?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Despite its broad range and high abundance, nest sites of the European robin Erithacus rubecula, a small forest passerine, are rarely characterised, and most studies refer to human-transformed habitats. In this paper, we describe the species’ nest sites in the natural conditions of a primeval forest. We also investigate robin preferences to address nest site selection which can only be assessed if the availability of potential nest sites is known. We studied robins’ nest sites in Białowieża National Park, eastern Poland, on three study plots (~30 ha each) in 2016–2019. Among all nests found (n = 165), the majority (118, ~72%) were found in tree holes, followed by ground nests (25, ~15%) and root-soil plates (22, ~13%). When the availability of cavities and root-soil plates is accounted for, it appears that robins preferred tree holes over plates. Robins appeared to be weakly selective in respect to tree species, preferring hornbeam Carpinus betulus, small-leaved lime Tilia cordata and Norway spruce Picea abies over other trees. Since both cavities and root-soil plates are superabundant in the Białowieża Forest, but scarce or absent in the heavily transformed robin breeding habitats of Western Europe (parks, orchards, hedgerows or managed forests), ground-nesting may predominate there simply due to these choice limits. In summary, our results provide a basis for understanding how original nest-site choices that evolved in natural, unmodified habitats can change when a species adapts to human-transformed habitats.
Introduction
The European robin Erithacus rubecula is small (c. 19 g), widespread and one of the most numerous forest passerines in Europe and Western Asia (Glutz Von Blotzheim & Bauer Citation1988; Cramp & Perrins Citation1993; Hegemeijer & Blair Citation1997). Despite its wide range and high abundance, surprisingly little is known of its breeding biology (Lack Citation1946, Citation1948, Citation1965a; Harper Citation1985; Cramp & Perrins Citation1993; Prokofeva Citation2006; Lebedeva & Lomadze Citation2007; Knysh Citation2008; Zimin Citation2009; Baranovskiy & Ivanov Citation2017). A search in the Web of Science databases (www.webofscience.com) for terms such as “European robin” plus “nest” in “all fields” resulted in just 27 records for European robin, while the same search for other numerous and frequently studied cavity nesters resulted in 5–26× more records (blue tit Cyanistes caeruleus – 362, great tit Parus major – 717, pied flycatcher Ficedula hypoleuca – 411, collared flycatcher Ficedula albicollis – 139). In particular, few studies deal with robins’ natural nesting places (Prokofeva Citation2006; Knysh Citation2008; Zimin Citation2009; Baranovskiy & Ivanov Citation2017), and all of them come from Russia or Ukraine and are not available in English. Nearly half of the published works refer to the British population (E. r. melophilus), which inhabits mostly human-transformed environments. In contrast to continental, nominate E. r. rubecula, British robins are mostly sedentary and highly synanthropic (Lack Citation1946, Citation1948, Citation1965a; Harper Citation1985; Cramp & Perrins Citation1993). The paucity of research related to the European robin’s nest sites may be due to its highly secretive behaviour during its nest building phase and difficulties in finding its nests other than nest-boxes (Cramp & Perrins Citation1993; Lebedeva & Lomadze Citation2007; Zimin Citation2009).
Robins show a prominent level of plasticity in nest site choice, both in forests and in anthropogenic environments (Cramp & Perrins Citation1993; Zimin Citation2009; Baranovskiy & Ivanov Citation2017). In Western Europe, where they occur in the vicinity of humans – in gardens, yards or midfield hedges – nests are most often located on the ground, under vegetation cover and in various structures such as nest boxes, postboxes, old buckets, shoes, cracks in buildings, under windowsills, in piles of stones, and even in a dead cat or human skull (Lack Citation1965a; Glutz Von Blotzheim & Bauer Citation1988; Schmidt Citation1990). In forest environments, they build nests across the full range of niches and heights, ranging from ground level to the tree crowns, including tree holes (Zimin Citation2009; Baranovskiy & Ivanov Citation2017). A striking feature of all robins’ nests is their perfect hiding (Zimin Citation2009).
Studies aiming to investigate nest site selection in natural conditions should be performed in unmodified environments (e.g. Lack Citation1965b; Tomiałojć et al. Citation1984, Wesołowski Citation2007a). Unfortunately, most of the data on robins’ nests comes from human-transformed environments, including forests with long-term intensive management. The range of potential nesting niches is strongly limited there, which affects the choice of breeding sites and increases both inter- and intra-specific competition (Bruns Citation1960; Newton Citation1998). In such environments it is hardly possible to discover the robins’ adaptations to the primeval forest conditions, where they evolved (e.g. Lack Citation1965a; Harper Citation1985; Prokofeva Citation2006; Knysh Citation2008). There are very few areas in lowland Europe where such unmodified conditions still exist. Perhaps the best known among them is the Białowieża Forest in eastern Poland, in particular its strictly protected area (Białowieża National Park, hereafter BNP) (Faliński Citation1968; Bobiec Citation2002), representing the last fragment of European primeval lowland temperate forest. This area provides a unique opportunity to study the ecology and behaviour of organisms under conditions that prevailed in European forests before their strong transformation by humans (Wesołowski Citation1983; Tomiałojć et al. Citation1984; Tomiałojć & Wesołowski Citation1990, Citation2005, Wesołowski Citation2007a).
To fill the gap in our knowledge on robins’ nesting places, in this paper we characterise nest sites of European robins breeding in natural conditions. Apart from fulfilling these descriptive purposes, we assess species nest site preferences for the first time in two main types of broad-leaved temperate forests in the BNP: ash–alder riverine and lime–hornbeam stands, differing in vegetation structure and the availability of various nesting niches. We discuss differences in patterns observed between these habitats and compare our results with other studies.
