dorsodistal seta (0.2x propodus), spine, and blade-like spine (0.3x propodus) ventrodistally; propodus 3.7 L:W, 3.3x dactylus and unguis combined length, with two serrate ventral setae and serrate dorsal seta (2.6x dactylus and unguiscombined length); dactylus 3.0 L:W, unguis small, hook-like.
Pereopod-5 ()) basis 5.0 L:W, 3.2x merus, with midventral penicillate seta; ischium with ventral seta; merus 2.8 L:W, 0.7x carpus, with ventrodistal spine; carpus 4.2 L:W, 0.9x propodus, with dorsodistal seta (0.15x propodus), spine and short blade-like spine (0.2x propodus) ventrodistally; propodus 6.8 L:W, 3.8x dactylus and unguiscombined length, with two serrate ventral setae (short and long) and one serrate dorsal seta (2.0x dactylus and unguis combined length; dactylus 3.0 L:W, unguis small, hook-like.
Pereopod-6 ()) basis 4.4 L:W, 3.8x merus (penicillate setae broken); ischium with ventral seta; merus 1.4 L:W, 0.5x carpus, with ventrodistal spine; carpus 3.1 L:W, 0.9x propodus, with seta (0.12x propodus), spine, and blade-like spine (0.2x propodus); propodus 5.3 L:W, 3.2x dactylus and unguiscombined length, with two serrate ventrodistal setae and two serrate dorsodistal setae (2.3x dactylus and unguis combined length); dactylus 3.0 L:W, unguis small, hook-like.
Pleopods ()) peduncle 1.2 L:W; endopod just shorter than exopod, 3.2 L:W, with four setae;, exopod 3.0 L:W, with eight setae.
Uropod ()) peduncle 0.8 L:W; exopod 0.6x endopod, article-1 2.8 L:W, with one penicillate seta, article-2 4.3 L:W, setae broken; endopod 5.3 L:W, with pseudo-articulation, article-1 3.0 L:W, with one simple and one penicillate setae, article-2 2.8 L:W, with three simple and one penicillate setae.
Juvenile male ()) similar to female but antennule thicker ()).
Distribution
Amundsen Sea, Southern Thule (Sandwich Is), 500–1113 m.
Remarks
Beksitanais vanhoeffeni sp. nov. is the third known species in the genus after B. apocalyptica that was recorded in a manganese nodule field in the Central Pacific (Jakiel et al. Citation2019) and the reclassification of B. abyssi (this paper). Beksitanais vanhoeffeni, with a two-articled uropod exopod, can be separated from B. apocalyptica where this is one-articled. Moreover, its short pereonite-1 (0.4x pereonite-2), is distinct from that of B. apocalyptica that is slightly shorter than pereonite-2 (0.8x). Both species can be distinguished by a thick rod seta on antenna article-6 that is present in B. vanhoeffeni but absent in B. apocalyptica. Finally, the length of the dorsal seta of the pereopod-4 propodus allows discrimination of the species: in B. vanhoeffeni it is 2.6x as long as the dactylus and unguis combined length, while in B. apocalyptica it is 3.2x.
Overall, the most similar species to B. vanhoeffeni is B. abyssi. However, the length of the pereopod-2 propodus relative to the dactylus and unguis combined length can distinguish both species, namely 2.0x in B. abyssi and 3.0x in B. vanhoeffeni. Additionally, the uropod endopod of B. apocalyptica is more robust than in B. vanhoeffeni (5.3 L:W) and slender in B. abyssi (>7.0 L:W). Finally, the setae on antenna articles 2–3 in B. vanhoeffeni are short (<0.6x article), and longer in B. abyssi (about 0.8x).
Genus Pseudotanais Sars, 1882
Remarks
Sieg and Heard (Citation1988) first pointed out that the morphology of P. kurchatovi Kudinova-Pasternak & Pasternak, Citation1978 did not match the definition of the genus. It lacks a blade-like spine in pereopods 2–6, as in the members of the genus Akanthinotanais. Moreover stout antennule, coronal molar of the mandibles and two setae on chela are characters which exclude P kurchatovi from that genus. Because there is no access to the collection studied by Kudinova-Pasternak it is impossible to resolve the systematic position of the P. kurchatovi until new material allows for thorough morphological analysis.
“affinis+longisetosus” group
Diagnosis
After Jakiel et al. (Citation2019).
Species included
Pseudotanais affinis Hansen, Citation1887; P. curieae Jakiel, Palero & Błażewicz, Citation2020; P. chanelae Jakiel, Palero & Błażewicz, Citation2020; P. gaiae Jakiel, Palero & Błażewicz, Citation2019; P. geralti Jakiel, Palero & Błażewicz, Citation2019; P. julietae Jakiel, Palero & Błażewicz, Citation2019; P. longisetosus Sieg, Citation1977; P. longispinus Bird & Holdich 1989; P. macrocheles Sars 1882; P. monroeae Jakiel, Palero & Błażewicz, Citation2020; P. nipponicus McLelland 2007; P. nordenskioldi Sieg, Citation1977; P. romeo Jakiel, Palero & Błażewicz, Citation2019; P. spatula Bird & Holdich 1989; P. scalpellum Bird & Holdich 1989; P. svavarssoni Jakiel, Stępień & Błażewicz 2018; P. szymborskae Jakiel, Palero & Błażewicz, Citation2020; P. uranos Jakiel, Palero & Błażewicz, Citation2019; P. vitjazi Kudinova-Pasternak 1966; P. yenneferae Jakiel, Palero & Błażewicz, Citation2019; Pseudotanais sp. O (sensu McLelland, Citation2008); Pseudotanais sp. P (sensu McLelland, Citation2008); P. rapunzelae sp. nov.; and P. shackletoni sp. nov.
Remarks
This is a complex group, with very subtle distinctions between the species. The (largely) diagnostic or useful characters of the species are summarised in .
Pseudotanais rapunzelae sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:6820E0B8-E3D3-4911-A4D9-39753B7FC245
Table II. Diagnostic features distinguishing the species of “affinis+denticulatus” group
Figure 13. Pseudotanais rapunzelae sp. nov., female holotype, (a, c), dorsal; (b, d), lateral; male, (c), lateral. Scale lines = 1 mm
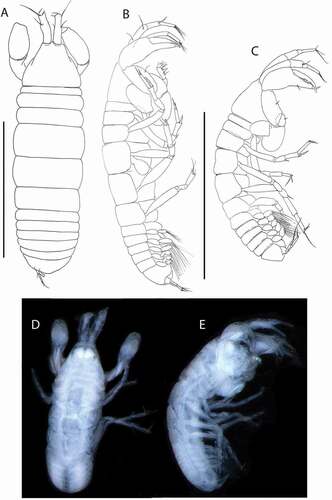
Figure 14. Pseudotanais rapunzelae sp. nov., (a), female antennule; (b), antenna; (c), antennule of subadult male; (d), labrum; (e), left mandible, with (e’), molar; (f), right mandible; (g), maxillule with (g’), maxillule palp; (h), maxilla, (i), epignath; (j), maxilliped and (j); maxilliped endites; (k), labium. Scale lines = 0.1 mm
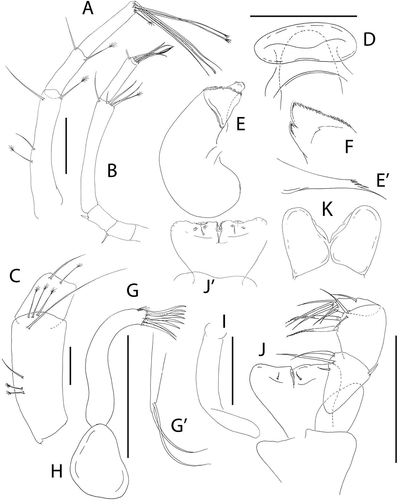
Figure 15. Pseudotanais rapunzelae sp. nov., (a), cheliped with (a') detail of chela; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), distal articles of pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
Material examined
Holotype, neuter 1.3 mm, ICUL8866, ST-EBS-3 (NHM UK.2021.108). Paratypes: juvenile male 1.2 mm, ICUL8868, EI-EBS-1 (NHM UK.2021.109); brooding female 1.6 mm, dissected, ICUL8897, EI-EBS-1 (NHM UK.2021.110); neuter, partly dissected, ICUL8865, ST-EBS-3 (NHM UK.2021.111); neuter (damaged) dissected, ICUL8904, ST-EBS-3 (NHM UK.2021.112); neuter 1.2 mm, juvenile female 1.5 mm, ICUL8867, ST-EBS-3B (NHM UK.2021.113).
Diagnosis
Pereopod-2 carpus with long blade-like spine (0.8x propodus). Pereopods 5–6 carpus with long rod seta (1.2x propodus). Uropod exopod 0.8x endopod.
Etymology
Rapunzel is a princess in a Brothers Grimm fairy tale who, imprisoned in a high tower, offered to let down her long hair so that her lover could climb up to her. This alludes to the unusually long dorsodistal seta on pereopods 5 and 6.
Description of neuter (holotype). BL = 1.3 mm. Body robust ()) 3.3 L:W. Cephalothorax 0.9 L:W, 1.0x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonite-1 less than half length of pereonites 2–3, pereonites-1–6: 0.1, 0.3, 0.3, 0.5, 0.4, and 0.3 L:W, respectively. Pleon 0.3x BL. Pleonites 0.8 L:W. Pleotelson 1.6x pleonite–5.
Antennule ()) article-1 3.9 L:W, 2.4x article-2, with one simple and one penicillate setae at mid-length, and one simple and one penicillate setae distally; article-2 3.3 L:W, 0.8x article-3, with one simple and one penicillate setae distally, both shorter than article; article-3 5.0 L:W, with two simple, two bifurcated, one penicillate setae, and one aesthetasc distally.
Antenna ()) as long as antennule; article-2 1.3 L:W; 1.0x article-3, with slender spine (0.5x article-2); article-3 1.3 L:W, 0.2x article-4, with stouter spine (0.4x article-2); article-4 5.7 L:W, 2.3x article-5, with four simple and one penicillate setae distally; article-5 4.0 L:W, 10.0x article-6, distal seta not seen; article-6 2.0 L:W, with five distal setae.
Labrum ()) rounded, flattened, naked.
Left mandible ()) incisor distal margin forms blunt irregular tooth, margin irregularly serrate; lacinia mobilis well developed, distally serrate; molar acuminate, with distal spines, one longer than the others ()).
Right mandible ()) incisor unequally bifid, distal margin serrate; molar as in left mandible.
Maxillule ()) endite with eight distal spines and outer subdistal tuft of setules; palp ()) with two distal setae.
Maxilla ()) almost rounded, naked.
Labium ()), simple, naked, widely cleft.
Epignath ()) simple, distally rounded.
Maxilliped ()) bases naked; palp article-1 naked, article-2 with fine outer seta and three inner setae (two longer and one short), article-3 with four inner setae (three long and one short), article-4 with five distal and one sub-distal setae, articles 3 and 4 inner setae distal half margin finely denticulate; endites ()) fused except for distal third, each with two gustatory cusps and subdistal seta.
Cheliped ()) basis slender, 1.9 L:W, naked; merus 0.75x carpus ventral margin, with ventral seta; carpus 1.7 L:W, 0.9x palm, with two ventral setae, and with one middorsal seta and one dorsodistal setae; chela 2.9 L:W, 1.7x carpus length, palm 1.6 L:W, with seta near dactylus insertion; fixed finger 3.3 L:W, 0.8x palm, with ventral seta, cutting edge almost simple, poorly calcified, with three setae; dactylus 5.7 L:W, cutting edge with two peg-like setae, without proximal seta.
Pereopod-1 ()) coxa with seta; basis 7.15 L:W, 3.8x merus, with one dorsoproximal, one midventral and one ventrodistal simple setae; ischium with ventral seta; merus 2.0 L:W and 0.8x carpus, with long dorsodistal seta as long as article; carpus 3.1 L:W, 0.6x propodus, with two (short and long) dorsodistal setae; propodus 5.6 L:W (no seta observed); dactylus and unguis broken.
Pereopod-2 ()) basis 5.5 L:W, 3.3x merus, with dorsoproximal penicillate seta and two simple ventroproximal setae; ischium with ventral seta; merus 1.8 L:W, 0.8x carpus, with seta and spine ventrodistally; carpus 2.2 L:W, 0.8x propodus, with one seta, one small spine and long blade-like spine (0.8x propodus); propodus 5.4 L:W, 1.6x dactylus and unguis combined length, with one distal seta (2.3x dactylus); dactylus 0.7x unguis.
Pereopod-3 ()) basis 5.8 L:W, 3.5x merus, with ventroproximal seta; ischium with ventral seta; merus 1.9 L:W, 0.8x carpus, with ventrodistal seta; carpus 2.5 L:W, 0.8x propodus, with one seta, one small spine and long blade-like spine (0.8x propodus); propodus 5.2 L:W, with one distal seta; dactylus and unguis broken.
Pereopod-4 ()) basis 5.4 L:W, 4.3x merus, with one dorsal penicillate and one simple ventral setae; ischium with two ventral setae (short and long); merus 1.9 L:W, 0.5x carpus, with one slender spine; carpus 3.5 L:W, 1.0x propodus, with one dorsodistal seta (0.2x propodus), one spine, one simple and one short blade-like spine (0.4x propodus); propodus 5.6 L:W, 2.3x dactylus and unguiscombined length, with two ventrodistal short and one dorsal long (1.7x dactylus and unguis combined length) setae; dactylus 5.0x unguis.
Pereopod-5 ()) basis 4.6 L:W, 4.6x merus, with dorsoproximal penicillate seta, one simple and one penicillate ventral setae; ischium with two ventral setae (short and long); merus 1.7 L:W, 0.4x carpus, with one spine (broken); carpus 5.4 L:W, 1.4x propodus, with one long (1.2x propodus) dorsodistal seta, one spine and one short blade-like spine (0.4x propodus); propodus 4.0 L:W, 2.0x dactylus and unguiscombined length, with dorsal penicillate seta, two ventrodistal and one dorsal seta (1.7x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-6 ()) basis broken; ischium setae not seen; merus 1.2 L:W, 0.4x carpus, with ventrodistal spine; carpus 3.4 L:W, 1.0x propodus, with long (1.1x propodus) seta dorsodistal seta, one spine and one short blade-like spine (0.4x propodus); propodus 4.5 L:W, 2.4x dactylus and unguiscombined length, with two ventrodistal setae and dorsal seta (2.2x dactylus and unguis combined length); dactylus 2.0x unguis.
Pleopods ()) endopod just shorter than exopod, 4.1 L:W, with seven setae; endopod 2.9 L:W, with nine setae.
Uropod ()) peduncle 1.5 L:W; exopod and endopod two-articled; exopod 0.8x endopod, article-1 3.3 L:W, with distal seta, article-2 4.7 L:W, with two simple setae; endopod article-1 4.0 L:W, with distal seta, article-2 3.8 L:W, with two penicillate setae; other setae broken.
Subadult male. Slightly smaller than female (); length 1.2 mm); proximal antennule article ()) stouter (2.5 L:W) with more penicillate setae, and distal antennule article with indication of subdivision.
Distribution
Elephant Island (South Shetland Is), Southern Thule (Sandwich Is), 500–1503 m.
Remarks
Pseudotanais rapunzelae sp. nov. is classified as a member of the “affinis+longisetosus” morphogroup because of the spine on antenna articles 2–3, relatively long distal spine on pereopods 2–3, long dorsodistal rod seta on the carpus of pereopods 5–6, and the uropod exopod at least 0.8x endopod.
Currently, 13 species of this morphogroup have been observed with a long rod seta on the carpus of pereopods 5–6 (P. chanelae, P. curieae, P. gaiae, P. julietae, P. longisetosus, P. longispinus, P. monroeae, P. nipponicus, P. spatula, P. szymborskae, P. romeo, P. uranos, and Pseudotanais sp. O. A short rod seta on the pereopod-4 carpus distinguishes the new species from P. chanelae, P. curieae and P. longisetosus, where this seta is long. Pseudotanais rapunzelae, with long carpal seta on carpus pereopod–1, can be distinguished from P. gaiae, P. monroeae and P. uranos, which have a short seta at this location.
Also, P. rapunzelae has one short and one long seta (0.5x merus) on the ischium on pereopods 4–6 in contrast to P. julietae, P. longispinus and P. spatula (with single seta), P. romeo (ischium lacking seta), and P. szymborskae (two short, equal seta). The new species is most similar to Pseudotanais sp. O (sensu McLelland) but the absence of a wide-based ventrodistal spine on the carpus pereopods 2–3 separates P. rapunzelae from Pseudotanais sp. O where this spine is present.
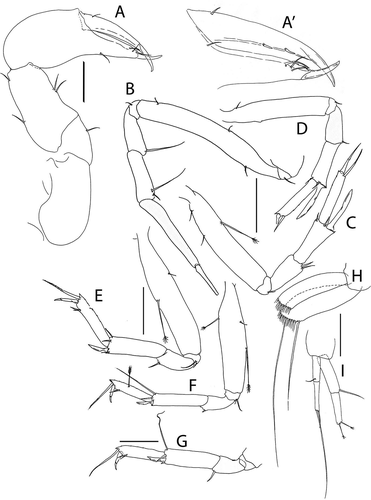
Pseudotanais shackletoni sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:04980C81-85FA-48C8-8F05-94FDA44FB881
Figure 16. Pseudotanais shackletoni sp. nov., female holotype, (a, b), dorsal; (c), latera. Scale line = 1 mm
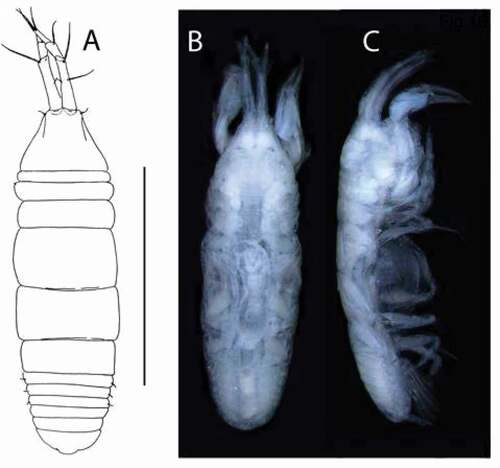
Figure 17. Pseudotanais shackletoni sp. nov., (a), antennule; (b), antenna; (c), left mandible; (d), labium; €, maxillule. Scale lines = 0.1 mm
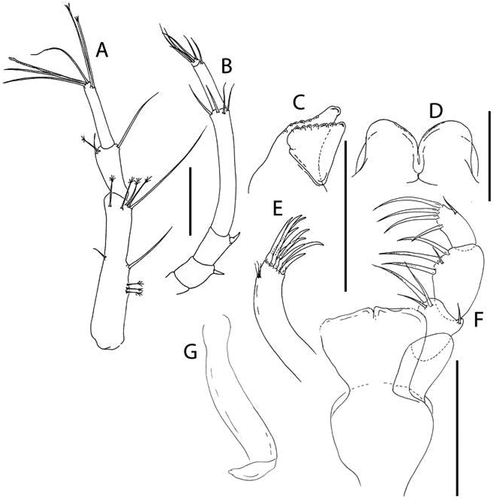
Figure 18. Pseudotanais shackletoni sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
Material examined
Holotype, female 1.6 mm, ICUL8875, SG-EBS-3E (NHM UK.2021.114). Paratypes: neuter (damaged) dissected, ICUL8917, SG-EBS-5E (NHM UK.2021.115); juvenile female 1.7 mm, dissected, ICUL8874, SR-EBS-6 (NHM UK.2021.116).
Diagnosis
Pereopod-2 carpus with long blade-like spine (0.6x propodus). Pereopods 4–6 carpus with short rod seta. Uropod exopod 0.8x endopod.
Etymology
The species is named in honour of Sir Ernest Henry Shackleton, the polar explorer and leader of heroic cruise of HMS Endurance.
Description of female. BL = 1.6 mm. Body robust ()) 3.2 L:W.
Cephalothorax 0.8 L:W, 1.1x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonites 1–3 progressively longer, pereonites-1–6: 0.1, 0.2, 0.3, 0.6, 0.5 and 0.4 L:W, respectively. Pleon 0.3x BL. Pleonites 0.7 L:W, pleonites 1–3 with lateral setae. Pleotelson 1.5x pleonite–5.
Antennule ()) article-1 4.8 L:W, 3.0x article-2, with two simple and group of penicillate setae at mid-length, and one simple and four penicillate setae distally; article-2 2.1 L:W, 0.8x article-3, with two simple and one penicillate setae distally; article-3 6.3 L:W, with three simple and two bifurcated setae and one aesthetasc.
Antenna ()) slightly shorter than antennule; article-2 1.1 L:W, 0.8x article-3, with spine (0.4x article-2); article-3 1.3 L:W, 0.3x article-4, with more robust spine (0.4x article-2); article-4 6.7 L:W, 2.6x article-5, with four distal setae; article-5 3.6 L:W, 7.2x article-6 with distal seta; article-6 0.8 L:W, with five setae.
Left mandible ()) incisor distal margin serrate, with blunt apex; lacinia mobilis well developed, distally serrate; molar not seen.
Labium ()) simple, glabrous.
Maxillule ()) endite with nine distal spines and outer subdistal tuft of setules.
Maxilla not seen.
Epignath ()) simple, distally rounded.
Maxilliped ()) bases as long as broad, naked, heart-shaped; palp article-1 naked; article-2 with fine outer and three inner two inner setae; article-3 with three long and small inner setae; article-4 with four distal and one outer setae; endites naked, almost fully fused, with minute central cleft.
Cheliped ()) basis 1.7 L:W, naked; merus 1.4x carpus ventral margin, with ventral seta; carpus 1.8 L:W, 1.1x palm, with two ventral, one middorsal and one dorsodistal setae; chela 2.5 L:W, 1.8x carpus length, palm 1.4 L:W, with small setae near dactylus insertion; fixed finger 2.8 L:W, 0.7x palm with ventral seta, cutting edge almost simple with three small setae, distal spine almost straight; dactylus 4.4 L:W, cutting edge smooth, with proximal seta.
Pereopod-1 ()) coxa with seta; basis 6.5 L:W, 4.3x merus, with one dorsoproximal and two midventral simple setae; ischium with ventral seta; merus 2.1 L:W and 0.7x carpus, with dorsodistal seta; carpus 1.8 L:W, 0.6x propodus, with two short dorsodistal setae; propodus 7.0 L:W, 1.1x dactylus and unguis combined length, with short ventrodistal seta; dactylus 0.7x unguis.
Pereopod-2 ()) basis 4.4 L:W, 2.9x merus, with middorsal penicillate seta, one dorsoproximal, one ventroproximal, and one ventrodistal simple setae; ischium with ventral seta; merus 2.0 L:W, 0.9x carpus, with seta and spine ventrodistally; carpus 2.2 L:W, 0.7x propodus, with one seta, one spine and long blade-like spine (0.6x propodus); propodus 6.2 L:W, 2.8x dactylus and unguis combined length, with one distal spine (1.6x dactylus); dactylus 1.0x unguis.
Pereopod-3 ()) basis 5.4 L:W, 3.2x merus, with one dorsoproximal penicillate seta, one ventroproximal and ventrodistal simple setae; ischium with ventral seta; merus 1.7 L:W, 0.9x carpus, with seta and spine ventrodistally; carpus 1.8 L:W, 0.7x propodus, with seta, spine, and long blade-like spine (0.7x propodus); propodus 6.5 L:W, with distal seta (1.6x dactylus); dactylus and unguis broken.
Pereopod-4 ()) basis 3.6 L:W, 4.4x merus, with two dorsoproximal and one midventral penicillate setae; ischium with two ventral setae (broken); merus 1.2 L:W, 0.5x carpus, with spine; carpus 3.6 L:W, 1.0x propodus, with dorsodistal seta (0.4x propodus), two spines and one short blade-like spine (0.25x propodus); propodus 5.6 L:W, 2.3x dactylus and unguis combined length, with dorsal penicillate seta, two ventrodistal and one dorsal setae (1.8x dactylus and unguis combined length); dactylus 2.0x unguis.
Pereopod-5 ()) basis 4.9 L:W, 4.0x merus, with two midventral penicillate setae; ischium with two ventral setae; merus 1.7 L:W, 0.6x carpus, with ventrodistal spine; carpus 3.6 L:W, 0.9x propodus, with dorsodistal seta (0.4x propodus), two setae and one short blade-like spine (0.2x propodus); propodus 6.6 L:W, 3.3x dactylus and unguis combined length, two ventrodistal and one dorsal setae (2.2x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-6 ()) basis 3.9 L:W, 4.1x merus, with two dorsal penicillate setae; ischium with two ventral setae; merus 1.7 L:W, 0.6x carpus, with ventrodistal spine; carpus 3.4 L:W, 1.0x propodus, with dorsodistal seta (0.4x propodus), two spines and short blade-like spine (0.3x propodus); propodus 5.4 L:W, 3.4x dactylus and unguis combined length, two ventrodistal and two dorsal setae (longer seta 2.4x dactylus and unguis combined length); dactylus 1.7x unguis.
Pleopods ()) peduncle 1.4 L:W; exoopod shorter than endopod, 4.2 L:W, with five setae; exopod 3.1 L:W, with eleven setae.
Uropod ()) peduncle 1.5 L:W; exopod two-articled, 0.8x endopod, article-1 4.3 L:W, with seta, article-2 7.0 L:W, with two simple setae; endopod two-articled, article-1 3.8 L:W, with long seta article-2 4.0 L:W, with three simple and two penicillate setae.
Distribution
Shag Rocks, South Georgia, 477–1027 m.
Remarks
Pseudotanais shackletoni sp. nov. (), with a long seta on the merus of pereopod-1, can be classified in the “affinis+longisetosus” morphogroup, but a short rod seta on the carpus of pereopods 5–6 differentiates it from Pseudotanais chanelae, P. curieae, P. gaiae, P. julietae, P. longisetosus, P. longispinus, P. monroeae, P. nipponicus, P. rapunzelae, P. romeo, P. spatula, P. uranos and Pseudotanais sp. O (sensu McLelland), which have a long seta. The new species, with a only a spine on the pereopods 4–6 merus, differs from P. geralti, P. nordenskioldi, P. svavarssoni, P. vitjazi, P. yenneferae and Pseudotanais sp. C (sensu McLelland), which have both a spine and seta (but seta only in P. svavarssoni and two spines and seta in P. vitjazi). Finally, P. shackletoni, bearing two setae on the ischium of pereopods 4–6, can be distinguished from P. scalpellum that has a single seta.
Pseudotanais shackletoni is most similar to P. affinis but differs in the proportion of the pereopod–5 dactylus to unguis, 0.6x, versus 0.3x in P. affinis. The carpus of pereopod–5 in P. shackletoni is shorter than the propodus, while in P. affinis it is at least as long. Finally, the long blade-like spine on the pereopod-2 carpus is 0.6x propodus, slightly shorter than in P. affinis where this is 0.7x.
“denticulatus+abathagastor” group
Diagnosis
After Jakiel et al. (Citation2019), amended. Antenna article 2–3 with spines, setae or naked. Mandible molar coronal or subcoronal/acuminate. Chela non-forcipate. Pereopod-1 basis with few (<3) setae; merus and carpus dorsodistal seta short. Pereopod-2 with short, semilong/intermediate or long blade-like spine on carpus. Pereopods 5–6 carpus dorsodistal seta short. Pereopods 4–6 unguis elongate. Uropod slender, exopod longer or slightly shorter than endopod.
Species included. Pseudotanais corollatus Bird & Holdich, 1989; P. denticulatus Bird & Holdich, 1989; P. abathagastor Błażewicz-Paszkowycz, Bamber & Jóźwiak, Citation2013; P. chopini Jakiel, Palero & Błażewicz, Citation2019; P. georgesandae Jakiel, Palero & Błażewicz, Citation2019; P. chaplini Jakiel, Palero & Błażewicz, Citation2019i; P. oloughlini Jakiel, Palero & Błażewicz, Citation2019; P. mariae Jakiel, Palero & Błażewicz, Citation2019; P. locueloae Jakiel, Palero & Błażewicz, Citation2019; P. amundseni sp. nov.; P. barnesi sp. nov.; P. biopearli sp. nov.; P. elephas sp. nov.; P. kitsoni sp. nov.; P. livingstoni sp. nov.; and P. palmeri sp. nov.
Remarks
As with the preceding “affinis+longisetosus” group, this is also complex, with species identification needing close attention. The (largely) diagnostic or useful characters of the species are summarised in .
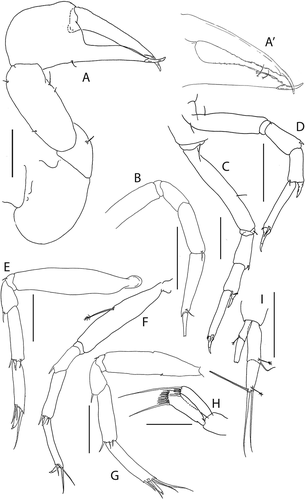
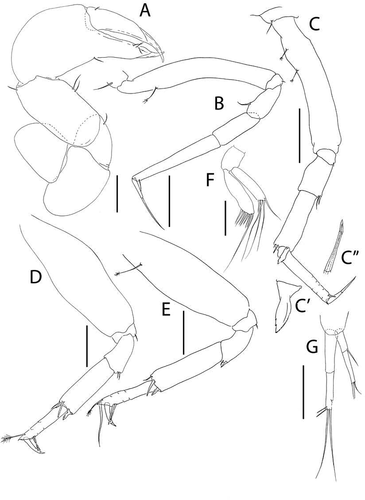
Pseudotanais amundseni sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:DBFC692D-1245-4C9E-BF75-CFEB5065876A
Figure 19. Pseudotanais amundseni sp. nov., female holotype, (a, c), dorsal; (b, d), lateral. Scale line = 1 mm
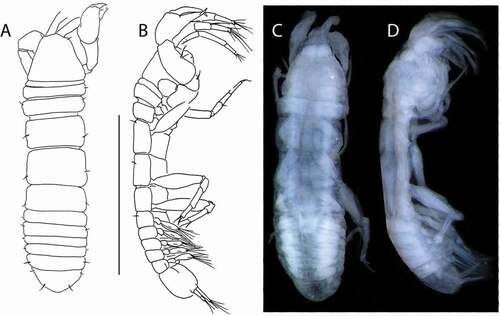
Figure 20. Pseudotanais amundseni sp. nov., (a), antennule; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible; (e’), molar; (f), maxillule; (g), labium; (h), epignath; (i), maxilla and maxilliped. Scale lines = 0.1 mm
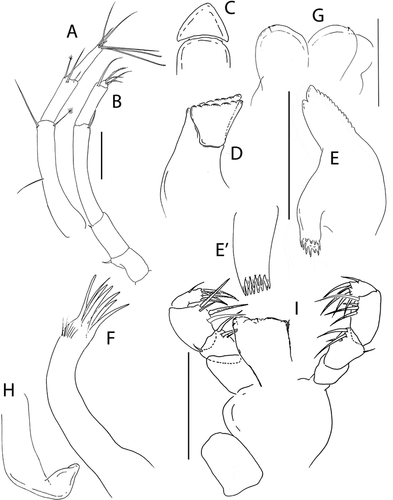
Figure 21. Pseudotanais amundseni sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2, with (c’), blade-like carpal spine and (c”), unguis; (d), pereopod-4; (e), pereopod-6; (f), pleopod; (g), uropod. Scale lines = 0.1 mm
Material examined
Holotype, neuter 1.4 mm, ICUL8826, BIO4-EBS-3B (NHM UK.2021.117). Paratypes: neuter 1.1 mm, ICUL8891, BIO4-EBS-3A (NHM UK.2021.118); two juvenile females 1.4–1.6 mm (one dissected in slides), ICUL8827, BIO4-EBS-3B (NHM UK.2021.119); neuter, 1.6 mm, dissected, ICUL8916, BIO4-EBS-3B (NHM UK.2021.120); juvenile female 1.4 mm, ICUL8833, BIO4-EBS-3D (NHM UK.2021.121); neuter 1.7 mm, ICUL8828, BIO5-EBS-2A (NHM UK.2021.122); neuter 1.6 mm, ICUL8829, BIO5-EBS-3B (NHM UK.2021.123); neuter 1.4 mm, ICUL8830, BIO5-EBS-3D (NHM UK.2021.124); juvenile female 1.5 mm, (1 microscope slide – NOT IN ORIGINAL MS _ LEH:); ICUL8831, BIO6-EBS-3A (NHM UK.2021.125); juvenile female 1.9 mm, ICUL8832, BIO6-EBS-3E (NHM UK. 2021.126).
Diagnosis
Antenna article–2 with thin spine; article–3 with seta. Mandible molar coronal. Pereopods 2–3 carpus blade-like spine short (0.3x propodus). Pereopods 4–6 ischium with seta; merus with spine and seta. Uropod exopod 0.7x endopod.
Etymology
The name of the species, whose type locality is the Amundsen Sea, is given in honour of Roald Engelbregt Gravning Amundsen, the Norwegian Polar pioneer.
Description of female. BL = 1.4 mm. Body robust ()) 4.1 L:W. Cephalothorax 0.8 L:W, 1.5x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonite-1 half as long as pereonites 2–3, pereonites-1–6: 0.1, 0.2, 0.2, 0.5, 0.6, and 0.4 L:W, respectively. All pereonites with small anterolateral setae. Pleon short, 0.3x BL. Pleonites 0.9 L:W, each with lateral seta. Pleotelson 1.4x pleonite-5, with lateral seta and laterodistal seta.
Antennule ()) article-1 5.0 L:W, 2.6x article-2, with simple mid-length seta, and one simple and one penicillate setae distally; article-2 2.9 L:W, 1.1x article-3, with two simple (short and long) and one penicillate setae distally; article-3 4.2 L:W, with one distally bifurcated and five simple setae and one aesthetasc (some setae broken).
Antenna ()) slightly longer than antennule; article-2 1.7 L:W; 0.9x article-3, with spine (0.3x article-2); article-3 2.2 L:W, 0.4x article-4, with minute seta (0.1x article-3); article-4 6.3 L:W, 2.4x article-5, with distal seta; article-5 3.0 L:W, 7.0x article-6, with seta; article-6 0.6 L:W, with two simple and two serrate setae distally.
Labrum ()) dome-shaped, naked.
Left mandible ()) lacinia mobilis well developed, distally serrate; incisor distal margin serrate.
Right mandible ()) incisor unequally bifid, margin serrate ()); molar coronal.
Labium ()) simple, naked.
Maxillule ()) endite with at least seven distal spines and outer subdistal tuft of setules.
Epignath ()) ribbon-like, distally rounded.
Maxilla ()) subrectangular, naked.
Maxilliped ()) bases naked; palp article-1 naked; article-2 with fine outer seta and three inner (one small) setae; article-3 with three long and one short inner setae, article-4 with five distal and one sub-distal setae; all setae with finely denticulate margins on their distal half; endites fused, but with central distal cleft, each with two small gustatory cusps.
Cheliped ()) robust, basis 1.5 L:W, naked; merus slightly shorter than carpus ventral margin, with ventral seta; carpus 1.7 L:W, 0.9x palm, with two midventral and one dorsodistal setae; chela 2.4 L:W, 1.7x carpus length, palm 1.4 L:W, with ventral seta; fixed finger 2.4 L:W, 0.8x palm, cutting edge almost simple, poorly calcified, with three inner setae, distal spine sharp but stout; dactylus 3.4 L:W, cutting edge smooth, with proximal seta.
Pereopod-1 ()) basis 6.8 L:W, 4.3x merus, with dorsoproximal penicillate seta; ischium with ventral seta; merus 1.6 L:W and 0.8x carpus, with dorsodistal seta; carpus 2.7 L:W, 0.5x propodus, naked; propodus 6.0 L:W, 1.4x dactylus and unguis combined length, with ventrodistal seta; dactylus 0.5x unguis; dactylus and unguis 0.6 as propodus.
Pereopod-2 ()) coxa with seta; basis 5.4 L:W, 3.8x merus, with two dorsoproximal penicillate setae; ischium with small ventral seta; merus 1.5 L:W, 0.6x carpus, with ventrodistal seta and spine; carpus 3.0 L:W, 1.0x propodus, with seta, spine and short blade-like spine (0.3x propodus) distally (); propodus 6.0 L:W, 1.4x dactylus and unguis combined length, with ventrodistal spine (0.4x dactylus); dactylus 0.8x unguis ()).
Pereopod-3 similar to pereopod-2.
Pereopod-4 ()) basis 2.9 L:W, 4.4x merus, naked; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with seta and small spine ventrodistally; carpus 2.8 L:W, 1.2x propodus, with dorsodistal seta (0.25x propodus), spine and short blade-like spine (0.3x propodus); propodus 4.8 L:W, 1.3x dactylus and unguis combined length, with dorsodistal penicillate seta and two ventrodistal serrate setae (dorsal seta broken); dactylus 5.0x unguis; unguis very short.
Pereopod-5 similar to pereopod-4.
Pereopod-6 ()) basis 3.0 L:W, 4.3x merus, with proximal penicillate seta; ischium with ventral seta; merus 1.5 L:W, 0.4x carpus, with seta and spine ventrodistally; carpus 3.5 L:W, 1.5x propodus, with dorsodistal seta (0.2x propodus), spine and blade-like spine (0.2x propodus) ventrodistally; propodus 4.8 L:W, 2.0x dactylus and unguis combined length, with dorsodistal penicillate seta, two ventral and one dorsal setae (1.9x dactylus and unguis combined length); dactylus 5.0x unguis.
Pleopods ()) peduncle 1.6 L:W; endopod just shorter than exopod, 4.4 L:W, with five setae; endopod 4.0 L:W, with eight setae.
Uropod ()) peduncle 0.8 L:W; exopod and endopod two-articled; exopod 0.3x endopod, article-1 2.8 L:W, with simple seta, article-2 5.0 L:W, with two distal setae; endopod article-1 3.3 L:W, article-2 5.7 L:W, with one subdistal, two simple and two penicillate distal setae.
Distribution
Amundsen Sea, 489–1046 m.
Remarks
Pseudotanais amundseni sp. nov. has a slender spine on antenna article-2 and can be separated from P. abathagastor and P. mariae that have a seta at this position. A small seta on antennal article-3 also distinguishes P. amundseni from all other members of the group, in which this article is armed with a spine. In addition, a short carpal blade-like spine (0.3x propodus) on pereopods 2–3 makes P. amundseni different from P. chopini, P. denticulatus, P. locueloae and P. oloughlini, where this spine is long or semilong/intermediate (at least 0.5x propodus). Finally, one seta on the ischium of pereopods 4–6 of P. amundseni differentiates it from P. georgesandae that has two setae on that article, while a spine and seta on the merus of pereopods 4–6 separates it from both P. georgesandae and P. chaplini.
Pseudotanais barnesi sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:A38C890A-B82E-4029-943B-8889288779FC
Figure 22. Pseudotanais barnesi sp. nov., juvenile male, (a), dorsal; (b), lateral; holotype female, (c, d), dorsal; €, lateral. Scale line = 0.1 mm
Figure 23. Pseudotanais barnesi sp. nov., (a), antennule; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible; (f), maxillule; (g), maxilla; (j), labium; (i), maxilliped; (h), epignath. Scale lines = 0.1 mm
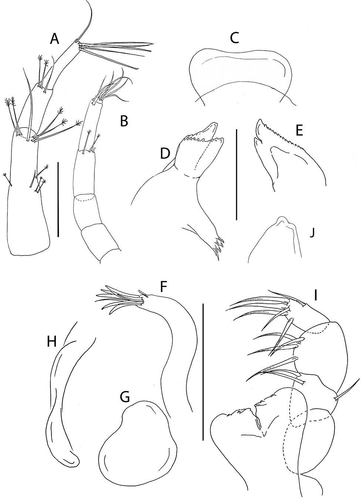
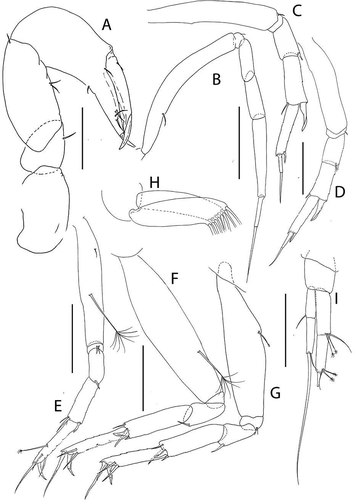
Figure 24. Pseudotanais barnesi sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
Material examined
Holotype, juvenile female 1.0 mm, ICUL8808, BIO5-EBS-3A (NHM UK.2021.127). Paratypes: neuter 1.3 mm, ICUL8816, BIO4-EBS-1A (NHM UK.2021.128); juvenile male 1.0 mm, ICUL8818, BIO4-EBS-2A(E) (NHM UK.2021.129); dissected neuter, ICUL8810, BIO4-EBS-3A (NHM UK.2021.130); juvenile female 1.3 mm, ICUL8817, BIO4-EBS-3A (NHM UK.2021.131); dissected neuter, ICUL8921, BIO4-EBS-3A (NHM UK.2021.132); juvenile male 0.9 mm, neuter 1.1 mm, ICUL8819, BIO4-EBS-3B (NHM UK.2021.133); two damaged juvenile females, ICUL8815, BIO5-EBS-1A (NHM UK.2021.134); neuter 1.2 mm, ICUL8814, BIO5-EBS-2A (NHM UK.2021.135); juvenile female 1.0 mm, juvenile male 1.0 mm, ICUL8812, BIO5-EBS-3A (NHM UK.2021.136); dissected neuter, ICUL8922, BIO5-EBS-3A (NHM UK.2021.137); male 1.2 mm, juvenile male 1.1 mm, neuter 0.8 mm, ICUL8813, BIO5-EBS-3B (NHM UK.2021.138); neuter 1.1 mm, ICUL8805, BIO5-EBS-3D (NHM UK.2021.139); juvenile male 1.2 mm, ICUL8809, BIO5-EBS-3D (NHM UK.2021.140); neuter 1.0 mm, ICUL8811, BIO5-EBS-3D (NHM UK.2021.141); dissected neuter, ICUL8920, BIO5-EBS-3D (NHM UK.2021.142).
Diagnosis
Antenna articles 2 and 3 naked, article-4 short (2.0 L:W). Pereopod-1 merus and carpus naked. Mandible molar coronal. Pereopods 2–3 carpus blade-like spine long (0.6x propodus). Uropod exopod 0.8x endopod.
Etymology
The name is in honour of David K.S. Barnes (British Antarctic Survey, UK), who collected the material and kindly made it available for our study.
Description of female. BL = 1.0 mm. Body robust ()) 3.4 L:W. Cephalothorax 0.9 L:W, 1.3x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonite-1 about half, or less, as long as pereonites 2–3, pereonites-1–6: 0.1, 0.17, 0.3, 0.4, 0.4, and 0.3 L:W, respectively. Pleon 0.9x BL. Pleonites 0.8 L:W; pleonite-5 with mid-lateral and pleotelson with distal pair of fine setae. Pleotelson 0.5 L:W, naked, tapering distally.
Antennule ()) article-1 3.2 L:W, 2.3x article-2, with one simple and four penicillate setae at mid-length, with one simple and five long penicillate distal setae; article-2 2.5 L:W, 0.9x article-3, with one simple and two penicillate distal setae; article-3 4.6 L:W, with four simple distal setae and one aesthetasc.
Antenna ()) shorter than antennule; article-2 1.4 L:W; 1.1x article-3, naked; article-3 1.2 L:W, 0.6x article-4, naked; article-4 2.0 L:W, 1.0x article-5, with one simple (broken) and two penicillate distal setae; article-5 3.9 L:W, 5.4x article-6, with distal seta; article-6 2.5 L:W, with six distal setae.