Materials and methods
Study area
The Białowieża Forest is located in north-eastern Poland on the Polish–Belarusian border (coordinates of the Białowieża village: 52°42′04″N, 23°52′00″E). It is situated in the transition zone between boreal and nemoral forests. The best-preserved parts of the Białowieża Forest have been under strict protec-tion since 1921 within BNP. In 1992, it was inscribed on the United Nations Educational, Scientific and Cultural Organization World Heritage List (Jaroszewicz et al. Citation2019).
The unique, well-preserved stands of the strict reserve are a remnant of the vast forests that once covered most of Europe. Old-growth stands of BNP are highly diverse, in both species and structure, with superabundant dead wood (Faliński Citation1968; Falińska Citation1991; Wesołowski et al. Citation2006; Wesołowski Citation2012). The trees reach large sizes and ages (up to few hundred years). The tallest are Norway spruce Picea abies, which can reach 57 m in height, while the thickest are pedunculate oak Quercus robur, reaching up to 230 cm in diameter at breast height (DBH) (Faliński Citation1977; Falińska Citation1991). Other organisms are also present in a variety that is unique in Europe. Species communities and relations between them and the environment have been well preserved (Wesołowski Citation2012). Studies conducted on other bird species show that tree holes occur in excess in the strict BNP reserve (Walankiewicz Citation2002, Wesołowski Citation2007a, Wesołowski & Martin Citation2018). All of this makes the strict BNP reserve a unique place for studying avian nest site preferences as its forest stands are currently free from direct human activity.
Field methods
Study plots
The research was conducted from 2016 to 2019. Fieldwork was carried out during the species’ breeding season: from the third week of March to the end of June each year. Three sample plots, each about 30 ha, were designated within larger permanent census plots established in 1975 for bird monitoring (Tomiałojć et al. Citation1984), with no or little human activity both in the forest stands and around them. One plot, designated plot K, was located in the ash–alder riverine forest Fraxino–Alnetum and bordered open, currently unmanaged tussocky meadows in the Narewka River valley at the edge of BNP. The two other plots comprised lime–hornbeam forest and were located either on the edge of the strict nature reserve (plot W, bordering meadows, partly mowed once a year) or ~3 km inside the forest (plot M). A detailed description of the plots can be consulted in e.g. Tomiałojć and Wesołowski (Citation1996) or Wesołowski et al. (Citation2015).
Nest site characteristics
Each year, from the earliest arriving birds,singing males and – later in the season – pairs were mapped on all three plots following a combined territory mapping approach (Tomiałojć Citation1980) every 2 days. Additionally, attempts to mist-net and colour-ring male robin were performed early in the season, and at the stage of nest building some 65% of males present were colour-ringed. Both these approaches were helpful in recognising the number of territories and delimiting their borders before the birds started nest building. Then, we searched for the robins’ nests exclusively by direct observation within the territories: by following adult, paired birds carrying building material or food to the chicks. Nests were found in 85% of the intensively searched territories on average (range 75–95%, depending on the plot and year), so the results are unlikely to be biased much, given that very few nests were missed. We classified a place as a nest site if a nest with at least one egg was found. After a nest was found, it was monitored at 5-day intervals until the young fledged or a nest loss occurred. During 2017 and 2018 all the nests were monitored with a camera-trap. Inspections of nests located up to 2.5 m above the ground were made with a flashlight and a mirror. A camera on an extension arm was used for the higher-placed nests in the tree holes. It was possible to check and monitor places up to approximately 10 m above the ground this way, and in a few higher locations the trees were climbed with spurs. Exact locations of all the nests were established using a Garmin 62s Global Positioning System.
Several measurements were recorded for each nest in a cavity: forest type (ash–alder vs lime–hornbeam forest), tree species, height of the nest location above the ground, tree condition (alive, dead or dying), tree tilt, DBH and cavity origin (woodpecker-made or other). After young fledged or a brood loss was recorded, the nest site was described with further measurements. The collected data differed for cavity and root-soil plates due to their different nature. The height and width along with the shape of the entrance of the hole were noted as well as the diameter of the tree under the hole. Hole types reflecting their origin were adopted after Wesołowski and Martin (Citation2018), with five categories: round, scar, fissure, rot hollow and bulb. The holes were measured inside by their depth with the nest inside and the total depth (after removing the nest). Accessibility of a nest for predators was measured as the distance between the nest and the edge of the entrance (the so-called “danger distance”) (Wesołowski Citation2002).
The description of the root-soil plates was different. Their maximum height and width were measured, and the tree species was noted. A five-grade decomposition scale was used to assess the degree of log decomposition (Brown Citation1974). To describe the potential suitability of the root-soil plate for birds, the presence of roots, soil and vegetation was assessed according to three more scales: root decay level (RDL) – 0 (0–25%), 2 (51–75%), 3 (76–100%), 4 (present but covered by soil); soil coverage degree (SCD) – 0 (old compacted soil), 1 (<5%), 2 (<25%), 3 (<50%), 4 (<75%), 5 (>75%); plant cover degree (PCD) – 0 (0–25%), 1 (26–50%), 2 (51–75%), 3 (76–100%). Due to the large variety in form and the absence of any regular concealment boundaries, it was impossible to record additional information on ground nests.
Statistics
One could reasonably assume that most traits of nest sites can be described by a normal distribution. However, since there were frequent outliers in the data, we simply describe them as they are to provide basic descriptive statistics. Where necessary, non-parametric methods (e.g. the bootstrap; Efron & Tibshirani Citation1998) not requiring assumptions in respect to underlying distributions were used to compare means. The general additive mixed models (GAMMs) used to estimate the mean number of holes for a given tree species in each DBH class included counts as a response, treated as having a Poisson distribution; these are described below.