Labrum ()) distally truncate, naked.
Left mandible ()) incisor with distally elongated blunt tooth, margin weakly serrate; lacinia mobilis well developed, margin serrate; molar coronal.
Right mandible ()) incisor unequally bifid, distal margin serrate; molar as left mandible.
Labium ()) with rounded distal cusp on each lobe.
Maxillule ()) endite with nine distal spines and outer subdistal tuft of setae.
Maxilla ()) semi-rounded, naked.
Maxilliped ()) palp article-1, naked; article-2 with one outer and two inner setae; article-3 with four (three longer and one shorter) inner setae, article-4 with five distal and one outer sub-distal setae; all setae on articles 3 and 4 with finely denticulate margins in their distal half; endites with distinct distal cleft, each with two small gustatory cusps.
Epignath ()) linguiform, naked.
Cheliped ()) basis 1.8 L:W, naked; merus 0.8x carpus ventral margin, with ventral seta; carpus 2.2 L:W, 1.3x palm, with two ventral seta and dorsodistal seta; chela 3.0 L:W, 1.5x carpus length, palm 1.5 L:W, with ventral seta and one seta near dactylus insertion; fixed finger 3.5 L:W, 0.95x palm, cutting edge with poorly calcified margin and three setae, distal spine robust; dactylus 7.6 L:W, cutting edge smooth, with proximal seta.
Pereopod-1 ()) basis 8.1 L:W, 6.8x merus, with dorsoproximal seta; ischium with ventral seta; merus 1.7 L:W and 0.6x carpus, naked; carpus 2.6 L:W, 0.5x propodus, naked; propodus 5.6 L:W, 0.9x dactylus and unguis combined length, naked: dactylus 0.6x unguis.
Pereopod-2 ()) basis 5.7 L:W, 3.8x merus, with ventral seta; ischium with ventral seta; merus 1.8 L:W, 0.6x carpus, with ventrodistal spine; carpus 2.5 L:W, 1.0x propodus, with dorsodistal seta and long blade-like spine (0.6x propodus); propodus 4.5 L:W, 1.0x dactylus and unguis combined length, with distal spine (0.6x dactylus); dactylus 0.6x unguis.
Pereopod-3 ()) basis 4.8 L:W, 3.3x merus; ischium with ventral seta; merus 1.6 L:W, 0.8x carpus, with ventrodistal seta and spine; carpus 2.1 L:W, 0.9x propodus, with dorsodistal seta and blade-like spine (0.6x propodus); propodus 3.8 L:W, 1.1x dactylus and unguis combined length, with distal spine (0.6x dactylus); dactylus 0.9x unguis.
Pereopod-4 ()) basis 5.5 L:W, 3.9x merus, with midventral penicillate seta; ischium with two ventral setae; merus 2.1 L:W, 0.7x carpus, with spine; carpus 3.0 L:W, 0.8x propodus, with seta (0.2x propodus), spine and short blade-like spine (0.2x propodus); propodus 5.0 L:W, 3.0x dactylus and unguis combined length, with subdistal dorsal penicillate seta, two ventrodistal serrate setae, and dorsal seta (2.1x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-5 ()) like pereopod-4.
Pereopod-6 ()) like pereopod-4, but propodus with two (long and short) dorsodistal setae.
Pleopods ()) peduncle 0.9 L:W; endopod just shorter than exopod, 3.9 L:W, with five setae; exopod 3.6 L:W, with eight setae.
Uropod ()) peduncle 1.3 L:W; exopod two-articled, 0.8x endopod, article-1 2.8 L:W, with simple seta, article-2 4.5 L:W, with two simple setae (one very long); endopod two-articled, article-1 3.1 L:W, with one simple and two penicillate setae, article-2 2.9 L:W, with two simple and two penicillate setae.
Juvenile male ()), body 1.0 mm. Similar to female, but with shorter (compact) pereonites and thicker antennule.
Distribution
Amundsen Sea, 478–1473 m.
Remarks
The coronal mandible molar and lack of setae on the merus and carpus of pereopod-1 in Pseudotanais barnesi sp. nov. make it a member of the “denticulatus+abathagastor” morphogroup. The apparently naked antenna articles 2–3 is a unique character, separating it from its congeners. Besides, the short antenna article-4 (2.0 L:W), is an autapomorphy which separates P. barnesi from other members of the group where it is at least 5.7 L:W.
Pseudotanais biopearli sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:69ED7E2A-FA5F-41AD-8118-33B65DC8D47E
Figure 25. Pseudotanais biopearli sp. nov., female holotype, (a, c), dorsal, (d), lateral; juvenile male, (b), dorsal. Scale lines = 1 mm
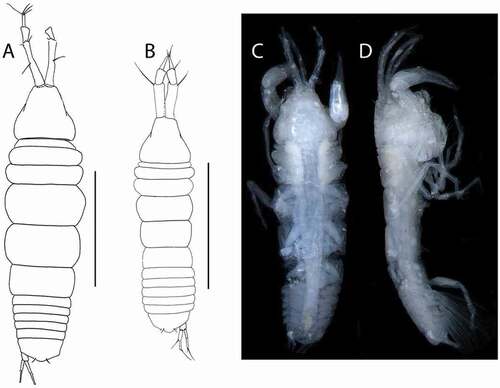
Figure 26. Pseudotanais biopearli sp. nov., (a), antennule of female; (b), antenna; (c), left mandible, with (c’), distal detail and (c”), molar; (d), right mandible; (e), labium; (f), maxillule; (g), maxillule endite; (h), maxilla; (i), maxilliped; (j), antennule of juvenile male. Scale lines = 0.1 mm
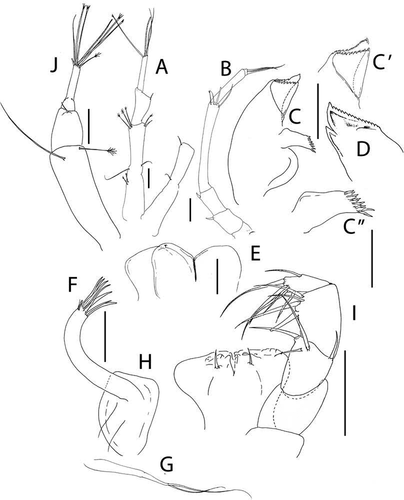
Figure 27. Pseudotanais biopearli sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod. Scale lines = 0.1 mm
Material examined
Holotype, neuter 2.4 mm, ICUL8834, BIO6-EBS-3A (NHM UK.2021.143). Paratypes: neuter 1.7 mm, dissected, ICUL8836, BIO6-EBS-1A (NHM UK.2021.144); 2 neuters 1.6–2.1 mm, ICUL8837, BIO6-EBS-1A (NHM UK.2021.145); neuter 1.7 mm, ICUL8838, BIO6-EBS-1A (NHM UK.2021.146); juvenile female (poor condition), dissected, ICUL8914, BIO6-EBS-1A (NHM UK.2021.147); male, dissected, ICUL8915, BIO6-EBS-1A (NHM UK.2021.148.); neuter 1.8 mm, ICUL8835, BIO6-EBS-2A (NHM UK.2021.149); one individual ICUL8896, BIO6-EBS-1A (NHM UK.2021.150).
Diagnosis
Antenna articles 2–3 with spines. Mandible molar coronal. Pereopods 2–3 carpus blade-like spine short (0.4x propodus). Pereopods 4–6 ischium with two setae; merus with spine and seta. Uropod exopod 0.8x endopod.
Etymology
The name of the species comes from the acronym BIOPEARL: Biodiversity dynamics: Phylogeography, Evolution and Radiation of Life in the Antarctica program.
Description of female. BL = 2.4 mm. Body robust ()) 3.4 L:W. Cephalothorax 0.9 L:W, 1.2x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonites-1–6: 0.14, 0.2, 0.3, 0.5, 0.5, and 0.5 L:W, respectively. Pleon short, 0.2x BL. Pleonites 0.7 L:W, pleonites 3–5. Pleotelson 2.1x pleonite-5, with laterodistal seta.
Antennule ()) article-1 4.3 L:W, 3.6x article-2, with two simple and two penicillate setae at mid-length, and one simple and four penicillate distal setae; article-2 1.4 L:W, 0.8x article-3, with distal seta; article-3 5.3 L:W, with one bifurcated and two simple setae and one aesthetasc.
Antenna ()) just longer than antennule; article-2 1.3 L:W; 1.0x article-3, with spine (0.3x article-2); article-3 1.5 L:W, 0.3x article-4, with spine (0.25x article-3); article-4 8.6 L:W, 2.0x article-5, with three simple and one penicillate distal setae; article-5 5.5 L:W, 5.5x article-6, distal seta not seen; article-6 0.5 L:W, with three distal setae.
Labrum not seen.
Left mandible ()) incisor distally pointed, margin serrate, molar coronal (); lacinia mobilis well developed, distally serrate.
Right mandible ()) incisor unequally bifid, distal margin serrate molar as left mandible.
Labium ()) simple, unornamented.
Maxillule ()) endite with seven distal spines and outer subdistal tuft of setae.
Maxilla ()) subrectangular, naked.
Maxilliped ()) palp article-1 naked; article-2 with fine outer and three inner (two long and one short) setae; article-3 with four inner setae; article-4 with five distal and one sub-distal setae, finely denticulate in distal half. Maxilliped endites fused apart from distal cleft, each with subdistal seta and two small gustatory cusps.
Cheliped ()) slender, basis 1.5 L:W, naked; merus 0.7x carpus ventral margin, with ventral seta; carpus 1.9 L:W, 1.0x palm, with two ventral setae, one middorsal and one dorsodistal setae; chela 3.1 L:W, 1.7x carpus length, palm 1.8 L:W; fixed finger 2.0 L:W, 0.9x palm, with short ventral seta, cutting edge weakly calcified, with three setae, distal spine slender; dactylus 5.0 L:W, cutting edge smooth, with proximal seta.
Pereopod-1 ()) basis 9.0 L:W, 5.3x merus, with two ventral setae, and one dorsoproximal seta; ischium with ventral seta; merus 2.1 L:W and 1.0x carpus, with small ventrodistal seta; carpus 2.4 L:W, 0.4x propodus, two dorsodistal setae; propodus 8.0 L:W, 0.7x dactylus and unguis combined length, with subdistal seta; dactylus 0.5x unguis.
Pereopod-2 ()) basis 7.2 L:W, 5.0x merus, with midventral simple seta; ischium with ventral seta and spine; merus 1.6 L:W, 0.6x carpus, with seta and spine ventrodistally; carpus 2.6 L:W, 0.8x propodus, with seta, two spines and short blade-like spine (0.4x propodus); propodus 6.7 L:W, with distal seta (broken); dactylus and unguis broken.
Pereopod-3 ()) basis 5.6 L:W, 3.7x merus, with midventral seta; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with ventrodistal seta and spine; carpus 2.5 L:W, with seta, two spines and short blade-like spine (0.4x propodus); propodus, dactylus and unguis broken.
Pereopod-4 ()) basis 4.7 L:W, 4.7x merus, naked; ischium with ventral seta (second seta broken); merus 1.5 L:W, 0.4x carpus, with spine (seta not seen); carpus 5.0 L:W, 1.5x propodus, with dorsodistal seta (0.23x propodus), two spines and short blade-like spine (0.3x propodus); propodus 4.7 L:W, 2.3x dactylus and unguis combined length, with dorsal penicillate seta, two ventrodistal serrate setae and dorsal seta (2.0x dactylus and unguis combined length); dactylus 1.5x unguis.
Pereopod-5 ()) basis 3.7 L:W, 3.7x merus, with midventral penicillate seta; ischium with seta; merus 2.1 L:W, 0.5x carpus, with seta and spine ventrodistally; carpus 4.3 L:W, 1.4x propodus, with dorsodistal seta (0.3x propodus), two spines and short blade-like spine (0.3x propodus); propodus 4.4 L:W, 4.4x dactylus and unguiscombined length, with dorsodistal penicillate seta, two ventrodistal serrate setae and dorsodistal dorsal seta (2.1x dactylus and unguis combined length); dactylus 3.0x unguis.
Pereopod-6 ()) basis 4.6 L:W, 5.9x merus, naked; ischium with two ventral setae; merus 1.6 L:W, 0.4x carpus, with spine (seta not seen); carpus 3.6 L:W, 1.2x propodus, with dorsodistal seta (0.2x propodus), two spines and short blade-like spine (0.4x propodus); propodus 4.4 L:W, 2.8x dactylus and unguis combined length, with two ventrodistal serrate setae and two dorsal setae (longer seta 1.6x dactylus and unguis combined length); dactylus 1.7x unguis.
Pleopod ()) peduncle 1.1 L:W; endopod just shorter than exopod, 4.3 L:W, with nine setae; exopod 3.4 L:W, with 17 setae.
Uropod ()) peduncle 1.5 L:W; exopod and endopod two-articled; exopod 0.8x endopod, article-1 3.5 L:W, article-2 5.3 L:W; endopod article-1 4.0 L:W, article-2 6.0 L:W.
Subadult male body ()) more parallel-sided and slightly smaller than that of female, figured specimen 1.8 mm long; cephalothorax as long as wide. Antennule ()) more robust, article-1 with simple distal seta much longer than article-2, article-3 showing signs of subdivision and with four bifurcated, one simple and one penicillate setae distally.
Distribution
Amundsen Sea, 477–1486 m.
Remarks
The combination of antenna articles 2–3 with spines, coronal mandible molar, short dorsodistal setae on the pereopod-1 merus and carpus, and slender uropods places P. biopearli sp. nov. in the “denticulatus+abathagastor” group. A spine present on the antenna article-2 distinguishes it from P. abathagastor and P. mariae, which have a seta, and from P. barnesi, which has this article naked. The slender basis of pereopod-5, that is less than 4 L:W, separates P. biopearli from P. chaplini, P. chopini, P. corollatus, P. denticulatus, P. georgesandae and P. oloughlini, where this proportion >4 L:W. Finally, P. biopearli has a shorter blade-like spine on pereropod-3 (0.4x propodus) than P. locueloae and P. amundseni (0.6x and 0.3x propodus, respectively).
Pseudotanais elephas sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:EA9E1C71-4CB3-410B-A008-E503F0FCD1EB
Figure 28. Pseudotanais elephas sp. nov., female holotype, (a, c), dorsal; (b, d) lateral. Scale line = 1 mm
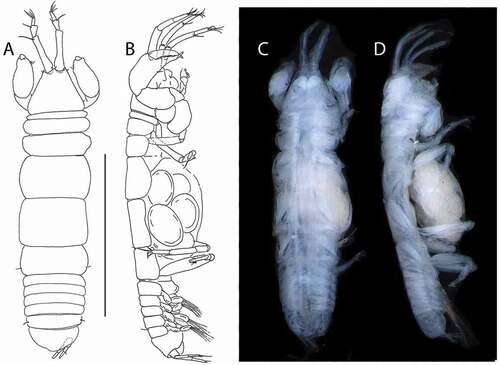
Figure 29. Pseudotanais elephas sp. nov., (a), antennule; (b), antenna, with (b’), distal antennal article; (c), labrum; (d), left mandible, with (d’), molar; (e), right mandible; (f), maxillule; (f’), maxillule endite, distal; (g), maxilliped; (g’), maxilliped palp; (h), labium. Scale lines = 0.1 mm
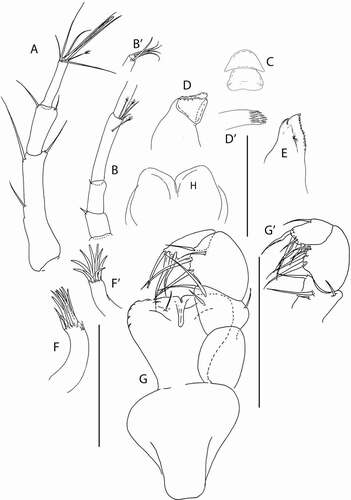
Figure 30. Pseudotanais elephas sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i)I, uropod. Scale lines = 0.1 mm
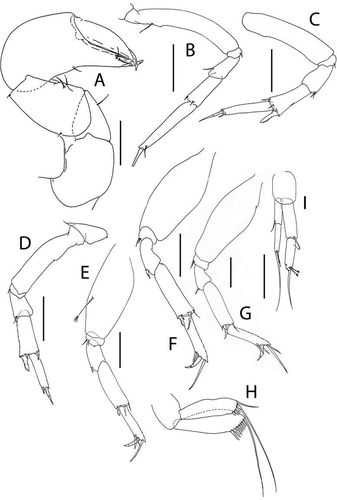
Material examined
Holotype female 1.6 mm, ICUL8860, EI-EBS-1 (NHM UK.2021.151). Paratypes: juvenile female 1.4 mm, neuter 1.2 mm, ICUL8861, EI-EBS-1 (NHM UK.2021.152); neuter, dissected, ICUL8919, EI-EBS-1 (NHM UK.2021.153); neuter, dissected, ICUL8925, EI-EBS-1 (NHM UK.2021.154); female 1.8 mm, juvenile male 1.5 mm, manca 0.8 mm, ICUL8888, EI-EBS-2 (NHM UK.2021.155); juvenile female 1.5 mm (partly dissected), ICUL8862, LI-EBS-3 (NHM UK.2021.156); juvenile female 1.6 mm, ICUL8863, ST-EBS-2 (NHM UK.2021.157); juvenile male 1.3 mm, ICUL8864, ST-EBS-2 (NHM UK.2021.158); three individuals, JR 144 EI-EBS-2E.
Diagnosis
Antenna article-2 with spine; article-3 with minute seta. Mandible molar coronal. Pereopod-1 merus with short dorsodistal seta. Pereopod-2 carpus blade-like spine short (0.4x propodus). Pereopods 4–6 ischium with two setae; merus with two spines; carpus seta short. Uropod exopod 0.8x endopod.
Etymology
The Latin noun elephas alluding to the type locality, Elephant Island.
Description of female. BL = 1.6 mm. Body robust ()) 3.9 L:W. Cephalothorax 0.8 L:W, 1.0x pereonites 1–3, 0.15x BL. Pereonites 0.6x BL, pereonite-1 at most half as long as pereonites 2–3, pereonites 1–6: 0.1, 02, 0.3, 0.6, 0.7, and 0.4 L:W, respectively. Pleon short, 0.3x BL. Pleonites 0.9 L:W, pleonite-5 with lateral setae. Pleotelson 1.5x pleonite-5, with conspicuous paired distal setae.
Antennule ()) article-1 3.5 L:W, 2.1x article-2, with seta at mid-length, and two distal setae; article-2 1.5 L:W, 1.0x article-3, with two simple distal setae; article-3 5.0 L:W, with six simple and one penicillate setae, and one aesthetasc.
Antenna ()) article-2 1.1 L:W, 0.7x article-3, with spine (0.4x article-2); article-3 2.2 L:W, 0.4x article-4, with minute seta (0.2x article-3); article-4 5.7 L:W, 2.1x article-5, with two simple and three penicillate distal setae; article-5 broken, with simple seta; article-6 1.5 L:W, with four distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor distally blunt, margin serrate; lacinia mobilis well developed, distally serrate; molar coronal ().
Right mandible ()) incisor unequally bifid, distal margin serrate.
Labium ()) lobe simple, unornamented.
Maxillule ()) endite with nine distal spines and outer subdistal tuft of setules.
Maxilliped ()) bases together chordate, as long as broad, naked; palp article-1 naked, article-2 with fine outer and three inner setae (two long and one short); article-3 with two long and two short inner setae; article-4 with five distal and one subdistal setae; endites fused but with mid-distal cleft, gustatory cusps not seen each with subdistal seta.
Cheliped ()) robust; basis 1.5 L:W, naked; merus as long a as carpus ventral margin, with ventral seta; carpus 1.6 L:W, 0.9x palm, with two ventral setae, one dorsoproximal and dorsodistal setae; chela 2.4 L:W, 1.75x carpus length, palm 1.5 L:W; fixed finger 2.3 L:W, 0.7x palm, with short ventral seta, cutting edge poorly calcified, with three setae; dactylus robust, 2.9 L:W, without fine proximal seta.
Pereopod-1 ()) basis 6.2 L:W, 4.1x merus, with dorsoproximal seta; ischium with ventral seta; merus 1.5 L:W and 0.7x carpus, with dorsodistal seta; carpus 2.8 L:W, 0.6x propodus, with two distal setae; propodus 5.3 L:W, with subdistal seta, unguis broken.
Pereopod-2 ()) basis 4.2 L:W, 2.8x merus, naked; ischium with ventral seta; merus 1.1 L:W, 0.8x carpus, with two ventrodistal setae; carpus 2.0 L:W, 0.8x propodus, with simple seta, two spines and semilong/intermediate blade-like spine (0.4x propodus); propodus 5.4 L:W, 1.8x dactylus and unguis combined length, with ventrodistal spine (0.7x dactylus); dactylus 0.7x unguis.
Pereopod-3 ()) basis 4.7 L:W, 3.9x merus, naked; ischium with ventral seta; merus 1.1 L:W, 0.5x carpus, with two ventrodistal setae; carpus 2.3 L:W, 1.2x propodus, with simple seta, spine and semilong/intermediate blade-like spine (0.5x propodus); propodus 4.0 L:W, 2.0x dactylus and unguis combined length, with ventrodistal spine (0.8x dactylus); dactylus 1.0x unguis.
Pereopod-4 ()) basis 2.5 L:W, 3.5x merus, with midventral penicillate seta; ischium with two ventral setae; merus 1.5 L:W, 0.6x carpus, with two ventrodistal spines; carpus 3.3 L:W, 1.2x propodus, with seta (0.2x propodus), two spines and short blade-like spine (0.3x propodus); propodus 4.4 L:W, 2.2x dactylus and unguis combined length, with two ventral setae and one dorsal seta (1.4x dactylus and unguis combined length); dactylus 4.0x unguis.
Pereopod-5 ()) basis 2.5 L:W, 3.2x merus, naked; ischium with two ventral setae; merus 1.7 L:W, 0.6x carpus, with two ventrodistal spines; carpus 3.3 L:W, 1.2x propodus, with dorsodistal seta (0.2x propodus), two spines and short blade-like spine (0.3x propodus); propodus 4.3 L:W, 2.4x dactylus and unguis combined length, with two ventral setae and dorsal seta (1.6x dactylus and unguis combined length); dactylus 2.7x unguis.
Pereopod-6 ()) basis 2.8 L:W, 4.3x merus, naked; ischium with two ventral setae; merus 1.4 L:W, 0.5x carpus, with two ventrodistal spines; carpus 3.6 L:W, 1.1x propodus, with dorsodistal seta (0.3x propodus), two regular spines and short blade-like spine (0.2x propodus); propodus 4.6 L:W, 2.3x dactylus and unguis combined length, with two serrate ventrodistal setae, two serrate dorsal setae (longer seta 1.8x dactylus and unguis combined length); dactylus 4.0x unguis.
Pleopods ()) peduncle 1.2 L:W; endopod just shorter than exopod, 3.6 L:W, with five setae, exopod 2.8 L:W, with eight setae.
Uropod ()) peduncle 1.5 L:W; exopod and endopod two-articled; exopod 0.8x endopod, article-1 3.8 L:W, with simple distal seta, article-2 5.0 L:W, with two distal setae; endopod article-1 3.1 L:W, with seta, article-2 3.6 L:W, with two penicillate setae and at least three further setae (all but one detached).
Distribution
Elephant Island and Livingston Island (South Shetland Is), 557–1503 m.
Remarks
Pseudotanais elephas sp. nov., which has a small seta on antenna article-3, a coronal mandible molar, short setae on pereopod-1 carpus, and slender uropod, may be easily placed in the “denticulatus+abathagastor” morphogroup. Because this species is most similar to P. amundseni, it can be distinguished from the other members of the group by the same set of characters (see P. amundseni remarks). The blade-like spine on the pereopod-2 carpus that is 0.4x propodus is slightly longer than in P. amundseni (0.27x). Additionally, two setae (long and short) on the merus of pereopod-2, differentiate P. elephas from P. amundseni that has two setae of the same length. Finally, the dactylus and unguis of pereopods 4–5 in P. elephas is stouter, at most 3.5 L:W, compared to P. amundseni at 5.0 L:W.
Pseudotanais kitsoni sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:C01D421D-7F64-4AAD-971B-B6642AEFF380
Figure 31. Pseudotanais kitsoni sp. nov., female holotype, (a, c), dorsal; (b, d), lateral. Scale line = 1 mm
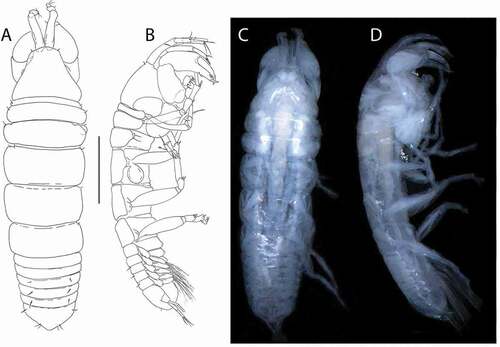
Figure 32. Pseudotanais kitsoni sp. nov., (a), antennule; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible, with E′, molar; (f), maxillule; (g), labium; (h) maxilla; (i), epignath; (j), maxilliped. Scale lines = 0.1 mm
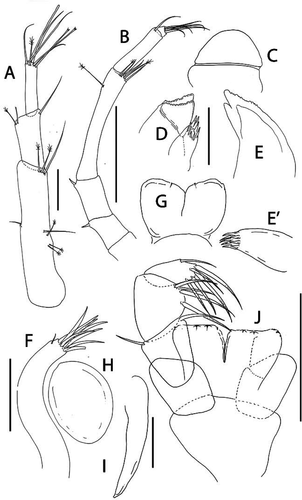
Figure 33. Pseudotanais kitsoni sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
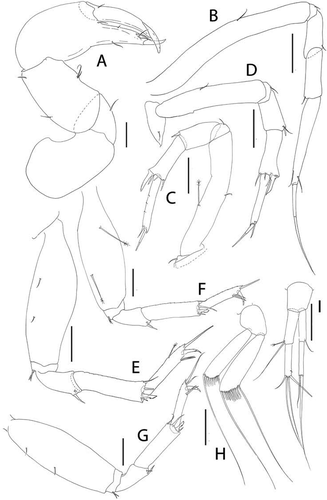
Material examined
Holotype juvenile female 2.1 mm, ICUL8799, BIO4-EBS-3D (NHM UK.2021.159). Paratypes: juvenile male 2.3 mm, ICUL8801, BIO3-EBS-1B (NHM UK.2021.160); juvenile female 2.2 mm, ICUL8882, BIO3-EBS-1B (NHM UK.2021.161); neuter 2.6 mm, ICUL8883, BIO4-EBS-1A (NHM UK.2021.162); neuter 1.3 mm, ICUL8804, BIO4-EBS-1B (NHM UK.2021.163); neuter 1.4 mm, ICUL8803, BIO4-EBS-3A (NHM UK.2021.164); neuter 1.8 mm, ICUL8807, BIO4-EBS-3A (NHM UK.2021.165); juvenile female 2.1 mm, ICUL8879, BIO4-EBS-3A (NHM UK.2021.166); neuter 1.8 mm, dissected, ICUL8900, BIO4-EBS-3A (NHM UK.2021.167); neuter 1.9 mm, dissected, ICUL8901, BIO4-EBS-3A (NHM UK.2021.168); LOST, ICUL8802, BIO5-EBS-2A (not registered); LOST, ICUL8806, BIO6-EBS-3A (not registered); juvenile male 2.4 mm, neuter 2.3 mm, ICUL8800, BIO6-EBS-3E (NHM UK.2021.171); neuter 1.9 mm, dissected ICUL8927, EI-EBS-2 (NHM UK.2021.172); one individual, ICUL8895, BIO5-EBS-2A (NHM UK.2021.173).
Diagnosis
Antenna articles 2–3 with spines. Mandible molar coronal. Pereopod-1 merus with ventrodistal seta. Pereopods 2–3 carpus blade-like spine short (0.4x propodus). Pereopods 4–6 ischium with two setae; merus with spine and seta. Uropod exopod 0.9x endopod.
Etymology
The species is named for Jane and Malcolm Kitson, for the years of friendship.
Description of female. BL = 2.1 mm. Body robust ()) 3.5 L:W. Cephalothorax 0.7 L:W, 1.0x pereonites 1–3, 0.3x BL. Pereonites 0.5x BL, pereonites-1–6: 0.08, 02, 0.3, 0.4, 0.5 and 0.5 L:W, respectively, all pereonites with small anterolateral setae. Pleon short, 0.2x BL. Pleonites 0.7 L:W, pleonites 3–5 with dorsolateral setae on each side; pleotelson 2.3x pleonite-5, with lateral setae and paired laterodistal setae.
Antennule ()) article-1 3.7 L:W, 2.5x article-2, with two simple and three penicillate setae at mid-length, and one simple and two penicillate distal setae; article-2 1.7 L:W, 1.1x article-3, with two simple (long and short) and one penicillate distal setae; article-3 4.5 L:W, with four simple, two bifurcated and one penicillate setae, and one aesthetasc.
Antenna ()) about as long as antennule; article-2 1.1 L:W; 0.8x article-3, with spine (0.3x article-2); article-3 1.6 L:W, 0.4x article-4, with spine (0.2x article-3); article-4 5.8 L:W, 1.9x article-5, with subdistal penicillate seta, three simple and two penicillate distal setae; article-5 4.4 L:W, 7.8x article-6, with simple seta; article-6 0.8 L:W, with five distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor distally in shape of blunt spine, margin irregularly serrate; lacinia mobilis well developed, distally serrate; molar coronal.
Right mandible ()) incisor unequally bifid, distal margin irregularly serrate; molar as left mandible.
Maxillule ()) endite with eight distal spines and outer subdistal setule.
Maxilla ()) almost circular, naked.
Labium ()) simple, rounded, glabrous.
Epignath ()) linguiform, simple, naked.
Maxilliped ()) bases together chordate, naked; palp article-1 naked, article-2 with one outer and one inner setae; article-3 with four (three long and one short) inner setae; article-4 with five distal and one subdistal setae; all setae with finely-denticulate in their distal half; endites mostly fused but with distinct central cleft, gustatory cusps very small.
Cheliped ()) robust, basis 1.5 L:W, naked; merus as long as carpus ventral margin, with ventral seta; carpus 1.8 L:W, 0.95x palm, with two midventral setae, one middorsal and one dorsodistal simple setae; chela 2.8 L:W, 1.8x carpus length, palm 1.5 L:W; fixed finger 3.0 L:W, 0.8x palm, with ventral seta, cutting edge poorly calcified with three setae; dactylus 6.3 L:W, cutting edge smooth, with proximal seta; unguis robust.
Pereopod-1 ()) basis 7.1 L:W, 3.5x merus, with midventral seta; ischium with ventral seta; merus 2.0 L:W and 0.8x carpus, with ventrodistal seta; carpus 2.7 L:W, 0.6x propodus, with dorsodistal short seta; propodus 6.9 L:W, with one dorsal and one ventral setae, dactylus 0.4x unguis.
Pereopod-2 ()) basis 5.9 L:W, 3.3x merus, with middorsal penicillate seta and midventral simple seta; ischium with ventral seta; merus 1.8 L:W, 0.8x carpus, with seta and spine ventrodistally; carpus 2.5 L:W, 0.8x propodus, with dorsodistal spine, seta and spine, and blade-like spine (0.4x propodus) distally; propodus 6.0 L:W with distal seta (1.0x dactylus); unguis broken.
Pereopod-3 ()) basis 5.5 L:W, 3.5x merus, with midventral simple seta; ischium with ventral seta; merus 1.5 L:W, 0.7x carpus, with seta and spine ventrodistally; carpus 2.3 L:W, 0.9x propodus, with dorsodistal seta, seta and spine and ventrodistal blade-like spine (0.4x propodus) distally; propodus 2.8 L:W, 1.8x dactylus and unguis combined length, with distal seta (1.4x dactylus); dactylus 0.8x unguis.
Pereopod-4 ()) basis 3.0 L:W, 3.8x merus, with two short setae; ischium with two ventral setae; merus 1.7 L:W, 0.5x carpus, with seta and spine ventrodistally; carpus 4.0 L:W, 1.25x propodus, with dorsodistal seta (0.25x propodus), two spines and short blade-like spine (0.3x propodus) distally; propodus 5.3 L:W, 2.9x dactylus and unguis combined length, with two serrate ventrodistal setae and serrate dorsal seta (1.8x dactylus and unguis combined length); dactylus 2.7x unguis.
Pereopod-5 ()) basis 3.0 L:W, 4.4x merus, with two penicillate setae; ischium with two ventral setae; merus 1.5 L:W, 0.5x carpus, with seta and spine ventrodistally; carpus 3.8 L:W, 1.2x propodus, with dorsodistal seta (0.2x propodus), two spines and short blade-like spine (0.2x propodus); propodus 5.0 L:W, 2.8x dactylus and unguis combined length, with two ventrodistal and dorsodistal setae; dactylus 3.5x unguis.
Pereopod-6 ()) basis 2.9 L:W, 4.6x merus, with two ventral submarginal setae; ischium with two ventral setae; merus 1.4 L:W, 0.5x carpus, with seta and spine ventrodistally; carpus 3.1 L:W, 1.0x propodus, with dorsodistal seta (0.3x propodus), two spines and short blade-like spine (0.25x propodus) distally; propodus 4.5 L:W, 3.4x dactylus and unguis combined length, with two ventrodistal setae and two dorsal setae (longer setae 2.6x dactylus and unguis combined length); dactylus 3.0x unguis.
Pleopods ()) peduncle 1.2 L:W; endopod slightly shorter than exopod, 4.7 L:W, with nine setae; endopod 3.9 L:W, with 10 setae.
Uropod ()) peduncle 1.2 L:W; exopod and endopod two-articled; exopod 0.9x endopod, article-1 4.3 L:W, with distal seta, article-2 6.7 L:W, with two setae (one very long); endopod article-1 3.3 L:W, with one simple and one penicillate setae, article-2 4.5 L:W, with one subdistal and four distal setae.
Distribution
Amundsen Sea, Elephant Island (South Shetland Is), 477–1473 m.
Remarks
The spines on antenna articles 2–3 allow differentiation of P. kitsoni sp. nov. from five species of the “denticulatus+abathagastor” group, particularly P. abathagastor and P. elephas, which have a spine and seta on antenna articles 2–3, respectively; P. mariae that has only setae, P. barnesi with both articles naked, and P. amundseni that has a spine and minute seta, respectively.
The uropod exopod that is slightly shorter than the endopod (0.9x) separates P. kitsoni from P. chaplini and P. oloughlini, where it is longer, while the coronal mandible molar separates P. kitsoni from P. chopini that has an subcoronal/acuminate molar. Two setae on pereopods 4–6 ischium distinguish it from P. denticulatus that has a spine and seta, while a spine and seta on the pereopods 4–6 merus separates P. kitsoni from P. corollatus (with two setae), and P. georgesandae and P. locueloae (both with one spine only). A short blade-like spine on the pereopod–6 carpus in P. kitsoni (0.25x propodus) is different from P. biopearli that has a longer spine (0.4x). Pseudotanais kitsoni lacks a subdistal seta on the maxilliped endite that is present in P. biopearli.
Pseudotanais livingstoni sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:0B774067-D568-4576-9E18-ACC983BC554E
Figure 34. Pseudotanais livingstoni sp. nov., female holotype, (a, b) dorsal; (c), lateral. Scale line = 1 mm
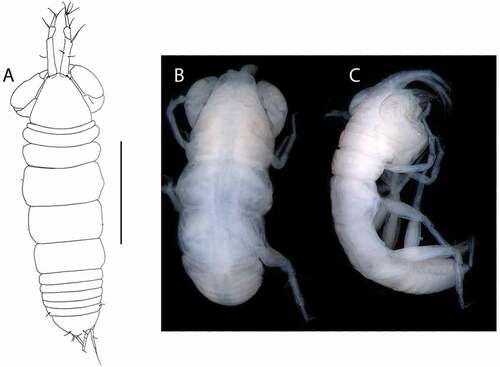
Figure 35. Pseudotanais livingstoni sp. nov., (a), antennule; (b), antenna; (c), labrum; (d), mandibular molar; (e), maxillule; (f), maxilla; (g), labium; (h), maxilliped. Scale lines = 0.1 mm
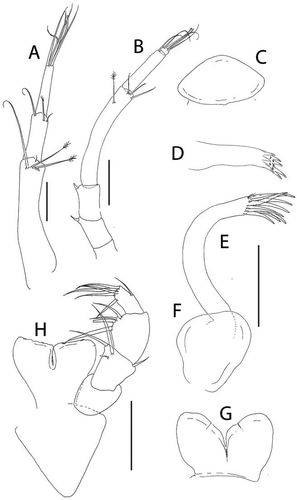
Figure 36. Pseudotanais livingstoni sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2, with (c’), detail of dactylus and unguis, and (c”), blade-like carpal spine; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
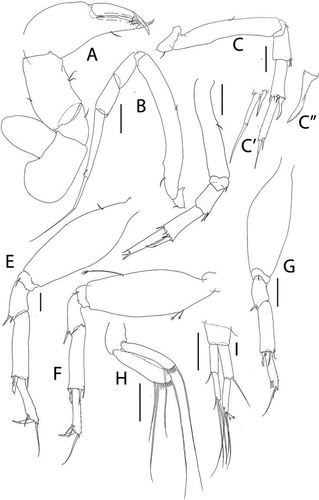
Material examined
Holotype neuter 2.4 mm, ICUL8870, LI-EBS-3 (NHM UK.2021.174); two neuters 2.2–2.4 mm, ICUL8871, EI-EBS-2 (NHM UK.2021.175); neuter, dissected, ICUL8880, EI-EBS-2 (NHM UK.2021.176); neuter, dissected, ICUL8924, EI-EBS-2 (NHM UK.2021.177); neuter, dissected, ICUL8926, EI-EBS-2 (NHM UK.2021.178); neuter 1.8 mm, ICUL8873, EI-EBS-4 (NHM UK.2021.179); neuter 2.3 mm, ICUL8869, LI-EBS-3 (NHM UK.2021.180); juvenile female 1.6 mm, neuter 1.8 mm, ICUL8872, ST-EBS-2 (NHM UK.2021.181); one individual, ICUL8898, EI-EBS-2 (NHM UK.2021.182).
Diagnosis
Antenna articles 2–3 with spines. Mandible molar coronal. Pereopod–1 merus with minute ventrodistal seta. Pereopod 3 carpus blade-like spine long (0.6x propodus). Pereopods 4–6 ischium with seta; merus with spine and seta. Uropod exopod 0.8x endopod.
Etymology
From the type locality, Livingston Island.
Description of female. BL = 2.4 mm. Body robust ()) 3.2 L:W. Cephalothorax 0.9 L:W, 0.7x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonite-1 half, or less, as long as pereonites 2–3, pereonites-1–6: 0.08, 0.2, 0.3, 0.5, 0.5, and 0.4 L:W, respectively. Pleon 0.3x BL. Pleonites 0.7 L:W, pleonite-5 with lateral setae. Pleotelson 3.0x pleonite-5, with distolateral setae.
Antennule ()) article-1 3.9 L:W, 2.5 article-2, with two simple and three penicillate distal setae; article-2 2.8 L:W, 1.0x article-3, with two simple and one penicillate distal setae; article-3 4.5 L:W, with five simple distal setae and one aesthetasc.
Antenna ()) as long as antennule; article-2 1.7 L:W, 0.9x article-3, with spine (0.3x article–2); article-3 1.7 L:W, 0.4x article-4, with spine (0.2x article–3); article-4 6.3 L:W, 1.9x article-5, with one subdistal penicillate seta and four simple distal setae; article-5 4.3 L:W, 7.5x article-6, with distal seta; article-6 0.8 L:W, with three simple and two bifurcated distal setae.
Labrum ()) rounded, naked.
Mandibular molar coronal ()). Other parts of mandibles not found.
Maxillule ()) endite with nine distal spines and outer subdistal tuft of setules.
Maxilla ()) semioval, simple.
Labium ()) simple, naked, widely cleft.
Maxilliped ()) bases together deltoid, as long as broad, naked; palp proximal article-1 naked, article-2 with fine outer and three inner (two longer and one shorter) setae, article-3 with four inner setae, article-4 with five distal and one sub-distal setae; all inner setae with finely-denticulate margins in their distal half; endites fused except for distal third, each with two small and shallow gustatory cusps.
Cheliped ()) basis 1.4 L:W, naked; merus just shorter than carpus ventral margin, with ventral seta; carpus 1.6 L:W, 0.9x palm, with two ventrodistal setae, one middorsal seta and one dorsodistal seta; chela 2.7 L:W, 1.8x carpus length, palm 1.7 L:W; fixed finger 3.0 L:W, 0.7x palm with one ventral seta, and with three setae on cutting edge; dactylus 5.3 L:W, cutting edge smooth, without proximal seta.
Pereopod-1 ()) coxa with seta; basis 8.0 L:W, 5.3x merus, with dorsoproximal and midventral simple setae; ischium with ventral seta; merus 1.7 L:W and 0.7x carpus, with minute ventrodistal seta; carpus 2.4 L:W, 0.6x propodus, with short dorsodistal seta; propodus 7.6 L:W, 0.8x dactylus and unguis combined length, with subdistal seta; dactylus 0.6x unguis.
Pereopod-2 ()) basis 5.7 L:W, 3.4x merus, with midventral seta and two simple ventral setae; ischium with ventral seta; merus 1.7 L:W, 0.8x carpus, with ventrodistal seta and spine; carpus 2.6 L:W, 0.8x propodus, with seta, spine and long blade-like spine (0.4x propodus) distally ()); propodus 6.8 L:W, 1.7x dactylus and unguis combined length, with distal seta (1.0x dactylus); dactylus 0.8x unguis ()).
Pereopod-3 ()) basis 5.5 L:W, 3.2x merus, with midventral seta; ischium with ventral seta; merus 1.7 L:W, 0.8x carpus, with ventrodistal seta and spine; carpus 2.2 L:W, 0.9x propodus, with seta, two spines and long blade-like spine (0.6x propodus) distally; propodus 4.8 L:W, with distal seta; dactylus and unguis broken.
Pereopod-4 ()) basis 2.8 L:W, 3.9x merus, with ventroproximal seta; ischium with ventral seta; merus 1.8 L:W, 0.5x carpus, with seta and spine; carpus 4.0 L:W, 1.4x propodus, with seta (0.4x propodus), spine and short blade-like spine (0.4x propodus) distally; propodus 5.0 L:W, 2.1x dactylus and unguis combined length, with two ventrodistal setae and one dorsal seta (1.7x dactylus and unguis combined length); dactylus 2.0x unguis.
Pereopod-5 ()) basis 2.8 L:W, 4.4x merus, with two dorsoproximal and one midventral penicillate setae; ischium with ventral seta; merus 1.6 L:W, 0.5x carpus, with seta and spine; carpus 4.4 L:W, 1.4x propodus, with seta (0.3x propodus), two spines and short blade-like spine (0.3x propodus); propodus 5.0 L:W, 2.8x dactylus and unguis combined length, with two ventrodistal and one dorsodistal seta (2.2x dactylus and unguis combined length); dactylus 2.0x unguis.
Pereopod-6 ()) basis 2.9 L:W, 4.2x merus, with two dorsoproximal and one midventral penicillate setae; ischium with ventral seta; merus 1.7 L:W, 0.6x carpus, with seta and spine; carpus 3.9 L:W, 1.2x propodus, with seta (0.2x propodus), two spines and short blade-like spine (0.4x propodus); propodus 4.0 L:W, with two ventrodistal and two dorsodistal setae; dactylus and unguis broken.