Nest site selection
We assessed nest site and tree species preferences of robins using Ivlev’s electivity index (Ivlev Citation1961). This method characterises the degree of selection (or electivity), given the proportions of objects present (available) to those actually used according to the equation
where ri is the relative abundance (i.e. proportion among all present) of objects of a given type available and pi is the proportion of objects used (among all used). The index has a straightforward interpretation: it takes values from the [–1, 1] range, with negative values indicating avoidance, positive indicating preference and zero indicating no selectivity (i.e. the use of a resource is proportional to its availability). In our case, ri’s were relative abundances of tree holes and root-soil plates present on the plots, while pi’s were proportions of robins’ nests in each nest site type. In the case of tree holes and selectivity for tree species (all plots combined), ri’s were estimates of the tree holes in each tree species, and pi’s reflected the proportions of nests in a given tree species. With nest sites, only selectivity for cavities and root-soil plates could be assessed, despite robins also having nested on the ground (it is impossible to assess the relative abundance of the ground, so ground nests were excluded from this analysis). Root-soil plates were fully censused and mapped in 2019 across all plots, totalling 1878, 266 and 413 plates at K, M and W plots, respectively, so that there is no uncertainty associated with these numbers. However, the number of cavities had to be estimated with survey sampling methods. We estimated the number of available tree holes in three steps: first, we estimated the number of trees of the eight most common species on each of the three plots for each of the 11 DBH (size) classes (see Estimation of tree abundance below). The mean number of holes per tree of a given species and for a given DBH class was estimated separately with a GAMM, using data from the Kapusta (Citation2019) survey (see Estimation of cavity numbers per tree below). The total abundance of tree holes on a given plot (or across the plots in each tree species in a given DBH class) potentially available to birds is, then, the product of the number of trees from each species-and-diameter combination on that plot times the number of cavities per tree in a given species-and-diameter combination. Both the number of trees of a given species in a given DBH class and the number of tree holes in that species-and-diameter class are estimates and have associated uncertainty measures. We addressed uncertainty in both these estimates with a resampling (bootstrap) technique (Efron & Tibshirani Citation1998) to obtain distributions of desired quantities and to fully propagate both sources of uncertainty into Ivlev’s electivity index (EI). Therefore, our EIestimates became distributions as well, which allowed us to estimate and report any uncertainty associated with the index itself. Calculation of EI’s was performed with the selectapref package (Jason Citation2020), built into a self-written loop over bootstrap resamples to estimate variation, in R 3.6.3 (R Core Team Citation2019).
Estimation of tree abundance
To obtain the number of trees on a plot, we used data from a survey-sampling of trees, performed in our study plots in 1999 (plot K), 2015 (plot W) and 2015–2016 (plot M). Censuses were performed on squares within the 50 × 50 m grid, chosen at random and covering the whole square area (0.25 ha) (Walankiewicz et al. Citation2007; Stański et al. Citation2021). At plot K, 32 squares were sampled this way (total area surveyed 8 out of 33.5 ha) totalling 5000 individual trees, while the respective numbers at W and M were 24 and 16 squares (6 and 4 ha surveyed, out of 35 and 30 ha, respectively), totalling 5632 and 2847 individual trees, respectively. Within each square, all trees with DBH ≥ 1 cm were counted and their DBH values measured, providing data on species and age composition of the tree stands at the time of survey. Original, continuous DBH values were then simplified into 11 classes (1–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 71–80, 81–90, 91–100 and ≥101 cm). We used bootstrapping and resampled the datasets from each plot 1000 times to obtain distributions of abundance of trees of a given species and in a given DBH class on a given plot, from which medians, interquartile ranges and 95% intervals were extracted as summary statistics. Although we bootstrapped the complete datasets (i.e. including trees too thin to bear holes suitable for robins), only trees with DBH ≥ 13 cm (the smallest diameter of a tree with a robin’s nest in a tree hole) were considered in further calculations.
Estimation of cavity numbers per tree
Kapusta (Citation2019) surveyed cavity abundance at 45 points (each 0.05 ha) on three sample plots on BNP, where all trees with DBH ≥ 10 cm and/or 130 cm height were measured and searched for cavities. In total, 107 cavities were found in 84 trees, with the minimal size limits for a cavity to be considered available for small, passerine cavity nesters set at 7 cm bottom diameter (sufficient to fit the nest of a small bird) and a 2.5 cm entrance width. These criteria fit the smallest-size cavities occupied by robins as found in our study (minimal entrance diameter 2.3 cm, minimal inside/bottom diameter 5.0 cm), which means that occasionally robins are able to occupy cavities even smaller than those classified by Kapusta (Citation2019) as “available to birds”. In turn, the possible number of cavities being available for robins may be a bit higher than found by Kapusta (Citation2019), since some of the smallest ones might have been omitted, but this difference is unlikely to be large and we are unable to correct it in any way. We used these data to estimate the number of cavities per tree with Poisson GAMM, including tree species as a fixed factor (with eight levels), tree diameter expressed as the number (1–11) as described above and modelled with a smooth function, and a plot random effect (see Table S1). We again used non-parametric bootstrapping (1000 resamples) of the original dataset and fitted GAMM to each resample. The predicted numbers of cavities per tree in a given DBH class were obtained with the predict function from the models fitted to each resample. Medians, along with 95% confidence intervals, were then extracted from the distribution of predicted values (Figure S1).
Estimation of total number of cavities
Distributions of the number of trees of a given species–diameter combination and the number of cavities per tree were multiplied to obtain the final quantity: the expected distribution of the total number of cavities on a plot or in a given tree species. EI was also used to assess preferences for the seven most common tree species, using the above-described approach (Tables S2–S4).