Pleopods ()) peduncle 1.5 L:W; endopod just shorter than exopod, 4.4 L:W, with seven setae; endopod 3.5 L:W, with ten setae.
Uropod ()) peduncle 0.9 L:W; exopod and endopod two-articled; exopod 0.8x endopod, article-1 3.4 L:W, with simple seta, article-2 4.7 L:W, with three simple setae; endopod article-1 3.3 L:W, with simple seta; article-2 5.7 L:W, with two simple and two penicillate setae.
Distribution
Elephant Island and Livingston Island (South Shetland Is), Southern Thule (Sandwich Is), 199–1038 m.
Remarks
Pseudotanais livingstoni sp. nov., with spines on antenna article-3, a coronal mandible molar, short setae on the pereopod-1 carpus, and a slender uropod, is classified to the “denticulatus+abathagastor” morphogroup.
Pseudotanais livingstoni is most similar to P. kitsoni and therefore can be distinguished from the other members of “denticulatus+abathagastor” group by the same set of characters (see Remarks P. kitsoni). These two species can be distinguished by:
- a blade-like spine on the carpus of pereopod-3 that is semilong/intermediate in P. livingstoni (0.6x propodus) but short in P. kistoni (0.4x propodus);
- the proportion between the dactylus and unguis of pereopod-5, 2.0x in P. livingstoni but 3.5x in P. kitsoni;
- the length of the blade-like spine on the carpus of pereopod–6, 0.4x propodus in P. livingstoni and 0.25x in P. kitsoni.
Pseudotanais palmeri sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:72077206-DC84-4C0B-89E6-1B550F104DCE
Figure 37. Pseudotanais palmeri sp. nov., female holotype, (a, c), dorsal; (b, d), lateral. Scale line = 1 mm
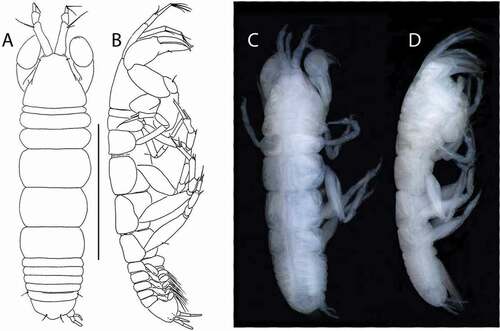
Figure 38. Pseudotanais palmeri sp. nov., (a), antennule of female; (b), antenna; (c), antennule of juvenile male; (d), labrum; (e), left mandible; (f), right mandible, with (f’), detail of pars molaris; (g), labium; (h), maxillule; (i), maxilliped. Scale lines = 0.1 mm
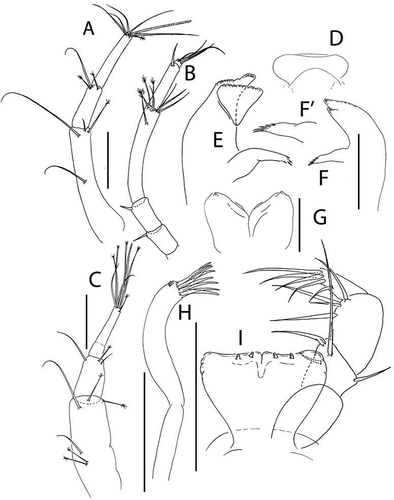
Figure 39. Pseudotanais palmeri sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
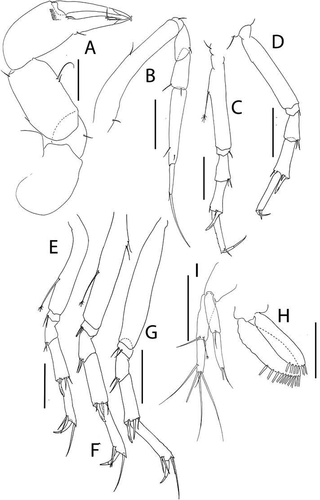
Material examined
Holotype, neuter 1.8 mm, ICUL8877, PB-EBS-4 (NHM UK.2021.183); female 1.4 mm, 2 juvenile males 1.2 mm, two neuters 1–1.5 mm, ICUL8878, PB-EBS-4 (NHM UK.2021.184); neuter, dissected, ICUL8909, PB-EBS-4 (NHM UK.2021.185); neuter, dissected, ICUL8910, PB-EBS-4 (NHM UK.2021.186); one individual, ICUL8894, PB-EBS-4 (NHM UK.2021.187).
Diagnosis
Antenna article 2–3 with spines; article-3 spine 0.4x article-3. Mandible molar subcoronal/acuminate. Maxilliped gustatory cusps conical. Pereopods carpus 2–3 blade-like semilong/intermediate (0.5x propodus). Uropod exopod as long as endopod.
Etymology
The species, whose type locality is the Palmer Bay, (South Orkney Islands) was named in honour of Nathaniel Brown Palmer, the sailing captain and ship designer.
Description of neuter. BL = 1.8 mm. Body robust ()) 3.9 L:W. Cephalothorax 0.9 L:W, 1.3x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonite-1 half, or less, as long as pereonites 2–3, pereonites 1–6: 0.1, 02, 0.3, 0.5, 0.8, and 0.5 L:W, respectively. Pleon short, 0.2x BL. Pleonites 0.7 L:W, pleonite-5 with lateral setae. Pleotelson 2.0x pleonite-5, with conspicuous paired distal setae.
Antennule ()) article-1 3.8 L:W, 2.7x article-2, with long seta at mid-length, and two simple (one long) and one penicillate setae distally; article-2 2.5 L:W, 0.7x article-3, with one simple and two penicillate setae distally; article-3 5.4 L:W, with four simple and one penicillate setae and one aesthetasc.
Antenna ()) just shorter than antennule; article-2 1.4 L:W; 0.8x article-3, with spine (0.5x article-2); article-3 1.9 L:W, 0.4x article-4, with spine (0.4x article-3); article-4 6.7 L:W, 2.1x article-5, with four simple and four penicillate setae distally; article-5 3.7 L:W, 7.3x article-6, with simple seta; article-6 1.0 L:W, with four distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor distally elongated with blunt tip, margin serrate; lacinia mobilis well developed, distally serrate; molar subcoronal/acuminate, with five distal spines.
Right mandible ()) incisor unequally bifid, margin weakly serrate; molar as in left mandible.
Maxillule ()) endite with eight distal spines and outer subdistal tuft of setules.
Labium ()) with apical cusps.
Maxilliped ()) palp article-1 naked, article-2 with one outer and three inner (two short and one long reaching distal tip of palp) inner setae, article-3 with four inner (three long and one short) setae, article-4 with five inner/distal setae; endites fused but with mid-distal cleft, each with two conical gustatory cusps.
Cheliped ()) basis 1.7 L:W; merus 0.7x carpus ventral margin, with ventral seta; carpus 1.8 L:W, 1.2x palm, with two midventral setae (one long), one dorsodistal and one mediodorsal setae; chela sub-forcipate, 3.0 L:W, 1.6x carpus L, palm 1.6 L:W; fixed finger 3.2 L:W, 0.8x palm with ventral seta, cutting edge weakly calcified, smooth with three setae; dactylus 4.2 L:W, cutting edge smooth, proximal seta not seen; unguis slender.
Pereopod-1 ()) basis 5.8 L:W, 4.7x merus, with dorsoproximal and midventral simple setae; ischium with ventral seta; merus 1.9 L:W and 0.7x carpus, with ventrodistal seta; carpus 2.4 L:W, 0.5x propodus, with two distal setae; propodus 5.8 L:W, 0.9x dactylus and unguis combined length, with two subdistal setae, dactylus 0.5x unguis.
Pereopod-2 ()) coxa with seta; basis 7.1 L:W, 3.8x merus, with dorsoproximal penicillate seta; ischium with ventral seta; merus 1.9 L:W, 0.9x carpus, with simple seta and spine ventrodistally; carpus 2.4 L:W, 0.8x propodus, with simple seta, small spine and semilong/intermediate blade-like spine (0.5x propodus); propodus 4.4 L:W, 3.7x dactylus and unguis combined length, with distal spine (1.0x dactylus); dactylus 0.7x unguis.
Pereopod-3 ()) coxa with seta; basis 5.6 L:W, 3.3x merus; ischium with seta; merus 2.1 L:W, 0.8x carpus, with seta and spine ventrodistally; carpus 2.0 L:W, 0.9x propodus, with setae, small spine and semilong/intermediate blade-like spine (0.5x propodus) distally; propodus 5.5 L:W, 2.8x dactylus and unguis combined length, with distal spine (1.3x dactylus); dactylus 0.8x unguis.
Pereopod-4 ()) basis 5.5 L:W, 4.6x merus, with midventral penicillate seta; ischium with two long ventral setae; merus 1.5 L:W, 0.5x carpus, with ventrodistal seta and spine; carpus 2.8 L:W, 0.9x propodus, with simple seta, seta (0.2x propodus), spine and short blade-like spine (0.4x propodus); propodus 5.0 L:W, 1.9x dactylus and unguis combined length, with two ventral setae, one dorsal seta (1.4x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-5 ()) basis 5.3 L:W, 5.3x merus, with two dorsoproximal setae and midventral penicillate seta; ischium with two long ventral setae; merus 1.2 L:W, 0.5x carpus, with ventrodistal seta and spine; carpus 3.3 L:W, 0.9x propodus, with one seta, one seta (0.2x propodus), spine and short blade-like spine (0.4x propodus); propodus 5.0 L:W, 3.8x dactylus and unguis combined length, with two ventrodistal setae, dorsodistal seta (1.6x dactylus and unguis combined length); dactylus 1.8x unguis.
Pereopod-6 ()) basis 5.5 L:W, 5.1x merus, naked; ischium with two long ventral setae; merus 1.8 L:W, 0.6x carpus, with ventrodistal seta and spine; carpus 2.7 L:W, 0.8x propodus, with simple seta, seta (0.2x propodus), spine and short blade-like spine (0.4x propodus) distally; propodus 5.0 L:W, 2.1x dactylus and unguis combined length, with two ventrodistal setae, two dorsodistal setae (longer seta 1.6x dactylus and unguis combined length); dactylus 1.8x unguis.
Pleopod ()) endopod just shorter than exopod, 3.8 L:W, with six setae; endopod 3.0 L:W, with eleven setae.
Uropod ()) peduncle 0.9 L:W; exopod and endopod two-articled; exopod 1.0x endopod, article-1 3.7 L:W, with simple seta, article-2 5.0 L:W, with two setae; endopod article-1 3.4 L:W, with one simple and one penicillate setae, article-2 3.6 L:W, with one subdistal seta and four distal setae.
Subadult male. Similar to female, but antennule ()) stouter, article-3 showing signs of subdivision and with four bifurcated distal setae.
Distribution
Palmer Bay (South Orkneys Is), 210–211 m.
Remarks
Pseudotanais palmeri sp. nov. has a uropod exopod as long as the endopod. In all the other species of the group this is slightly shorter (0.9x) or slightly longer (P. chaplini and P. oloughlini). The spines on antenna article 2–3 distinguish P. palmeri from P. abathagastor, P. amundseni, P. chopini, P. elephas and P. mariae, which have a combination of seta and spine on these articles; however no species has a spine on both articles and P. barnesi has naked antennule articles 2 and 3. The relatively long and slender spine on antenna article-3 (0.4x article-3) separates P. palmeri from all but one members of the “denticulatus+abathagastor” group. The exception is P. corollatus, that has antennal spines similar to P. palmeri; nevertheless P. corollatus has pereonites 1–2, almost equally long, while P. palmeri has pereonite-1 clearly shorter than pereonite-2.
“forcipatus” group
Diagnosis
After Bird & Holdich (Citation1989a). Antenna articles 2–3 with spine or strong seta. Mandible molar acuminate. Chela forcipate, cutting edges smooth.
Species included. P. artoo Błażewicz-Paszkowycz & Stępień, 2015; P. californiensis Dojiri & Sieg, 1997; P. forcipatus Lilljeborg, 1864 [type species of Pseudotanais]; P. falcicula Bird & Holdich, 1989; P. isabelae García-Herrero, Sánchez, García-Gómez, Pardos & Martínez, 2017; P. jonesi Sieg, Citation1977; P. mediterraneus Sars, 1882; P. mexikolpos Sieg & Heard, Citation1988; P. misericorde Jakiel, Stępień & Błażewicz, 2018; P. soja Błażewicz-Paszkowycz, Bamber & Jóźwiak, Citation2013; P. stiletto Bamber, 2009; P. unicus Sieg, Citation1977; P. vulsella Bird & Holdich, 1989; P. discoveryae sp. nov.; P. enduranceae sp. nov.; and P. scotti sp. nov.
Pseudotanais discoveryae sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:983D7B12-856F-4E76-BA23-31282521377A
Figure 40. Pseudotanais discoveryae sp. nov., juvenile female holotype, (a, c), dorsal; (d), lateral; neuter, (b), dorsal. Scale line = 0.1 mm
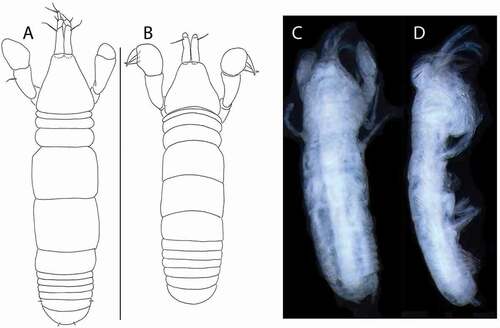
Figure 41. Pseudotanais discoveryae sp. nov., (a), antennule; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible; (f), maxillule; (f′), maxillule endite, distal; (g), maxilla; (h), maxilliped. Scale lines = 0.1 mm
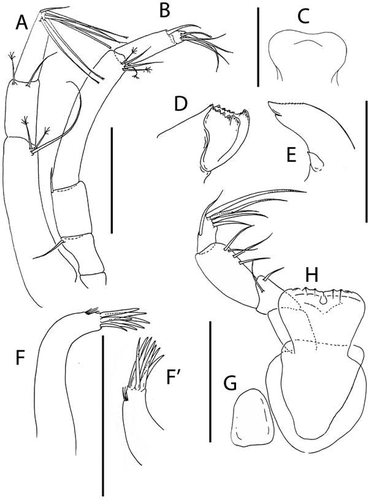
Figure 42. Pseudotanais discoveryae sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4 and (e’) detail of dactylus and unguis; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
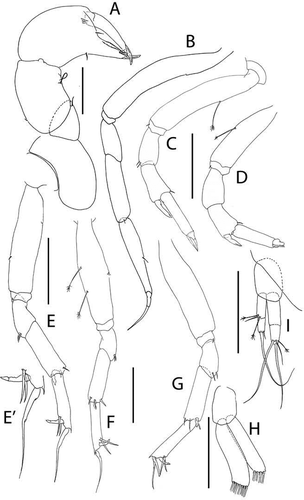
Material examined
Holotype, juvenile female 1.2 mm, ICUL8859, ST-EBS-3 (NHM UK.2021.188); brooding female 1.1 mm, 3 neuters 0.9–1.3 mm, juvenile female 1.1 mm, ICUL8856, ST-EBS-3 (NHM UK.2021.189); two females 1.2-1.5 mm, two neuters 1.1–1.2 mm, ICUL8857, ST-EBS-3 (NHM UK.2021.190); brooding female 1.1 mm, two neuters 0.9–1.2 mm ICUL8858, ST-EBS-3B (NHM UK.2021.191); three females 1.0–1.2 mm, two neuters 0.8–1.0 mm, ICUL8889, ST-EBS-3B (NHM UK.2021.192); 1.2 mm neuter, dissected, ICUL8908, ST-EBS-3B (NHM UK.2021.193).
Diagnosis
Antenna articles 2–3 with seta. Pereopods 2–3 carpus blade-like spine 0.5x propodus. Uropod exopod 0.8x endopod.
Etymology
The species named after the steam barque RRS Discovery. Her first mission known as Discovery Expedition was led by Robert Falcon Scott and Sir Ernest Shackleton who explored the Southern Ocean.
Description of juvenile female. BL = 1.2 mm. Body robust ()) 4.2 L:W. Cephalothorax 1.1 L:W, 1.8x pereonites 1–3, 0.2x BL. Pereonites 0.6x BL, pereonites-1–6: 0.1, 0.15, 0.3, 0.8, 0.8, and 0.4 L:W, respectively. Pleon short, 0.2x BL. Pleonites 0.7 L:W, pleonite-5 with lateral setae. Pleotelson 1.1x pleonite-5.
Antennule ()) article-1 3.3 L:W, 2.9x article-2, with one long and two penicillate distal setae; article-2 1.8 L:W, 0.8x article-3, with one simple and one penicillate distal setae; article-3 3.2 L:W, with two simple and four bifurcated setae and one aesthetasc.
Antenna ()) about as long as antennule; article-2 1.4 L:W; 0.8x article-3, with seta (0.8x article-2); article-3 1.5 L:W, 0.3x article-4, with seta (0.5x article-3); article-4 5.9 L:W, 2.5x article-5, with two simple and three penicillate distal setae; article-5 2.9 L:W, 5.2x article-6, with simple seta; article-6 1.3 L:W, with five distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor margin serrate, with blunt apex; lacinia mobilis well developed, distally serrate.
Right mandible ()) incisor unequally bifid, distal margin weakly serrate; molar acuminate, simple, short.
Maxillule ()) endite with at least seven distal spines and outer subdistal tuft of setules.
Maxilla ()) subrectangular, naked.
Maxilliped ()) bases together chordate, as long as broad, naked; palp article-1 naked, article-2 with two inner setae, article-3 with slender inner setae, article-4 with five long distal and one outer subdistal setae; distal setae with finely denticulate margins in their distal half; endites almost completely fused, with shallow medial cleft, and with two small gustatory cusps on distal margin.
Cheliped ()) robust; basis 1.5 L:W; merus slightly longer than carpus ventral margin, with ventral seta; carpus 1.6 L:W, 1.0x palm, with two midventral setae and one mediodorsal seta; chela 2.3 L:W, 1.7x carpus length, palm 1.4 L:W; fixed finger 2.7 L:W, 0.7x palm, with short ventral seta, cutting edge finely crenulate, with three setae; dactylus 4.6 L:W, cutting edge smooth, without proximal seta.
Pereopod-1 ()) basis 6.3 L:W, 4.0x merus, naked; ischium naked; merus 1.9 L:W and 0.5x carpus, naked; carpus 3.9 L:W, 0.8x propodus, with small distal seta; propodus 5.3 L:W, 1.7x dactylus and unguiscombined length, with small middorsal seta, dactylus 0.5x unguis.
Pereopod-2 ()) basis 5.2 L:W, 3.6x merus, with middorsal penicillate seta; ischium with ventral seta; merus 1.5 L:W, 0.8x carpus, with two ventrodistal setae; carpus 2.0 L:W, 0.9x propodus, with simple dorsodistal seta and semilong/intermediate blade-like spine (0.5x propodus); propodus 4.2 L:W, 2.3x dactylus and unguis combined length, with distal seta (2.5x dactylus); dactylus 0.6x unguis.
Pereopod-3 ()) basis 3.7 L:W, 2.2x merus, with midventral penicillate seta; ischium with ventral seta; merus 1.7 L:W, 1.2x carpus, with two ventrodistal setae; carpus 1.4 L:W, 0.8x propodus, with short seta and semilong/intermediate blade-like spine (0.5x propodus); propodus 3.0 L:W, with distal seta; dactylus and unguis broken.
Pereopod-4 ()) basis 5.7 L:W, 3.2x merus, with two setae; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with two ventrodistal setae; carpus 2.5 L:W, 0.8x propodus, with seta, one seta (0.12x propodus) and short blade-like spine (0.2x propodus); propodus 5.5 L:W, 3.3x dactylus and unguis combined length, with two ventral setae and one dorsal seta (3.3x dactylus and unguis combined length); dactylus 2.3x unguis.
Pereopod-5 ()) basis 3.5 L:W, 3.7x merus, with ventral (penicillate?) seta and two dorsal penicillate setae; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with two ventrodistal setae; carpus 3.3 L:W, 1.2x propodus, with simple seta, seta (0.18x propodus) and short blade-like spine (0.2x propodus); propodus 3.7 L:W, 3.1x dactylus and unguis combined length, with two ventral setae and one dorsal seta (3.0x dactylus and unguis combined length); dactylus 2.5x unguis.
Pereopod-6 ()) basis 3.7 L:W, 2.8x merus; ischium with one ventral seta; merus 2.0 L:W, 0.7x carpus, with two ventrodistal setae; carpus 3.0 L:W, 1.1x propodus, with one simple seta, one seta (0.15x propodus and one short blade-like spine (0.2x propodus); propodus 3.9 L:W, 3.9x dactylus and unguis combined length, with two ventral serrate setae ventrally and two dorsal serrate setae; dactylus 3.7x unguis.
Pleopods ()) peduncle 1.5 L:W; endopod shorter than exopod, 4.9 L:W, with four setae; endopod 4.0 L:W, with seven setae.
Uropod ()) peduncle 1.5 L:W; exopod and endopod two-articled; exopod 0.8x endopod, article-1 2.6 L:W, with one simple and two penicillate setae, article-2 2.0 L:W, two setae (short and long); endopod article-1 2.6 L:W, article-2 1.7 L:W, with one penicillate and three simple setae.
Neuter ()). Generally similar to juvenile female but has different body-proportions ()): pereonite-3 is only 1.4x pereonite-2 and 4.0 L:W; pereonites 4 and 5 are subequal, 2.3x pereonite-3 and 1.7 L:W; pereonite-6 is 2x pereonite-3, and 2 L:W.
Distribution
Southern Thule (Sandwich Is), 500–543 m.
Remarks
Pseudotanais discoveryae sp. nov. is the ninth blind forcipate-chela species of Pseudotanais. The character of the uropods allows it to be distinguished from the other members of “forcipatus group”. The proportion of the uropod exopod to the endopod, 0.8x in P. discoveryae, separates it from the species P. artoo, P. californiensis, P. forcipate, P. jonesi and P. soja, where the exopod reaches only half length of the endopod uropod. Moreover, relatively short uropods with an exopod 4.7 L:W can separate P. discoveryae from P. falcicula, P. misericorde, and P. vulsella, which have an exopod at least 5 L:W, Additionally, the long seta on antenna article-2 (as long as article-2) distinguishes P. discoveryae from other members of the “forcipatus group”, where this seta is at most 0.8x article-2.
Pseudotanais enduranceae sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:105CD261-77DF-49CF-BB7B-017A5702072F
Figure 43. Pseudotanais enduranceae sp. nov., holotype female, (a), (b), dorsal; (c), lateral. Scale line = 1 mm
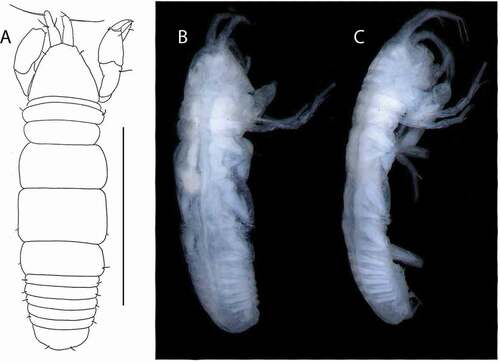
Figure 44. Pseudotanais enduranceae sp. nov., (a), antennule of female; (b), antenna; (c), labrum; (d), left mandible; (e), right mandible; (f), maxillule, (g), maxilla; (h), maxilliped; (i), antennule of juvenile male A. Scale lines = 0.1 mm
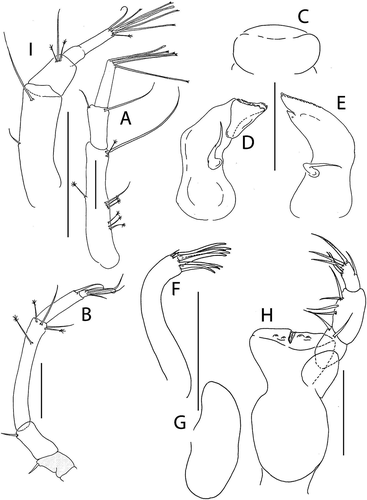
Figure 45. Pseudotanais enduranceae sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-6; (g), pleopod; (h), uropod. Scale lines = 0.1 mm
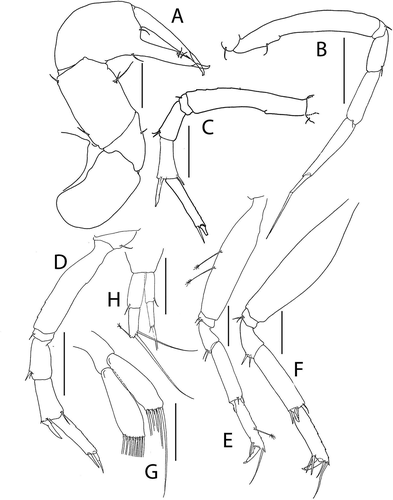
Diagnosis
Antenna articles 2–3 with seta. Pereopods 2–3 carpus blade-like spine 0.5x propodus. Uropod exopod 0.9x endopod.
Material examined
Holotype, neuter 1.7 mm, ICUL8845, BIO4-EBS-3B (NHM UK.2021.194); neuter 1.5 mm, ICUL8846, BIO4-EBS-3D (NHM UK.2021.195); male, dissected, ICUL8911, BIO4-EBS-3D (NHM UK.2021.196); female, dissected, ICUL8912, BIO4-EBS-3D (NHM UK.2021.197).
Etymology
The species name refers to the steam barquentine HMS Endurance, famous for her last catastrophic expedition to Antarctic (1914–1915) led by Sir Ernest Henry Shackleton.
Description of neuter. BL = 1.7 mm. Body robust ()) 3.6 L:W. Cephalothorax 0.8 L:W, 1.1x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonite-1 half, or less, as long as pereonites 2–3, pereonites-1–6: 0.07, 0.2, 0.3, 0.5, 0.6 and 0.4 L:W, respectively. Pleon short, 0.2x BL. Pleonites 0.7 L:W each with lateral setae. Pleotelson 2.2x pleonite-5.
Antennule ()) as long as antennule; article-1 5.1 L:W, 2.8x article-2, with mid-inner penicillate seta, three penicillate setae in proximal half, outer margin with two groups of simple and penicillate setae, one simple and two penicillate setae distally; article-2 2.2 L:W, 0.9x article-3, with one inner and one longer outer distal setae; article-3 3.7 L:W, with four bifurcated distal setae and one aesthetasc.
Antenna ()) shorter than antennule; article-2 1.1 L:W; 0.6x article-3, with seta (0.6x article-2); article-3 1.9 L:W, 0.4x article-4, with seta (0.4x article-3); article-4 7.1 L:W, 2.3x article-5, with two simple and three penicillate distal setae; article-5 3.7 L:W, 7.3x article-6 with simple seta; article-6 1.0 L:W, with five distal setae.
Labrum ()) rounded, naked.
Left mandible ()) incisor distally with two small teeth, margin weakly serrate; lacinia mobilis well developed, distally serrate; molar slender, elongate, and acuminate.
Right mandible ()) incisor unequally bifid, distal margin serrate, as in left mandible; molar as left mandible.
Labium not seen.
Maxillule ()) endite with eight distal spines and outer subdistal tuft of setae.
Maxilla ()) ovoid, naked.
Maxilliped ()) bases together ovoid, longer than broad, naked; palp article-1 naked; article-2 with two inner setae, outer seta not seen; article-3 with four inner setae; article-4 with five distal and one sub-distal setae. endites cleft, each with two distal gustatory cusps.
Cheliped ()) basis 1.8 L:W; merus as long as carpus ventral margin, with ventral seta; carpus 1.5 L:W, 0.9x palm, with two midventral setae, and one mediodorsal and one dorsodistal setae; chela 2.7 L:W, 1.8x carpus L, palm 1.6 L:W; fixed finger 3.4 L:W, 0.7x palm with ventral seta, cutting edge simple with three small setae, distal spine straight and slender, with tip bent up; dactylus 5.4 L:W, cutting edge smooth, without proximal seta; unguis straight and slender, with tip bent down.
Pereopod-1 ()) coxa with seta; basis 7.0 L:W, 4.1x merus with dorsoproximal seta; ischium with ventral seta; merus 2.1 L:W and 0.8x carpus, with ventrodistal seta; carpus 3.1 L:W, 0.6x propodus, with ventrodistal seta; propodus 5.7 L:W, 0.9x dactylus and unguis combined length, naked; dactylus 0.5x unguis.
Pereopod-2 ()) coxa with seta; basis 5.7 L:W, 3.6x merus, naked; ischium with ventral seta; merus 2.0 L:W, 0.9x carpus, with spine and seta ventrodistally; carpus 1.8 L:W, 0.7x propodus, with seta, and spine, and long blade-like spine (0.6x propodus); propodus 5.4 L:W, with one distal spine (1.5x dactylus); unguis broken.
Pereopod-3 ()) coxa with seta; basis 4.4 L:W, 2.8x merus, naked; ischium with ventral seta; merus 1.7 L:W, 0.9x carpus, with one ventrodistal seta and one spine; carpus 1.7 L:W, 0.8x propodus, with two simple setae and one semilong/intermediate blade-like spine (0.5x propodus); propodus 4.2 L:W, 2.1x dactylus and unguis combined length, with one distal seta (0.8x dactylus); dactylus 0.7x unguis.
Pereopod-4 ()) basis 4.5 L:W, 5.3x merus, with two ventral penicillate setae; ischium with two ventral setae; merus 1.5 L:W, 0.4x carpus, with two ventrodistal spines; carpus 4.1 L:W, 1.2x propodus, with one simple seta, one seta (0.2x propodus) and one short blade-like spine (0.2x propodus); propodus 4.2 L:W, 2.3x dactylus and unguis combined length, with one middorsal penicillate seta, two serrate ventral setae, one serrate dorsal seta (1.7x dactylus and unguis combined length); dactylus 4.5x unguis.
Pereopod-5 missing.
Pereopod-6 ()) basis 4.5 L:W, 5.1x merus, naked; ischium with two ventral setae; merus 1.5 L:W, 0.4x carpus, with two ventrodistal spines; carpus 3.9 L:W, 1.2x propodus, with one simple seta, one seta (0.2x propodus) and one short blade-like spine (0.2x propodus); propodus 4.1 L:W, 2.6x dactylus and unguis combined length, with two serrate ventral setae, two serrate dorsal setae (longer seta 1.6x dactylus and unguis combined length); dactylus 10.0x unguis.
Pleopods ()) peduncle 1.5 L:W; endopod just shorter than exopod, 3.4 L:W, with five setae; endopod 2.9 L:W, with eight setae.
Uropod ()) peduncle 0.8 L:W; exopod and endopod two-articled; exopod 0.9x endopod, article-1 2.8 L:W, with one simple seta, article-2 4.3 L:W, with two setae (short and long); endopod article-1 2.4 L:W, with one simple seta, article-2 2.8 L:W, with two simple and one penicillate seta.
Subadult male. Similar to female, but antennule ()) stouter, article-3 showing signs of subdivision.
Distribution
Amundsen Sea, 489–507 m.
Remarks
Pseudotanais enduranceae sp. nov., with its uropod exopod 0.9x as long as the endopod, can be distinguished from P. artoo, P. californiensis, P. forcipatus, P. jonesi and P. soja, where it is relatively shorter (0.5x). It is most similar to P. discoveryae although its uropod endopod is 4.3 L:W (4.7 L:W in P. discoveryae), the seta on antenna article-2 is 0.5x as long as article-3 (as long as article-3 in P. discoveryae) and the exopod uropod is 0.9x as long as endopod (0.8x in P. discoveryae).
Pseudotanais scotti sp. nov.()http://www.zoobank.org/urn:lsid:zoobank.org:act:C3C4C558-DB67-4A3E-9DC4-F59B5DA278B1F59B5DA278B1
Figure 46. Pseudotanais scotti sp. nov., holotype female, (a, c), dorsal; (b, d), lateral. Scale line = 0.1 mm
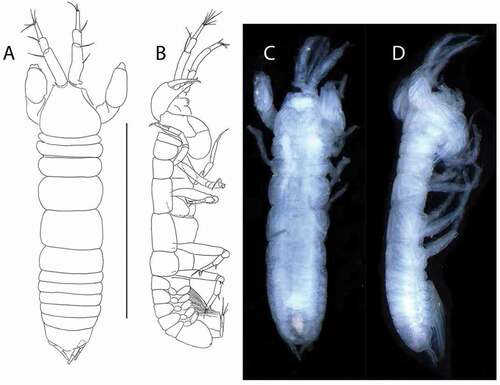
Figure 47. Pseudotanais scotti sp. nov., (a), antennule; (b), antenna; (c), left mandible, distal; (c’), mandible molar; (d), maxillule endite; (e), maxilliped. Scale lines = 0.1 mm
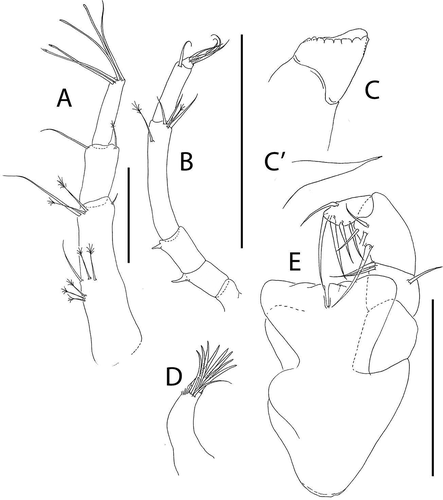
Figure 48. Pseudotanais scotti sp. nov., (a), cheliped; (b), pereopod-1; (c), pereopod-2; (d), pereopod-3; (e), pereopod-4; (f), pereopod-5; (g), pereopod-6; (h), pleopod; (i), uropod. Scale lines = 0.1 mm
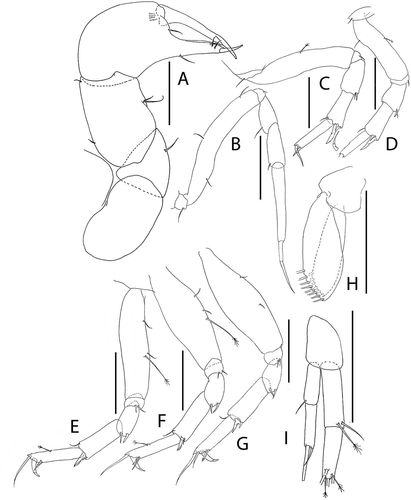
Material examined
Holotype, juvenile female 1.2 mm, ICUL8851, ST-EBS-3 (NHM UK.2021.198). Paratypes: juvenile female 1.2 mm, ICUL8850, ST-EBS-3 (NHM UK.2021.199); Paratypes: two neuters 0.8–1.2 mm, juvenile male 1.2 mm, juvenile female 1 mm, ICUL8853, ST-EBS-3 (NHM UK.2021.200); two neuters 0.9-1.2 mm, juvenile female 1.1 mm, manca 0.8 mm, ICUL8854, ST-EBS-3 (NHM UK.2021.201); juvenile female 1.5 mm, ICUL8855, ST-EBS-3 (NHM UK.2021.202); neuter, dissected ICUL8905, ST-EBS-3B (NHM UK.2021.203).
Etymology
The species named in honour of Capitan Robert Falcon Scott, an officer of the British Royal Navy, the explorer of the Antarctic and leader of the Discovery Expedition.
Diagnosis
Antenna articles 2–3 with spines. Pereopods 2–3 carpus blade-like spine 0.6x propodus. Uropod exopod 0.7x endopod.
Description of female. BL = 1.2 mm. Body robust ()) 4.1 L:W. Cephalothorax 0.9 L:W, 1.4x pereonites 1–3, 0.2x BL. Pereonites 0.5x BL, pereonites-1–6: 0.12, 0.18, 0.3, 0.5, 0.6 and 0.3 L:W, respectively. Pleon short, 0.3x BL. Pleonites 0.9 L:W each with lateral setae. Pleotelson 1.8x pleonite-5.
Antennule ()) article-1 5.1 L:W, 2.6x article-2, with one simple and five penicillate setae at mid-length, and two (long and short) simple and two penicillate setae distally; article-2 1.9 L:W, 0.9x article-3, with simple and penicillate; article-3 4.0 L:W, with four simple and one aesthetasc.
Antenna ()) just shorter than antennule; article-2 1.2 L:W; 0.9x article-3, with spine (0.5x article-2); article-3 1.1 L:W, 0.3x article-4, with spine (0.4x article-3); article-4 5.9 L:W, 1.9x article-5, with three simple and two penicillate distal setae distally and subdistally; article-5 3.0 L:W, 8.0x article-6 with seta; article-6 0.8 L:W, with four distal setae.
Left mandible ()) incisor distally rounded, margin simple; lacinia mobilis distal margin crenulate; molar narrow long, acute (’)).
Maxillule ()) endite with eight distal spines and outer subdistal tuft of setules.
Maxilliped ()) bases together chordate, about as long as broad, naked; palp article-1 naked, article-2 with two inner setae and one long outer seta, article-3 with four inner setae, article-4 with one outer sub-distal and four inner-distal setae; endites with distal cleft, distal gustatory cusps not seen.
Cheliped ()) robust; basis 1.8 L:W; merus 1.2x carpus ventral margin, with ventral seta; carpus 1.4 L:W, 1.0x palm, with two ventral setae, one mediodorsal and one dorsodistal setae; chela 2.5 L:W, 1.9x carpus length, palm 1.3 L:W with four small setae on inner side and one seta near dactylus insertion; fixed finger 3.2 L:W, 0.8x palm, with ventral seta, cutting edge simple, with three small setae; dactylus 4.2 L:W, cutting edge smooth, without proximal seta; unguis almost straight and pointed.
Pereopod-1 ()) coxa with seta; basis 6.7 L:W, 5.0x merus with one dorsoproximal and one ventral simple seta; ischium with ventral seta; merus 1.5 L:W, 0.6x carpus, with dorsodistal seta; carpus 2.9 L:W, 0.5x propodus, with dorsodistal seta; propodus 7.4 L:W, 1.4x dactylus and unguis combined length, naked; dactylus 0.6x unguis.
Pereopod-2 ()) coxa with seta; basis 4.5 L:W, 3.0x merus, with ventral penicillate seta; ischium with ventral seta; merus 1.5 L:W, 1.0x carpus, with seta and small spine ventrodistally; carpus 1.9 L:W, 0.75x propodus, with seta, spine and semilong/intermediate blade-like spine (0.5x propodus) distally; propodus 4.0 L:W, 2.0x dactylus and unguis combined length, with distal spine (0.5x dactylus); dactylus 0.7x unguis.
Pereopod-3 ()) coxa with seta; basis 3.7 L:W, 3.0x merus, with ventral seta; ischium with ventral seta; merus 1.1 L:W, 0.7x carpus, with seta and small spine ventrodistally; carpus 1.9 L:W, 0.7x propodus, with two setae and blade-like spine (0.7x propodus); propodus 4.0 L:W, 2.0x dactylus and unguis combined length (distal spine not observed); dactylus 0.7x unguis.
Pereopod-4 ()) basis 4.2 L:W, 2.9x merus, with two simple (short) and one penicillate (long) ventral setae; ischium with two ventral setae; merus 2.1 L:W, 0.8x carpus, with two ventrodistal spines; carpus 2.6 L:W, 0.9x propodus, with simple seta, rod setae (0.2x propodus) and short blade-like spine (0.4x propodus); propodus 4.4 L:W, 2.4x dactylus and unguis combined length, with middorsal penicillate seta, two ventral setae and one dorsal seta (1.9x dactylus and unguis combined length); dactylus 2.0x unguis.
Pereopod-5 ()) as pereopod-4.
Pereopod-6 ()) basis 3.2 L:W, 4.3x merus, with two ventral setae; ischium with two ventral setae; merus 1.4 L:W, 0.6x carpus, with two ventrodistal spines; carpus 2.8 L:W, 1.0x propodus, with simple seta, seta (0.3x propodus) and short blade-like spine (0.3x propodus); propodus 3.7 L:W, 2.4x dactylus and unguis combined length, with two ventral setae and two dorsal setae (longer seta 2.1x dactylus and unguis combined length); dactylus 3.5x unguis.
Pleopods ()) peduncle 0.9 L:W; endopod just shorter than exopod, 3.6 L:W, with six setae; endopod 2.6 L:W, with eight setae.
Uropod ()) peduncle 1.4 L:W; exopod and endopod two-articled; exopod 0.7x endopod, article-1 3.2 L:W, with simple seta, article-2 5.0 L:W, two setae (short and long); endopod article-1 3.3 L:W, with one simple and one penicillate setae, article-2 3.3 L:W, with four simple and two penicillate setae.
Distribution
Southern Thule (Sandwich Is), 500–543 m.
Remarks
Pseudotanais scotti sp. nov. is the eleventh blind forcipate pseudotanaid. The length of the uropod exopod to the endopod (0.8x) separates it from P. artoo, P. californiensis, P. forcipatus, P. jonesi and P. soja, with a uropod exopod half as long as the endopod. From other members of the “forcipatus group” that have a seta on the antennule articles 2 and 3 it can be distinguished by the presence of a spine.
1. Blade-like spines on carpus of pereopods 2–6:
absent, simple or bayonet-like ()) 2
present ())4
2. Eyes:
presentAkanthinotanais guillei
absent (or not pigmented) ()3
3. Uropod exopod; ischium of pereopod-1; dactylus of pereopod-1:
two-segmented; naked; with long proximal seta ())A. gaussi
one-segmented; one seta; naked ()) A. rossi sp. nov.
4. Chela:
forcipate ())5
not-forcipate ())8
5. Antenna article-6 thick rod seta; chela cutting edge:
present; serrate (; )B. vanhoeffeni sp. nov.
absent; smooth (), )) (“forcipatus” gp.) 6
6. Antenna articles 2–3 with:
spines ())P. scotti sp. nov.
setae ()) 7
7. Antenna article-2 seta; uropod endopod:
0.5x article-3; 4.3 L:W (); )P. enduranceae sp. nov.
as long as article-3, 4.7 L:W (; )) P. discoveryae sp. nov.
8. Pereopod-2 propodus seta:
clearly longer than dactylus ())(“affinis+longisetosus” gp.) 9
as long as or shorter than dactylus ()) (“denticulatus+abathagastor” gp.) 12
9. Pereopods 5–6 carpus dorsodistal seta:
long ())10
short ())11
10. Pereopod-4 carpus dorsodistal seta:
longP. longisetosus
short ()) P. rapunzelae sp. nov.
11. Pereopods 4–6 ischium with; pereopods 4–6 merus with; pereopod-5 proportion carpus to propodus:
one seta; spine and seta; at least as long as P. nordenskioldi
two setae; spine; shorter ())P. shackletoni sp. nov.
12. Antenna article-2:
naked ())P. barnesi sp. nov.
with spine ())13
13. Antenna article-3 with:
minute seta ()) 14
spine ()) 15
14. Pereopod-2 merus with: carpus blade like spine; pereopods 4–5 proportion dactylus to unguis:
two seta same length; 0.27x propodus; 5.0x (); ))P. amundseni sp. nov.
two setae (short and long); 0.4x propodus; at most 3.5x (; ) P. elephas sp. nov.
15. Uropod exopod:
as long as endopod ())P. palmeri sp. nov.
shorter than endopod ())16
16. Pereopod-6 carpus blade like spine:
0.25x propodus ()) P. kitsoni sp. nov.
0.4x propodus ()) 17
17. Pereopod-3 carpus blade-like spine:
0.6x propodus. ()) P. livingstoni sp. nov.
0.4x propodus ())P. biopearli sp. nov.