Results
Nest types
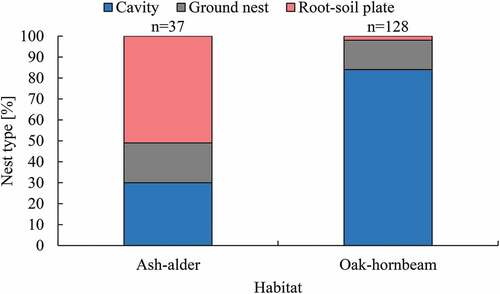
Three types of robin’s nests were recognised in BNP: ground nests, cavity nests and root-soil plate nests. Cavities were the most numerous nest type found in lime–hornbeam habitat (plots M and W), and the second most numerous in the ash–alder plot (K) (). The majority of robins’ nests in root-soil plates were found in ash–alder forest, while they were less numerous in lime–hornbeam. Ground nests were the least represented nest type in both forest types. Differences in nest-type proportions between the two habitats were significant (χ2 = 66.09, df = 2, P < .001).
Ground nests
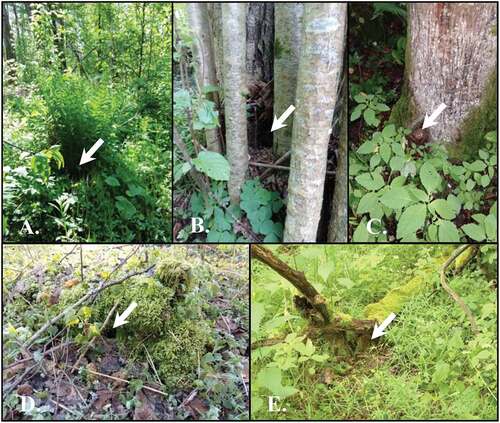
Due to both the difficulties in characterising ground nests by measurements and their small number (n = 22), we only characterised these nests descriptively. Five types of ground nests could be recognised. The first (n = 2) type that could be distinguished were the ones built in clumps of grass and fern. These were very well-hidden nests, invisible to human eyes from a distance of 2 m. In this case, robins utilised the space between grass stalks and fallen grass leaves ()). “Hazel nest” was the second (n = 5) type of ground nest – these nests often had no top cover and were tightly fitted, low over the ground, between hazel trunks. All such nests were built low at the base of trunks ()). Also, five ground nests were found in root crowns of several tree species. Nests of this type were usually located within a root crown, close to or just under the main trunk overhang and more or less hidden ()). The most numerous ground nest type (n = 6) was the “rotten stump” nest, localised under the overhang of highly decomposed deadwood, most often in the form of a stump ()). Finally, the last type was found integrated into the litter under fallen branches. In these nests (n = 5), the branch formed a cover and roof for the nest ()).
Nests in tree holes
The majority of robins’ nests were found in non-excavated cavities, mostly in living trees (). Robin cavity nests were found in 11 tree species. The greatest number of cavity nests were situated in hornbeams in lime–hornbeam habitats and in alders in ash–alder forest –these tree species predominate in their respective habitats, suggesting that robins are opportunistic and may simply occupy cavities in the most abundant trees (). However, when the availability of cavities in a particular tree species is considered, it appears that there are clear preferences for Norway spruce, hornbeam and lime, all with positive Ivlev’s electivity indices, while no avoidance is evident ().This might, however, be due to extremely small sample sizes in some cases resulting in wide uncertainty around our EI estimates.
Table I. Robins’ nest cavity type proportions (%) relative to the vitality of the nesting tree, and the cavity creation process. n - sample size
Figure 3. Location of European robins’ nests in different tree species in ash–alder and oak–hornbeam habitats
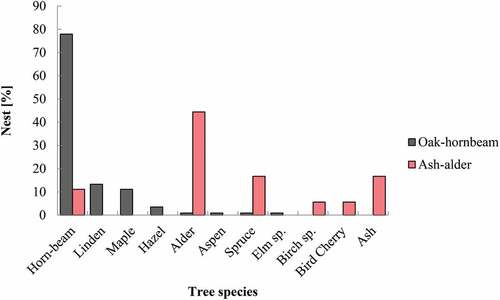
Table II. Robin’s nest site selection as measured with Ivlev’s electivity indices for the eight tree species, the most common in the studied plots (data combined for all three plots), in which 94% of all robin’s nests in cavities were found (n = 132). Availability (total) and nest numbers (used) are given. Totals represent the median estimated number of cavities (25–75% interquartile range in parentheses) in all trees of a given species with DBH ≥13 cm and age structure considered (see Methods). EI is the Ivlev electivity index, while P preferred is the probability that EI is positive (i.e. the proportion of estimates >0). No nests were found in oaks, so the EI is −1 by default (complete avoidance)
Table III. Characteristics of the European robin cavity nests in Białowieża National Park in 2016–2019
In BNP, robins occupied cavities highly variable in size, in respect to both the entrance and the inside. The smallest diameters of the entrance ranged from 2.3 to 17.0 cm (median 5.0 cm), whereas the greatest diameters varied from 5.0 to 73.0 cm (median 14.0 cm) ().