Key to the identification of Antarctic pseudotanaids (females)
Results
(i). Diversity. The 169 pseudotanaids analysed in this paper revealed 15 species belonging to three genera: Akanthinotanais, Beksitanais, and Pseudotanais, with seven species recorded in the Amundsen Sea and eight species in the Scotia Sea (; Appendix 2). Only one species, Akanthinotanais gaussi, was formerly known from the Antarctic coast (Vanhöffen Citation1914; Kudinova-Pasternak Citation1975); the fourteen others were new to science and represent three morphogroups: “affinis+longisetosus”, “denticulatus+abathagastor” and “forcipatus” (Bird & Holdich Citation1989a; Jakiel et al. Citation2019).
Table III. Distribution of Pseudotanaidae species in studied area
All the pseudotanaid species recorded in the Amundsen Sea were considered to be unique for the region because they are mostly absent elsewhere (, ) and grouped within clade 1 (similarity cluster, ). The exception was P. kitsoni that was present also at Elephant Island, and B. vanhoeffeni recorded also in the South Sandwich Islands. The cluster analysis revealed five robust groups of samples distinguished at 20% of similarity, i.e., (1) Amundsen Sea (12 stations), (2) Amundsen Sea (2 stations), (3) Elephant Island + Livingstone + S. Sandwich (4 stations), (4) Southern Tule + Elephant Island (3 stations), and (5) South Georgia + Shag Rocks (5 stations). One station from Palmer Bay (South Orkney Islands) was represented by only one species (P. palmeri) and was an outlier.
Figure 49. Geographic distribution of newly described Pseudotanaidae from the BIOPEARL 1 and 2 expeditions
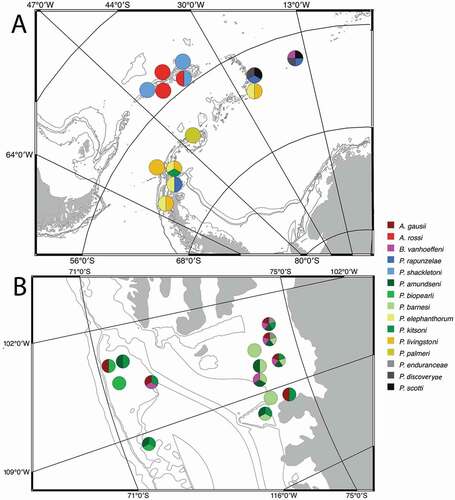
Figure 50. (a) Dendrogram of similarity between the stations based on pseudotanaid presence-absence data, the Bray-Curtis algorithm, and group average clustering. (b) A Heat Map of similarity/dissimilarity between the samples based on pseudotanaids presence-absence data: intensity of colour corresponds to level of similarity
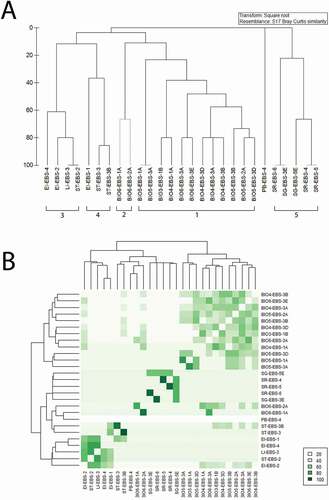
The most similar grouping within clade 1 of ten samples (all BIO4, three BIO5, two BIO6, one BIO3) was characterised by the presence of five species: B. vanhoeffeni, P. amundseni, P. barnesi, P. kitsoni and A. gaussi. A pair of stations (BIO5-EBS-1A and BIO5-EBS-3A) made a distinct subclade distinguished by the presence of B. biopearli. Two stations (BIO6-EBS-1A and BIO6-EBS-2A) from clade 2 shared low diversity (one or three species). Clade 4 was indicated by the presence of P. elephas and P. livingstoni, with clade 5 by P. rapunzelae, P. enduranceae and P. discoveryae. Finally, clade 5 was defined by A. rossi and P. shackletoni.
An ANOSIM analysis confirms a general difference in pseudotanaids species composition between the areas (Global R = 0.567, p < 0.01). The strongest dissimilarity (R = >0. 61, p < 0.001) was between the Amundsen Sea and Southern Tule/Shag Rock, and between the Amundsen Sea and Elephant Island R = 0,579; p < 0.05. Heat map
The number of the pseudotanaids individuals representing each species varied between 3 and 21 (Median = 11) (). In the Amundsen Sea the most abundant species were: P. barnesi (21 ind.), P. kitsoni (16 ind.), A. gaussi (15 ind.) and B. vanhoeffeni (14 ind.), and in the Scotia Sea — P. discoveryae (19 ind.) and P. scotti (12 ind.). The least abundant species were P. shackletoni (3 ind.) and P. enduranceae (4 ind.).
(ii). Depth gradient. Except for Akanthinotanais guillei, which is a truly shallow water species (10–32 m), all the pseudotanaids occurred deeper than the Antarctic shelf (>500 m) () and showed relatively wide bathymetric spans, between 300 and 1300 m. The distribution of only one species, P. nordenskioldi, covers a depth range of 6050 m (Kudinova-Pasternak Citation1993).
(iii). Feeding. Food preferences of pseudotanaids have been rarely studied, and there are no studies applying biochemical methods to indicate their position in Antarctic food-webs. Direct observation of pseudotanaids reveals an empty digestive system in most specimens, which together with weak sclerotinisation of the integument make internal observation feasible. In general, shallow-water tanaidaceans in the Antarctic were classified as unselective detritivores (Błażewicz-Paszkowycz & Ligowski Citation2002) or bacterivorous (Delille et al. Citation1985) although they have been proved to prey on meiofauna or their larval stage including polychaetes or echinoderms, as well as on some nematodes or copepods (Larsen Citation2005) or they parasite the tegument of holothurians (Alvaro et al. Citation2011). The coronal or piercing mandibular molars present in pseudotanaids implies that they might be non-opportunistic and non-selective predators.
Most of the pseudotanaids that we studied revealed empty digestive tracts, however, in two species, A. rossi and P. kitsoni, fragments of harpacticoid copepods, intact foraminiferan tests and other unidentified possible animal tissue fragments were identified (). The foraminiferan tests broke into fine pieces under a cover glass, possibly suggesting their semi-digested stage.
Discussion
(i). Species composition. We have increased the number of currently known Antarctic Pseudotanaidae by the fourteen species recorded in the Amundsen and Scotia Seas, bringing the total number to 20 species (Vanhöffen Citation1914; Kudinova-Pasternak Citation1975, Citation1990, Citation1993; Sieg Citation1977, Citation1986a, Citation1986b; Shiino Citation1978) (Appendix 1). The updated taxonomical position of B. abyssi and description of B. vanhoeffeni are the first records of the genus in the Southern Ocean.
Previously, the Pseudotanaidae were known from six species in Antarctic waters. i.e., Akanthinotanais gaussi, A. guillei, Beksitanais abyssi, Pseudotanais affinis, P. nordenskioldi, and P. longisetosus. Beksitanais abyssi originally described from the Arctic (Hansen Citation1913) was later recorded in the Antarctic (Vanhöffen Citation1914; Sieg Citation1986a; Kudinova-Pasternak Citation1993). The same situation concerns P. affinis that was primarily recorded in the Arctic but was later discovered in the Scotia Sea (Kudinova-Pasternak Citation1990), . There are several hypotheses (including geological transport, anti-tropical distribution, or equatorial submergence) that rationalize bipolar distribution of taxa with high dispersal capability or planktonic stages (e.g., Stepanjants et al. Citation2006; Havermans et al. Citation2013; Moles et al. Citation2017) but none can be satisfactorily applied for the deep-sea Pseudotanaidae. Considering the typical tanaidacean peracarid brooding mode of reproduction, and their rather limited population connectivity, a bipolar distribution of any macrofauna lacking planktonic stages is highly improbable (Brandt et al. Citation2012; Riehl & Kaiser Citation2012; Kaiser et al. Citation2013). Therefore, it is more likely that the Antarctic records of P. affinis represent a distinct species.
(ii). Distribution. Our data, collated with literature records, reveal a clear difference between the pseudotanaid fauna on either side of the Drake Passage (, , ). Except for P. nordenskioldi, which has an apparently wide bathymetric and latitudinal zoogeographical distribution, all the pseudotanaids recorded along the Scotia Arch (Livingston I., Elephant I., South Orkney Is, and partly South Sandwich Is) were absent from South Georgia, Shag Rock and Patagonia. However, P. shackletoni and A. rossi were recorded only at South Georgia and Shag Rock and were absent south of the Polar Front. Here, we can speculate about restricted migration in south-north (or reversal) direction and about the role of Antarctic Current (ACC) as zoogeographical factor affecting distribution of pseudotanaids inhabiting the West Antarctic. Also, we could postulate that, if passive transport from north to south direction is possible for pseudotanaids, we should expect the presence of eye-bearing pseudotanaids, like A. guillei, on the Antarctic shelf.
The spatial distribution of Pseudotanaidae in the Amundsen Sea, as well as along the Antarctic Peninsula, and Scotia Arch up to the South Sandwich Islands suggests rather restricted zoogeographical ranges, although a potential for gene flow cannot be excluded. The Amundsen Sea fauna, characterized by distinctive set of the pseudotanaid species, may not be unique. Beksitanais vanhoeffeni and P. kitsoni were also found frequently in the South Sandwich Islands and Elephant Island environments, respectively. This pattern might imply their potentially wider or continuous distribution concealed within unsampled or under-sampled areas between these localities.
We could further speculate that B. abyssi has a continuous distribution along the whole Antarctic slope (see ). The continuous-Circumantarctic ranges for Antarctic fauna associated with the ACC have been postulated for many invertebrate groups (Brey et al. Citation1996) and validated in a species-habitat modelling approach 7(O’Hara et al. Citation2011). Several shallow-water tanaidacean species (e.g., Nototanais dimorphus Beddard, 1886) associated with algal habitats might have been passively distributed around the Antarctic (Błażewicz-Paszkowycz Citation2014). In the deeper waters, the connectivity between physically separated populations is proposed to be sustained by the oceanic currents. The ACC, which extends down to 4000 m (https://oceancurrents.rsmas.miami.edu/southern/antarctic-cp.html), could facilitate gene flow within the deeper living fauna, but evidence for circumpolar distribution is equivocal and has been questioned in genetic studies for several groups of benthic invertebrates (Held Citation2003; Held & Wägele Citation2005; Brandão et al. Citation2010; Allcock et al. Citation2011; Arango et al. Citation2011). Therefore, in the alternative scenario, the records of B. vanhoeffeni and P. kitsoni outside Amundsen Sea could represent separate populations of closely related and morphologically indistinguishable (=cryptic) species.
(iii). Habitat preferences. Unfortunately, not much is known about tanaidacean habitat preferences, but observation from the upper slope of North Atlantic indicates that the quality of the sediments and their dynamics along a benthic boundary layer influence pseudotanaids, hence “muddy-sand or other muddy-substrata”, have been indicated as clearly favourable (Bird & Holdich Citation1989a). Supposedly, the character of the sediments is an essential condition for tube-building fauna (Hassack & Holdich Citation1987), although pseudotanaids might use the tubes only for reproduction. Some authors consider them as fairly mobile tanaidaceans, well-adapted for walking assuming their elongated pereopods and reduced to one pair of oostegites marsupium (Bird & Holdich Citation1989a, Citation1989b; Jakiel et al. Citation2019). It is worth noting that soft sediments generally make a favourable habitat for harpacticoid copepods and might be an important element of the pseudotanaid diet ().
Figure 51. Stomach content of Pseudotanais kitsoni sp. nov. (a) and Akanthinotanais rossi sp. nov. (b). Apart from a foraminiferan tests (shells) now disintegrated under the coverslip, fragments of crustaceans (left) and unidentified fragments of “worms” are observed (right)
Figure 52. Bathymetric depth of Antarctic Pseudotanaidae based on the literature and current data. (Vanhöffen Citation1914; Shiino Citation1978; Sieg Citation1986a, Citation1986b; Kudinova-Pasternak Citation1993; Błażewicz-Paszkowycz & Siciński Citation2014; Pabis et al. Citation2014)
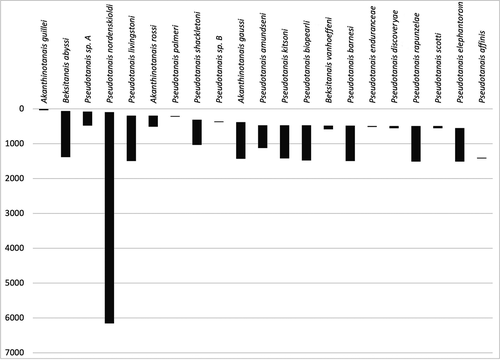
(iv). Diversity. From our study Pseudotanaidae are perceived to be an important component of the Antarctic macrobenthic communities on the deeper shelf and are certainly a less significant component of shallow water communities. Most of the previous records of Antarctic Pseudotanaidae were located at deeper shelf levels (>500 m) along the western side of the Antarctic Peninsula and along the Scotia Arch (). Sieg (Citation1986a, Citation1986b), who produced two large monographs on Antarctic tanaidaceans from the upper shelf of the Antarctic Peninsula and Weddell Sea, recorded only two pseudotanaids, Beksitanais abyssi (Weddell Sea) and P. nordenskioldi (Bransfield Strait), out of a total of 47 tanaidacean species. Pseudotanaids were not recorded in the shallow and extensively sampled bays like Admiralty Bay (South Shetland Is.) (Błażewicz-Paszkowycz & Sekulska-Nalewajko Citation2004; Siciński et al. Citation2011), Anvers Island (Lowry Citation1975), Deception Island (Gallardo Citation1987), and Marguerite Bay (Sieg Citation1984a), as well as the Franklin and Ross Islands (Ross Sea) (Sieg Citation1983a).
On the East Antarctic shelf pseudotanaids were noted only three times, with two nominal species and two morpho-species (Vanhöffen Citation1914; Sieg Citation1986b; Błażewicz-Paszkowycz & Siciński Citation2014). Additionally, 2–3 species were recorded on the shelf of the Ross Sea (Błażewicz-Paszkowycz & Siciński Citation2014; Pabis et al. Citation2015), although they were identified only to morpho-species level. In studies of deeper shelf and upper slope of East Antarctic, pseudotanaids were represented by seven, eight and nine species in the Ross Sea, Amundsen Sea and Scotia Sea, respectively (Pabis et al. Citation2015; this study). In three EBS samples taken out of the Ross Sea at the depth 3212–3490 m, tanaidaceans were the dominant peracarids (Lörz et al. Citation2013), five of the 31 morpho-species listed were Pseudotanaidae (Pabis et al. Citation2015).
To complete the picture on the Antarctic pseudotanaids it must be emphasised that the Antarctic abyss stays almost completely unknown in terms of the Pseudotanaidae, although field investigations off the Ross Sea imply that they are as equally diverse component of benthic assemblages as in any other part of the world Ocean (Bird & Holdich Citation1989a; Jakiel et al. Citation2019, Citation2020). A high diversity of pseudotanaids in Antarctic abyssal and slope simply demonstrates our deficiency in knowledge of these peracarids and the Antarctic macrobenthos in general. The asymmetry between the diversity of Pseudotanaidae in the East and West Antarctic is striking and reveals the paucity and rarity of the Pseudotanaidae in the former. For this reason, the noticeable rareness of pseudotanaids in the East Antarctic should be interpreted as result of lesser scientific attention being given to that part of the Southern Ocean. The low diversity of Pseudotanaidae on the shelf contrasting with their high diversity on the slope, could imply a deep-sea origin of the family on the Antarctic shelf, at least for the blind species.
Episodes of glacial interglacial cycles for the last 30 million years have created the unique character of the Antarctic fauna (Clarke et al. Citation2004; Bentley & Hodgson Citation2009; Kaiser et al. Citation2013) (Clarke et al. Citation2004; Bentley and Hodgson Citation2009; Kaiser et al. Citation2013). Fragmented by the advance of the Antarctic icecap populations on the shelf are supposed to have survived glacial maxima in bathyal shelters and later undergone an adaptive radiation into vacant shelf habitats. The descendants of those events are currently represented by discrete phylogenetic lineages (Raupach et al. Citation2009). The concept of glacial shelters is commonly accepted and given as justification for the wider bathymetric ranges of the Antarctic fauna (Brey et al. Citation1996). Sieg (Sieg Citation1983b) has pointed out that most of the tanaidaceans on the Antarctic shelf are blind (Błażewicz-Paszkowycz Citation2014) suggesting their deep-sea origin, however this fact does not exclude passive migration from the area outside the Polar Front (Sands et al. Citation2015). Passive transport with brown-algae as a vector is not precluded for justification of several eye-bearded species which are associated with algal habitats, (e.g., Nototanais dimorphus, Zeuxo phytalensis Sieg, 1980, Zeuxoides ohlini, Stebbing, 1914 or Zeuxoides troncosoi Esquete & Bamber, 2012 (Sieg Citation1986a; Esquete et al. Citation2012; Błażewicz-Paszkowycz Citation2014; Błażewicz-Paszkowycz & Siciński Citation2014). In the case of pseudotanaids living in deeper shelf which rich in soft sediments (Bird & Holdich Citation1989a, Citation1989b), rafting or ship hauling would seem to be unlikely.
Conclusions
The explorations of the Antarctic benthos on the deeper shelf and shelf break, with sampling gear appropriate for the small and light fraction of macrobenthos (Brandt & Barthel Citation1995; Frutos et al. Citation2016) have revealed a remarkable diversity of peracarids, of which the Pseudotanaidae constitute a diverse and clearly underestimated component (Błażewicz-Paszkowycz Citation2007; Brandt et al. Citation2007b; Pabis et al. Citation2014). Our results increase the number of the previously known pseudotanaids species from six to 20 known from the Antarctic, and from 75 to 89 known in the world ocean. Except for the clearly shallow water subantarctic species A. guillei, most of the Antarctic pseudotanaids have bathymetric ranges of less than a thousand meters. The exception is P. nordenskioldi, whose wide bathymetric range (6000 m) raises suspicion about the conspecific character of its records. The spatial distribution of Pseudotanaidae reveals evidence for disjunct geographical ranges of fauna inhabiting Subantarctic and West Antarctic Regions (De Broyer & Danis Citation2011).
The Antarctic slope and abyssal are indicated as the domain of peracarid diversity with a confound number of the species (Clark & Johnston Citation2003; Brandt et al. Citation2007a). Moreover, it is assumed that the Antarctic bathyal zone served as a glacial refuge for recent macrobenthos (Thatje et al. Citation2005; Ingels et al. Citation2012; Kaiser et al. Citation2013). Without genetic data and adequate geographic coverage, firm conclusions about the origin of the Antarctic Pseudotanaidae cannot be made. However, the higher diversity below the shelf break, and the presence of blind representatives of the family, suggest they could have survived the Cenozoic glaciations in bathyal shelters or have an abyssal origin.
Supplemental Material
Download MS Excel (17 KB)Supplemental Material
Download MS Excel (29.3 KB)Acknowledgements
We are grateful to Jerry McLelland for access to a copy of his unpublished PhD Dissertation (McLelland Citation2008) and also to David Barnes and BAS for collecting material and making it available for our studies.
Disclosure statement
No financial interest or benefit has arisen from the direct applications of this research.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Allcock AL, Barratt I, Eléaume M, Linse K, Norman MD, Smith PJ, Steinke D, Stevens DW, Strugnell JM. 2011. Cryptic speciation and the circumpolarity debate: A case study on endemic Southern Ocean octopuses using the COI barcode of life. Deep-Sea Research Part II: Topical Studies in Oceanography 58(1–2):242–249. DOI: 10.1016/j.dsr2.2010.05.016.
- Alvaro MC, Błażewicz-Paszkowycz M, Davey N, Schiaparelli S. 2011. Skin-digging tanaids: The unusual parasitic behaviour of Exspina typica in Antarctic waters and worldwide deep basins. Antarctic Science Cambridge University Press 23(4):343–348. Available: http://www.journals.cambridge.org/abstract_S0954102011000186.
- Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko CB, Brandão SN, Bray RA, Bruce NL, Cairns SD, Chan TY, Cheng L, Collins AG, Cribb T, Curini-Galletti M, Dahdouh-Guebas F, Davie PJF, Dawson MN, De Clerck O, Decock W, De Grave S, De Voogd NJ, Domning DP, Emig CC, Erséus C, Eschmeyer W, Fauchald K, Fautin DG, Feist SW, Fransen CHJM, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gómez-Daglio L, Gordon DP, Guiry MD, Hernandez F, Hoeksema BW, Hopcroft RR, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb JB, Kristensen RM, Kroh A, Lambert G, Lazarus DB, Lemaitre R, Longshaw M, Lowry J, MacPherson E, Madin LP, Mah C, Mapstone G, McLaughlin PA, Mees J, Meland K, Messing CG, Mills CE, Molodtsova TN, Mooi R, Neuhaus B, Ng PKL, Nielsen C, Norenburg J, Opresko DM, Osawa M, Paulay G, Perrin W, Pilger JF, Poore GCB, Pugh P, Read GB, Reimer JD, Rius M, Rocha RM, Saiz-Salinas JI, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel KE, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker ML, Thuesen EV, Timm T, Todaro MA, Turon X, Tyler S, Uetz P, Van Der Land J, Vanhoorne B, Van Ofwegen LP, Van Soest RWM, Vanaverbeke J, Walker-Smith G, Walter TC, Warren A, Williams GC, Wilson SP, Costello MJ. 2012. The magnitude of global marine species diversity. Current Biology 22(23):2189–2202. DOI: 10.1016/j.cub.2012.09.036.
- Arango CP, Soler-Membrives A, Miller KJ. 2011. Genetic differentiation in the circum—Antarctic sea spider Nymphon australe (Pycnogonida; Nymphonidae). Deep-Sea Research Part II: Topical Studies in Oceanography Elsevier 58(1–2):212–219. DOI: 10.1016/j.dsr2.2010.05.019.
- Bentley MJ, Hodgson DA. 2009. Antarctic ice sheet and climate history since the last Glacial Maximum. Pages News 17(1):28–29. Available: http://pages-dataportal.unibe.ch/products/newsletters/2009-1/specialsection/sciencehighlights/Bentley+Hodgson_2009-1(28-29).pdf.
- Bird GJ, Holdich DM. 1989a. Tanaidacea (Crustacea) of the north‐east Atlantic: The subfamily Pseudotanainae (Pseudotanaidae) and the family Nototanaidae. Zoological Journal of the Linnean Society 97(3):233–298. DOI: 10.1111/j.1096-3642.1989.tb00548.x.
- Bird GJ, Holdich DM. 1989b. Recolonisation of artificial sediments in the deep Bay of Biscay by tanaidaceans (Crustacea: Peracarida), with a description of a new species of Pseudotanais. Journal of the Marine Biological Association of the United Kingdom 69(2):307–317. DOI: 10.1017/S0025315400029428.
- Bird GJ, Larsen K. 2009. Tanaidacean phylogeny – The second step: The basal paratanaoidean families (Crustacea: Malacostraca). Arthropod Systematics and Phylogeny 67:137–158.
- Błażewicz M, Jóźwiak P, Menot L, Pabis K. 2019. High species richness and unique composition of the tanaidacean communities associated with five areas in the Pacific polymetallic nodule fields. Progress in Oceanography 176:102141. DOI: 10.1016/j.pocean.2019.102141.
- Błażewicz-Paszkowycz M. 2007. A revision of the family Typhlotanaidae Sieg 1984 (Crustacea: Tanaidacea) with the remarks on the Nototanaidae Sieg, 1976. Zootaxa 1598(1):1–141. DOI: 10.11646/zootaxa.1598.1.1.
- Błażewicz-Paszkowycz M, Bamber RN, Jóźwiak J. 2013. Tanaidaceans (Crustacea: Peracarida) from the SoJaBio Joint Expedition in slope and deeper waters in the Sea of Japan. Deep Sea Research Part II: Topical Studies in Oceanography 86–87(2):181–213. DOI: 10.1016/J.DSR2.2012.08.006.
- Błażewicz-Paszkowycz M. 2014. The biogeographic Atlas of the Southern Ocean. In: Biogeographic Atlas of the Southern Ocean. De Broyer C, Koubbi P, Griffiths HJ, Raymond B, Udekem d’Acoz CD, et al., editors. Cambridge: Census of Antarctic Marine Life SCAR-Marine Biodiversity Information Network. pp. 173–180. Available: https://www.researchgate.net/publication/265258194.
- Błażewicz-Paszkowycz M, Bamber RN, Anderson G. 2012. Diversity of Tanaidacea (Crustacea: Peracarida) in the World’s Oceans – How far have we come? PLoS ONE 7(4):e33068. DOI: 10.1371/journal.pone.0033068.
- Błażewicz-Paszkowycz M, Ligowski R. 2002. Diatoms as food source indicator for some Antarctic Cumacea and Tanaidacea (Crustacea). Antarctic Science 14(1):11–15. DOI: 10.1017/S0954102002000524.
- Błażewicz-Paszkowycz M, Sekulska-Nalewajko J. 2004. Tanaidacea (Crustacea, Malacostraca) of two polar fjords: Kongsfjorden (Arctic) and Admiralty Bay (Antarctic). Polar Biology 27(4):222–230. DOI: 10.1007/s00300-003-0585-x.
- Błażewicz-Paszkowycz M, Siciński J. 2014. Diversity and distribution of Tanaidacea (Crustacea) along the Victoria Land Transect (Ross Sea, Southern Ocean). Polar Biology Springer Berlin Heidelberg 37:519–529. DOI: 10.1007/s00300-014-1452-7.
- Brandão SN, Sauer J, Schön I. 2010. Circumantarctic distribution in Southern Ocean benthos? A genetic test using the genus Macroscapha (Crustacea, Ostracoda) as a model. Molecular Phylogenetics and Evolution Elsevier Inc 55(3):1055–1069. DOI: 10.1016/j.ympev.2010.01.014.
- Brandt A, Barthel D. 1995. An improved supra- and epibenthic sledge for catching Peracarida (Crustacea, Malacostraca). Ophelia, Taylor & Francis Group 43:15–23. DOI: 10.1080/00785326.1995.10430574.
- Brandt A, Błażewicz-Paszkowycz M, Bamber RN, Mühlenhardt-Siegel U, Malyutina MV, Kaiser S, de Broyer C, Havermans C. 2012. Are there widespread peracarid species in the deep sea (Crustacea: Malacostraca)? Polish Polar Research 33(2):139–162. Available: http://journals.pan.pl/Content/99519/mainfile.pdf.
- Brandt A, Brix S, Brökeland W, Choudhury M, Kaiser S, Malyutina M. 2007a. Deep-sea isopod biodiversity, abundance, and endemism in the Atlantic sector of the Southern Ocean. Results from the ANDEEP I-III expeditions. Deep-Sea Research Part II: Topical Studies in Oceanography 54(16–17):1760–1775. DOI: 10.1016/j.dsr2.2007.07.015.
- Brandt A, Gooday AJ, Brandão SN, Brix S, Brökeland W, Cedhagen T, Choudhury M, Cornelius N, Danis B, De Mesel I, Diaz RJ, Gillan DC, Ebbe B, Howe JA, Janussen D, Kaiser S, Linse K, Malyutina M, Pawlowski J, Raupach M, Vanreusel A. 2007b. First insights into the biodiversity and biogeography of the Southern Ocean deep sea. Nature 447(7142):307–311. DOI: 10.1038/nature05827.
- Brey T, Dahm C, Gorny M, Klages M, Stiller M, Arntz WE. 1996. Do Antarctic benthic invertebrates show an extended level of eurybathy? Antarctic Science 8(1):3–6. DOI: 10.1017/S0954102096000028.
- Clark A, Johnston NM. 2003. Antarctic marine benthic diversity. Oceanography and Marine Biology: An Annual Review 41:47–114.
- Clarke A, Aronson RB, Alistair Crame J, Gili JM, Blake DB. 2004. Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarctic Science 16(4):559–568. DOI: 10.1017/S0954102004002329.
- Clarke KR, Gorley RN. 2015. Primer v7: User manual/tutorial. Plymouth, UK: Primer-E Ltd. pp. 93.
- De Broyer C, Danis B. 2011. How many species in the Southern Ocean? Towards a dynamic inventory of the Antarctic marine species. Deep Sea Research Part II: Topical Studies in Oceanography Pergamon 58(1–2):5–17. DOI: 10.1016/j.dsr2.2010.10.007.
- Delille D, Guidi LD, Soyer J. 1985. Nutrition of Allotanais hirsutus (Crustacea: Tanaidacea) at Kerguelen Island Antarctic Nutrient Cycles and Food Webb. Berlin Heidelberg: Springer-Verlag. pp. 378–380.
- Esquete P, Bamber RN, Aldea C. 2012. On some shallow-water Tanaidomorpha (Crustacea: Peracarida: Tanaidacea) of Chilean fjords, with description of a new species of Zeuxoides Sieg, 1980. Zootaxa 55(1):38–55. DOI: 10.11646/zootaxa.3257.1.3.
- Frutos I, Brandt A, Sorbe JC. 2016. Deep-sea suprabenthic communities: The forgotten biodiversity. In: Rossi S, Bramanti L, Gori A, Orejas Saco del Valle C, editors. Marine Animal Forests. Cham: Springer International Publishing. pp. 1–29.
- Gallardo VA. 1987. The sublittoral macrofaunal benthos of the Antarctic shelf. Environment International 13(1):71–81. DOI: 10.1016/0160-4120(87)90045-6.
- Golovan OA, Błażewicz-Paszkowycz M, Brandt A, Budnikova LL, Elsner NO, Ivin VV, Lavrenteva AV, Malyutina MV, Petryashov VV, Tzareva LA. 2013. Diversity and distribution of peracarid crustaceans (Malacostraca) from the continental slope and the deep-sea basin of the Sea of Japan. Deep-Sea Research Part II: Topical Studies in Oceanography 86–87:66–78. DOI: 10.1016/j.dsr2.2012.08.002.
- Hansen HJ. 1887. Oversigt over de paa Dijmphna-Togtet indsamlede Krebsdyr. C.F., Dijmphna-Togtets Zoologisk-Botaniske Udbytte. Kjøbenhavn: I Kommission hos H. Hagerup. pp. 183–286.
- Hansen HJ. 1913. Crustacea Malacostraca. The Danish Ingolf-Expedition Printed by Bianco Luno, Copenhagen 3:1–145. https://www.biodiversitylibrary.org/item/40413.
- Hassack E, Holdich DM. 1987. The tubicolous habit amongst the Tanaidacea (Crustacea, Peracarida) with particular reference to deep-sea species. Zoologica Scripta Wiley/Blackwell (10.1111) 16(3):223–233. DOI: 10.1111/j.1463-6409.1987.tb00069.x.
- Havermans C, Sonet G, d’Udekem d’Acoz C, Nagy ZT, Martin P, Brix S, Riehl T, Agrawal S, Held C. 2013. Genetic and morphological divergences in the cosmopolitan deep-sea amphipod Eurythenes gryllus reveal a diverse abyss and a bipolar species. PLoS ONE 8(9):e74218. DOI: 10.1371/journal.pone.0074218.
- Held C. 2003. Molecular evidence for cryptic speciation within the widespread Antarctic crustacean Ceratoserolis trilobitoides (Crustacea, Isopoda). Antarctic Biology in a Global Context, Proceedings 3:135–139 338.
- Held C, Wägele JW. 2005. Cryptic speciation in the giant Antarctic isopod Glyptonotus antarcticus (Isopoda, Valvifera, Chaetiliidae). Scientia Marina 69(S2):175–181. Available: http://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/321/321.
- Holdich DM, Bird GJ. 1985. A preliminary report on ‘Dikonophoran’ tanaids (Crustacea). In: Peuplements profonds du Golfe de Gascogne. Campagnes BIOGAS. Brest, IFREMER. pp. 441–448.
- Ingels J, Vanreusel A, Brandt A, Catarino AI, David B, De Ridder Ch, Dubois P, et al. 2012. Possible effects of global environmental changes on Antarctic benthos: A synthesis across five major taxa. Ecology and Evolution 2 (2):453–85. DOI:10.1002/ece3.96
- Jakiel A, Palero F, Błażewicz M. 2019. Deep ocean seascape and Pseudotanaidae (Crustacea: Tanaidacea) diversity at the Clarion-Clipperton Fracture Zone. Scientific Reports 9(1):17305. DOI: 10.1038/s41598-019-51434-z.
- Jakiel A, Palero F, Błażewicz M. 2020. Secrets from the deep: Pseudotanaidae (Crustacea: Tanaidacea) diversity from the Kuril–Kamchatka Trench. Progress in Oceanography Elsevier 183:102288. DOI: 10.1016/j.pocean.2020.102288.
- Jóźwiak P, Pabis K, Brandt A, Błażewicz M. 2020. Epibenthic sled versus giant box corer – Comparison of sampling gears for tanaidacean species richness assessment in the abyssal benthic ecosystem. Progress in Oceanography 181:102255. DOI: 10.1016/j.pocean.2019.102255.
- Kaiser S, Brandão SN, Brix S, Barnes DK, Bowden DA, Ingels J, Leese F, Schiaparelli S, Arango CP, Badhe R, Bax N, Błażewicz-Paszkowycz M, Brandt A, Brenke N, Catarino AI, David B, De Ridder C, Dubois P, Ellingsen KE, Glover AG, Griffiths HJ, Gutt J, Halanych KM, Havermans C, Held C, Janussen D, Anne-Nina L, Pierrat B, Riehl T, Rose A, Sands CJ, Soler-Membrives A, Schuller M, Strugnell JM, Venreusel A, Veit-Köhler G, Wilson NG, Yasuhara M. 2013. Patterns, processes and vulnerability of Southern Ocean benthos: A decadal leap in knowledge and understanding. Marine Biology 160(9):2259–2317. DOI: 10.1007/s00227-013-2232-6.
- Kudinova-Pasternak R. 1975. Tanaidacea (Crustacea, Malacostraca) from the Atlantic sector of Antarctic and Subantartic. Trudy Instituta Okeanologii, Akademiya Nauk SSSR 103:194–229.
- Kudinova-Pasternak R. 1990. Tanaidacea (Crustacea, Malacostraca) of the southeastern part of Atlantic Ocean and the region to the north off Mordvinov (Elephant) Island. Trudy Instituta Okeanologii, Akademiya Nauk SSSR 126:90–107.
- Kudinova-Pasternak R. 1993. Tanaidacea (Crustacea, Malacostraca) collected in the 43 cruise of the R/V “Dmitri Mendeleev” in the South-western Atlantic and the Weddell Sea Trudy Instituta Okeanologii. Akademiya Nauk SSSR 127:134–145.
- Kudinova-Pasternak R, Pasternak FA. 1978. Deep-sea Tanaidacea, Caribbean Sea, Puerto Rico Trench. Trudy Instituta Okeanologii, Akademiya Nauk SSSR 113:178–197.
- Larsen K. 2005. Deep-Sea Tanaidacea From the Gulf of Mexico. Leiden, Boston: Brill.
- Larsen K, Nagaoka R, Froufe E. 2012. Tanaidacea (Crustacea) from Macaronesia III. The shallow-water Tanaidomorpha from the Cape Verde archipelago. Zootaxa 3498(1):24–44. DOI: 10.11646/zootaxa.3498.1.2.
- Larsen K, Wilson GDF. 2002. Tanaidacean phylogeny, the first step: The superfamily Paratanaidoidea. Journal of Zoological Systematics and Evolutionary Research 40(4):205–222. DOI: 10.1046/j.1439-0469.2002.00193.x.
- Lörz AN, Kaiser S, Bowden D. 2013. Macrofaunal crustaceans in the benthic boundary layer from the shelf break to abyssal depths in the Ross Sea (Antarctica). Polar Biology 36(3):445–451. DOI: 10.1007/s00300-012-1269-1.
- Lowry JK. 1975. Soft bottom macrobenthic community of Arthur Harbor, Antarctica Antarctic research series: Biological and life sciences. In: Biology of the Antarctic Seas; Antarctic Research. Biology of the Antarctic seas. American Geophysical Union. pp. 1–19. https://www.amazon.com/Macrobenthic-Community-Antarctica-Antarctic-Research/dp/0875901239
- McCallum AW, Woolley S, Błażewicz-Paszkowycz M, Browne J, Gerken S, Kloser R, Poore GCB, Staples D, Syme A, Taylor J, Walker-Smith G, Williams A. 2015. Productivity enhances benthic species richness along an oligotrophic Indian Ocean continental margin. Global Ecology and Biogeography Wiley/Blackwell (10.1111) 24(4):462–471. DOI: 10.1111/geb.12255.
- McLelland JA. 2008. A systematic and taxonomic review of the family Pseudotanaidae (Crustacea: Peracarida: Tanaidacea) based primarily on morphometric cladistic analyses. Doctoral Dissertation, University of Southern Mississippi. pp 298.
- Moles J, Wägele H, Uhl G, Avila C. 2017. Bipolarity in sea slugs: A new species of Doridunculus (Mollusca: Nudibranchia: Onchidoridoidea) from Antarctica. Organisms Diversity and Evolution Organisms Diversity & Evolution 17(1):101–109. DOI: 10.1007/s13127-016-0309-z.
- O’Hara TD, Rowden AA, Bax NJ. 2011. A Southern Hemisphere bathyal fauna is distributed in latitudinal bands. Current Biology Elsevier Ltd 21(3):226–230. DOI: 10.1016/j.cub.2011.01.002.
- Pabis K, Błażewicz-Paszkowycz M, Jóźwiak P, Barnes DKA. 2014. Tanaidacea of the Amundsen and Scotia seas: An unexplored diversity. Antarctic Science 27(1):19–30. DOI: 10.1017/S0954102014000303.
- Pabis K, Jóźwiak P, Lörz A-N-N, Schnabel K, Błażewicz-Paszkowycz M. 2015. First insights into the deep-sea tanaidacean fauna of the Ross Sea: Species richness and composition across the shelf break, slope and abyss. Polar Biology Springer Berlin Heidelberg 38:1429–1437. DOI: 10.1007/s00300-015-1706-z.
- Poore GCB, Avery L, Błażewicz-Paszkowycz M, Browne J, Bruce NL, Gerken S, Glasby C, Greaves E, McCallum AW, Staples D, Syme A, Taylor J, Walker-Smith G, Warne M, Watson C, Williams A, Wilson RS, Woolley S. 2015. Invertebrate diversity of the unexplored marine western margin of Australia: Taxonomy and implications for global biodiversity. Marine Biodiversity Springer Berlin Heidelberg 45(2):271–286. DOI: 10.1007/s12526-014-0255-y.
- Raupach MJ, Mayer C, Malyutina M, Wägele J-W. 2009. Multiple origins of deep-sea Asellota (Crustacea: Isopoda) from shallow waters revealed by molecular data. Proceedings of the Royal Society B: Biological Sciences 276(1658):799–808. DOI: 10.1098/rspb.2008.1063.
- Riehl T, Kaiser S. 2012. Conquered from the deep sea? A new deep-sea isopod species from the Antarctic shelf shows pattern of recent colonization. PLoS ONE 7(11):36–38. DOI: 10.1371/journal.pone.0049354.
- Sands CJ, O’Hara TD, Barnes DKA, Martín-Ledo R. 2015. Against the flow: Evidence of multiple recent invasions of warmer continental shelf waters by a Southern Ocean brittle star. Frontiers in Ecology and Evolution 3. DOI: 10.3389/fevo.2015.00063/abstract.
- Segadilha JL, Araújo-Silva CL. 2015. Two new species of Tanaopsis (Tanaidacea: Tanaopsidae) from Admiralty Bay (Antarctica), with an identification key. Nauplius 23(1):31–45. DOI: 10.1590/S0104-64972015002315.
- Segadilha JL, Dos Santos KC, Serejo CS. 2017. Two new species of Colletteidae (Crustacea: Tanaidacea: Tanaidomorpha) from Bransfield Strait, Antarctica. European Journal of Taxonomy. 1–16. Available: http://www.europeanjournaloftaxonomy.eu/index.php/ejt/article/view/489.
- Shiino SM. 1978. Tanaidacea collected by French scientists on board the survey ship “Marion-Dufresne” in the regions around the Kerguelen Islands and other Subantarctic islands in 1972, ’74, ’75, ’76. Shima Marineland. pp. 797–798.
- Siciński J, Jażdżewski K, De Broyer C, Presler P, Ligowski R, Nonato EF, Corbisier TN, Petti MAV, Brito TAS, Lavrado HP, Błażewicz-Paszkowycz M, Pabis K, Jażdżewska A, Campos LS. 2011. Admiralty Bay Benthos Diversity - A census of a complex polar ecosystem. Deep-Sea Research Part II: Topical Studies in Oceanography 58(1–2):30–48. DOI: 10.1016/j.dsr2.2010.09.005.
- Sieg J. 1977. Taxonomische Monographie der Familie Pseudotanaidae (Crustacea, Tanaidacea). Mitteilungen aus dem Museum für Naturkunde in Berlin. Zoologisches Museum und Institut für Spezielle Zoologie (Berlin) Wiley-Blackwell 53(1):3–109. DOI: 10.1002/mmnz.19770530102.
- Sieg J. 1983a. Tanaidomorpha (Crustacea: Tanaidacea) from the Ross Sea, Antarctica. Journal of the Royal Society of New Zealand 13(4):395–418. DOI: 10.1080/03036758.1983.10420805.
- Sieg J. 1983b. Evolution of Tanaidacea. In: Schram FR, editor. Crustacean phylogeny. Rotterdam: A.A. Balkema. pp. 229–256.
- Sieg J. 1984a. Tanaidacea of the United States Navy’s 1947-1948 Antarctic Expedition (Crustacea). Journal of Crustacean Biology 4(2):298–306. DOI: 10.2307/1548027.
- Sieg J. 1984b. Neuere Erkenntnisse zum natürlichen System der Tanaidacea. Eine phylogenetische Studie. Zoologica 136:1–132.
- Sieg J. 1986a. Tanaidacea of the Antarctic and Subantarctic 1. On Material collected at Tierra del Fuego, Isla de los Estados, and the West Coast of the Antarctic. Antarctic Research Series 180. American Geophysical Union.
- Sieg J. 1986b. Tanaidacea (Crustacea) von der Antarktic and Subantarktis. II. Tanaidacea gesammelt von Dr. J. W. Wägele während der Deutschen Antarktis Expedition 1983. Mitteilungen aus dem Zoologischen Museum de Universität Kiel II. Zoologisches Museum der Christian-Albrechts-Universität zu Kiel. pp. 1–80.
- Sieg J, Heard RW. 1988. Tanaidacea (Crustacea: Peracarida) of the Gulf of Mexico. V. The family Pseudotanaidae from less than 200 m, with the description of Pseudotanais mexikolpos, n.sp. and a key to the known genera and species of the world. Proceedings of the Biological Society of Washington 101:39–59.
- Stepanjants S, Cortese G, Kruglikova S, Bjørklund K. 2006. A review of bipolarity concepts: History and examples from Radiolaria and Medusozoa (Cnidaria). Marine Biology Research 2(3):200–241. DOI: 10.1080/17451000600781767.
- Stępień A, Pabis K, Błażewicz M. 2019. Tanaidacean faunas of the Sea of Okhotsk and northern slope of the Kuril-Kamchatka Trench. Progress in Oceanography Pergamon 178:102196. DOI:10.1016/j.pocean.2019.102196.
- Thatje S, Hillenbrand CD, Larter R. 2005. On the origin of Antarctic marine benthic community structure. Trends in Ecology and Evolution 20(10):534–540. DOI: 10.1016/j.tree.2005.07.010.
- Vanhöffen E. 1914. Die Isopoden. In: Von Drygalski E, editor. Deutsche Südpolar-Expedition 1901-1903 im Auftrage des Reichsamtes des Innern. Berlin: Druck und Verlag von Geotg Reimer. pp. 1–154.