Table IV. Characteristics of robin’s nests in root-soil plates in the BNP
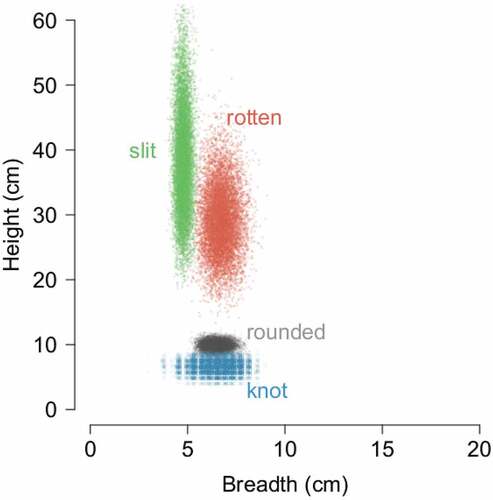
The most common hole entrance shape found during this research was a slit hole, with a narrow and tall entrance. Robins also built their nests in rot holes with irregular shape, probably mostly created by fungi in the wood decay process. A marginally lower share of robin nests were round holes, and these included mainly old woodpecker holes or perfectly round non-excavated cavities. Other types of cavity entrance shapes constituted less than 10% of the total: knotholes (where a branch was detached in a living tree, Wesołowski & Martin Citation2018) and crack holes – an opened scar after frost created a scratch and chimney – i.e. the top of a broken tree. Entrance sizes (height and breadth) were clearly associated with hole origin ().
Figure 4. Height and breadth of robins’ nesting tree hole entrance in ash–alder and lime–hornbeam forest. Points show means estimated at individual bootstrap resamples of the original data to visualise variation in both dimensions
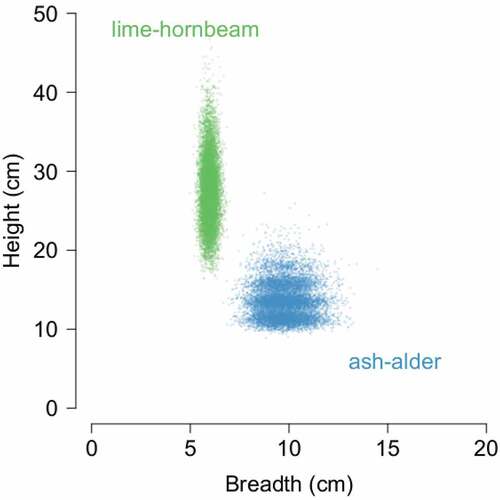
Figure 5. Height and breadth of robin’s tree hole entrances in the three most common tree nesting species. Points show means estimated at individual bootstrap resamples of the original data to visualise variation in both dimensions
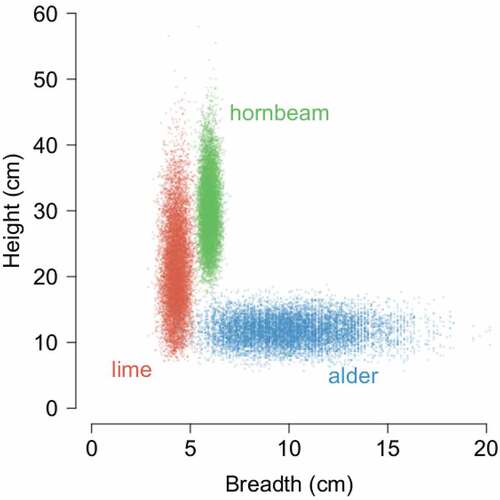
Figure 6. Height and breadth of robin’s tree hole entrances in relation to the configuration of the hole. Points show means estimated at individual bootstrap resamples of the original data to visualise variation in both dimensions
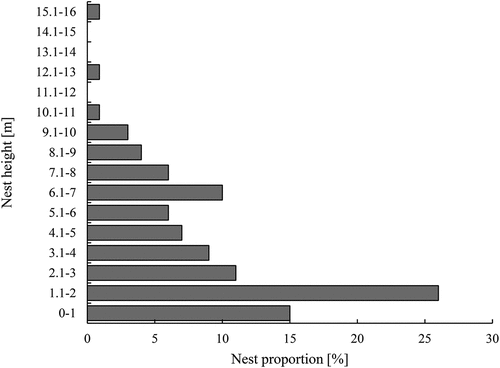
European robins occupied cavities located between 0.1 and 16 m above the ground. The majority of robins’ nests in cavities (71% of all nest types) were located low, up to 2 m above the ground ().
Root-soil plate nests
European robins’ nests in root-soil plates were well hidden among clods of soil and tangled roots. Root-soil plates chosen by robins to build nests strongly varied in size: lengths ranged from 60.0 to 670.0 cm (median 245.0 cm), whereas heights ranged from 42.0 to 370.0 cm (median 130.0 cm). Nests in root-soil plates were almost always located in recesses – i.e. places resembling a tree hole. However, birds were able to construct an “entrance tunnel” with rotten leaves and moss, creating a cavity-like structure. There were only few cases (n = 4) where robins built semi-open nests in plates (unlike cavities). The dimensions of root-soil plate nests differed from those of nests in cavities (). The mean entrance area was larger in cavities, but the danger distance was ~5 cm greater in comparison to root-soil plate nests ().
Nest site preference
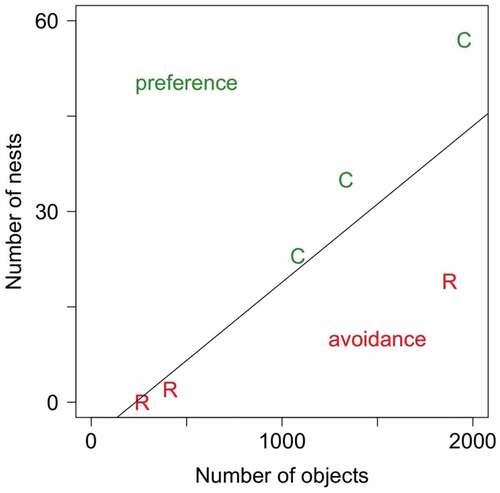
There was a significant difference in the frequency of hole-nesting vs root-nesting robins between habitats. In the two lime–hornbeam plots (M and W), 0 of 25 and 2 of 59 nests (0% and ~3.5%), respectively, were on root-soil plates, while the remaining pairs nested in tree holes. At the K plot (riverine), 19 nests were on root-soil plates (~45%) and 23 in cavities. These numbers did not follow the available number of either nest site type, suggesting significant nest site selection: in riverine forest robins preferred cavities (Ivlev’s index of 0.24 indicates their more intense use than expected given their availability in the forest) and avoided roots (Ivlev’s index −0.13; used less frequently than expected given availability), but both these trends were rather weak. In lime–hornbeam forests (M and W) these patterns were much stronger: roots were strongly avoided (Ivlev’s indices of −1 and −0.80; either not used at all or used much less frequently than expected given their availability), while cavities were moderately preferred (Ivlev’s indices of 0.55 and 0.51) (; ).
Table V. Robin’s nest site selection measured as Ivlev’s electivity indices for the two nest site types (cavities vs root-soil plates) at the three plots. For both nest site types, availability (total) and nest numbers (used) are given. Totals for roots come from a complete census, are estimates for cavities (see Methods) which include all trees with DBH ≥13 cm (95% confidence intervals in parentheses). EI – Ivlev’s electivity index. No nests were found in roots at M so the EI equals −1 by default (complete avoidance)
Figure 8. Relationship between the number of robins’ nests in root-soil plates (R) and cavities (C) and the number of available objects for the three plots. Green points above the line (1:1 relationship indicating usage strictly proportional to nest site type availability) indicate preference (more frequent usage than expected) while red points below the line indicate avoidance (less frequent usage than expected)
Discussion
Robins are often classified as a nest site opportunist or ground nester (Cramp & Perrins Citation1993; Prokofeva Citation2006; Knysh Citation2008) or a facultative cavity-nester (Wesołowski Citation2007a, Wesołowski & Martin Citation2018; Zawadzka Citation2018). Our results suggest that this species prefers to nest in cavities, rather than in other places imitating holes. The results suggest a specific category for this species: “flexible cavity nester”, since robins facultatively build nests in other places, but the most preferred nesting places are still cavities (). Our studies in Białowieża Forest also indicate that this species is extremely timid in the breeding season. A female robin may put nest material in a different place to mislead an intruder if she is feeling observed, or she could drop nest material from her bill and, for example, start foraging. Sometimes this species can build a nest in just a few hours (Lack Citation1965a; Glutz Von Blotzheim & Bauer Citation1988), which makes search much more difficult as there is less time to follow birds carrying nest material.
Ground nests
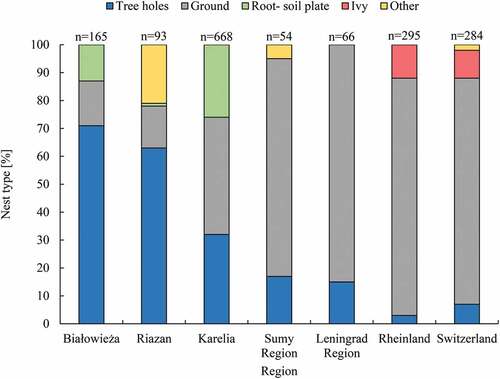
Among the three types of robins’ nests, the most difficult to characterise were ground ones due to their wide variety (). Results similar to these presented in our study from Białowieża NP were obtained by Zimin (Citation2009) in Karelia (taiga zone, NW Russia), Prokofeva (Citation2006) in the St. Petersburg region (NW Russia), Knysh (Citation2008) in a forest-steppe zone (E Ukraine) and Baranovskiy and Ivanov (Citation2017) in Riazan region in suburban, managed forests and a city area (central European Russia). Apart from nests similar to ground nest types as found in the Białowieża NP (a variety of rotten stumps, clumps of plants, root crowns and nests among roots and branches, under litter or old leaves) these authors also describe ground nests as follows: nests in walls of steep and low slopes, in small dimples and under fallen trunks. Contrary to our study, in many regions the most numerous robins’ nest type was ground nests ().This may be a result of low availability of tree holes, as in the Russian Karelia taiga forest (Zimin Citation2009), in managed forest in the Russian Leningrad Region (Prokofeva Citation2006) or Sumy Ukraine region (Knysh Citation2008) and in anthropogenic habitats in Switzerland (Glutz Von Blotzheim & Bauer Citation1988). Low availability of suitable tree holes could also affect European robin nest site choice in Great Britain, where studies were carried out mostly in university gardens and hedges (Lack Citation1965a). Zimin (Citation2009) stresses the problem with searching tree-hole nests: finding ground nests is much easier than finding nests high in the trees.
Figure 9. Proportion of nest types of European robin in different regions of Europe. Data sources: (Riazan – Baranovskiy & Ivanov Citation2017, Karelia – Zimin Citation2009, Sumy region – Knysh Citation2008, Leningrad Region – Prokof’eva Citation2006, Rheinland – Mildenberger Citation1984, and Switzerland – Glutz Von Blotzheim & Bauer Citation1988)
Importance of root-soil plates
The root-soil plate robins’ nests in Białowieża NP were most often found in ash–alder habitats. In spite of such results, cavities were still the most preferred nest site in both habitats (ash–alder and oak–lime–hornbeam forest). Nests were situated mostly in the low, ground part of the root plate, but also on the side, and in the turfy back of the root plate. Robins nesting in such places have also been reported from Karelia (Zimin Citation2009), Riazan region (Baranovskiy & Ivanov Citation2017) and SE Poland (Zabłocie reserve – oak–hornbeam forest) (Wojton & Pitucha Citation2020). In taiga, root-soil plates seem to play a significant role as a nesting place for robins (26% of all nests), perhaps because tree holes are much less abundant, but still, plates were the least used nesting location (Zimin Citation2009) (). Root-soil plates in Riazan region were used by robins as nesting places only in 1.1% of cases (Baranovskiy & Ivanov Citation2017). In robins of SE Poland, 2.1% out of over 180 searched root-soil plates were occupied. Few studies have addressed the importance of root-soil plates as nesting places for birds, especially for the wren Troglodytes troglodytes, which builds its nests most often in these structures (Wesołowski Citation1983; Tomiałojć & Wesołowski Citation2005; Czeszczewik & Walankiewicz Citation2016; Wojton & Pitucha Citation2020). Our study shows for the first time that robins choose root-soil plates for nesting, particularly where these are superabundant, but they are used less frequently than would be expected given their availability.
Robins as a cavity nester
Indisputably, in both studied habitats, the most preferred nest location for European robin in the Białowieża NP were tree holes. Białowieża Forest offers a vast variety of available tree holes (e.g. Tomiałojć & Wesołowski Citation2004; Wesołowski Citation2011, Citation2012; Walankiewicz et al. Citation2007). The long-term monitoring studies of cavity fates by Wesołowski (Citation2007a, Citation2007b) leaves little doubt that competition might be missing as an important mechanism here. There are many more cavities than cavity-nesters in Białowieża Forest, and the former are extremely variable. The birds do not compete for cavities in this forest. In contrast to most managed forests, conditions in Białowieża are strikingly different – so much so that mechanisms widely recognised as limiting may not act here.
The preference is therefore expected, since cavities are most likely the safest breeding place in the forest (Wesołowski & Tomiałojć Citation2005; Wesołowski & Martin Citation2018). Nests of European robin in the Białowieża NP were mostly located in slit tree-holes (cavities with a long vertical diameter and a short horizontal diameter). Similarly to our results, other authors have identified many nest-cavity types like those presented in our study (Zimin Citation2009; Baranovskiy & Ivanov Citation2017).
The only studies reporting a substantial proportion of robins’ nests in cavities were found in the Riazan region, Russia (~65%, Baranovskiy & Ivanov Citation2017, compared to ~72% in the Białowieża NP, this study). Those authors emphasise the importance of cavities as nesting places for robins in that area. All other studies from continental Europe reported much lower percentages (1–30%) of all nests in cavities (), which may simply stem from their unavailability as a result of forest management. There are no published data on measurements of cavities occupied by robins, and the only information we could find was reported in collective studies (Glutz Von Blotzheim & Bauer Citation1988; Cramp & Perrins Citation1993).
Figure 10. Cavity dimensions occupied by European robin in comparison to other cavity-nest species in BNP (ER –Erithacus rubecula; PP – Poecile palustris; SE – Sitta europeae; FH – Ficedula hypoleuca; FA – Ficedula albicollis; PM – Parus major; PE – Cyanistes careuleus) (Wesołowski Citation1996, Czeszczewik & Walankiewicz Citation2003; Wesołowski & Rowiński Citation2004, Citation2012; Walankiewicz et al. Citation2007; Maziarz Citation2012). Medians shown (cm)
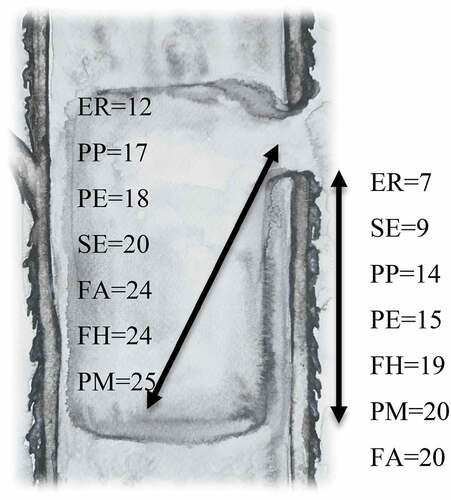
Cavity nesters have evolved many ways to minimise predation risk. For example, nuthatches Sitta europaea choose voluminous cavities, which makes it harder for a predator to reach for chicks, and they cover the entrance with clay and mud to reduce its size (Wesołowski & Rowiński Citation2004; Rowiński Citation2013). Titmice Paridae and flycatchers Muscicapidae choose narrow cavities with a relatively small entrance; predators have more difficulty accessing such holes (Wesołowski Citation2002; Czeszczewik & Walankiewicz Citation2003; Maziarz Citation2012; Wesołowski & Rowiński Citation2012; Maziarz et al. Citation2015) (). Titmice evolved hissing behaviour (Löhrl Citation1964; Rowiński Citation2013; Zub et al. Citation2017), which is helpful in deterring predators, as it is difficult for tit mice to escape from deep hollows when threatened (Adamík & Král Citation2008). The relatively large entrance size, short entrance–nest distance and “danger distance” in cavities chosen by robins () could reflect yet another antipredator strategy: birds can easily escape such cavities when attacked, by sacrificing the brood instead of their own life. In addition, robins are a species with good night vision (Bolshakov & Rezvyi Citation1998), so they have a non-zero chance to escape when attacked during the night, in comparison to birds with poor night vision such as titmice (Cramp & Perrins Citation1993). This might be important as the vast majority of avian broods in the Białowieża NP are predated at night (Rowiński Citation2013). Interestingly, another species known as a facultative cavity nester, also studied in the Białowieża NP, shows similar tendencies: the blackbird Turdus merula (Czeszczewik & Walankiewicz Citation2016). Despite being well known for nesting in forks of branches, near tree trunks or in concavities, ~50% of its nests were located in tree-holes or in decaying snags in the Białowieża NP (Tomiałojć Citation1993).
In the primeval forest of the Białowieża NP, a vast number of bird species avoid cavities in dead wood, most likely due to prominent levels of predation (Wesołowski Citation2007a, Maziarz et al. Citation2015; Wesołowski & Martin Citation2018). The robin case seems to confirm this, with over 60% of nests located in living trees (). Dead wood is softer, and it is much easier for predators to reach the brood (Christman & Dhondt Citation1997; Walankiewicz Citation2002; Rowiński Citation2013). Similarly, tree holes excavated by woodpeckers are also more vulnerable to attacks by predators (woodpeckers themselves visit old tree-holes and rob nests found there), and thus are less frequently occupied by small, passerine non-excavators (Bai et al. Citation2005, Remm et al. Citation2006; Wesołowski Citation2007a, Walankiewicz et al. Citation2007; Maziarz Citation2012; Rowiński Citation2013), including the European robin: ~90.2% of nests in cavities were in non-excavated cavities ().
Nest height
European robin nest cavities were found in the Białowieża NP mostly within 0.1–2 m above the ground, but a single robin’s nest was placed as high as 16 m above the ground. Authors from other regions found robins’ nests mostly at lower heights: up to 2 m in the Caucasus region (Lebedeva & Lomadze Citation2007), up to ~4 m in St. Petersburg (Prokofeva Citation2006) and Riazan regions (Baranovskiy & Ivanov Citation2017), and up to 8 m in Karelia (Zimin Citation2009). Zimin (Citation2009) found robins’ nests also down to 6 cm below the ground, but 93% of nests were located up to 1 m above the ground level. Zimin (Citation2009) indicates that there is no nest philopatry among this species. Nest choice is believed to be dictated by conditions of habitat and other circumstances. The same marked birds could occupy a ground nest in the first brood and a cavity in the second one (Zimin Citation2009). Data obtained in the BNP seems to confirm such facts – seven out of 10 birds changed their nest type during the second brood in the season.
Conclusions
Our results indicate that robins are not nest site opportunists under natural conditions: they appear to be moderately to highly selective in respect to nest sites. This selectivity seems to be of variable magnitude in different forest types, but with evident preference for cavities and the avoidance of root-soil plates. Therefore, the frequency of use of particular nest sites in mostly human-transformed habitats in Western Europe might not reflect robins’ true preferences.
Acknowledgements
We thank the Białowieża National Park for their kind cooperation.
Disclosure statement
The study complied with current Polish laws and was permitted by the Ministry of the Environment [DOP-WPN.286.30.2017.AN].
Additional information
Funding
References
- Adamík P, Král M. 2008. Climate and resource driven long term changes in dormice populations negatively affect hole-nesting songbirds. Journal of Zoology 275(3):209–215. DOI: 10.1111/j.1469-7998.2008.00415.x.
- Bai ML, Wichmann F, Mühlenberg M. 2005. Nest-site characteristics of hole-nesting birds in a primeval boreal forest of Mongolia. Acta Ornithologica 40(1):1–14. DOI: 10.3161/068.040.0105.
- Baranovskiy AV, Ivanov ES. 2017. Features of reproductive biology of robins (Erithacus rubecula) in anthropogenic habitats (for example, the city of Ryazan). Principy Èkologii 6(4):17–25. (in Russian, English summary).
- Bobiec A. 2002. Living stands and dead wood in the Bialowieza Forest: Suggestions for restoration management. Forest Ecology and Management 165(1–3):125–140. DOI: 10.1016/S0378-1127(01)00655-7.
- Bolshakov CV, Rezvyi SP. 1998. Time of nocturnal flight initiation (take-off activity) in the European Robin Erithacus rubecula during spring migration: Visual observations between sunset and darkness. Avian Ecology and Behaviour 1:37–49. (in Russian).
- Brown LK. 1974. Handbook for inventorying downed woody material. Ogden: USDA Forest Service, Intermountain Forest and Range Experiment Station. pp. 24. Gen. Tech. Rep. INT-16.
- Bruns H. 1960. The economic importance of birds in forests. Bird Study 7(4):193–208. DOI: 10.1080/00063656009475972.
- Christman BJ, Dhondt AA. 1997. Nest predation in Black–Capped Chickadees: How safe are cavity nests? The Auk 114(4):769–773. DOI: 10.2307/4089299.
- Cramp S, Perrins CM, eds. 1993. The birds of the Western Palearctic. Vol. 7. Oxford: Oxford University Press.
- Czeszczewik D, Walankiewicz W. 2003. Natural nest sites of the Pied Flycatcher in a primeval forest. Ardea 91:221–230.
- Czeszczewik D, Walankiewicz W. 2016. Ekologia i biologia ptaków Puszczy Białowieskiej z perspektywy czterdziestoletnich badań (Ecology and biology of birds in the Białowieża Forest: A 40-year perspective). Leśne Prace Badawcze 77(4):332–340. DOI: 10.1515/frp-2016-0034.
- Efron B, Tibshirani RJ. 1998. An introduction to the bootstrap. Boca Raton: Chapman & Hall/CRC.
- Falińska K. 1991. Plant demography in vegetation succession. Task for Vegetation Science. Vol. 26. Dordrecht: Kluver Academic Publishers.
- Faliński JB. 1977. Research on vegetation and plant populations dynamics conducted by Białowieża Primeval Forest the Warsaw University in the Białowieża Primeval Forest 1952–1977. Phytocoenosis 6(1/2):1–148.
- Faliński JB. 1968. Stan i prognoza neofityzmu w szacie roślinnej Puszczy Białowoeskiej. The state and prognosis of neophytism in the plant cover of the Białowieża Primeval Forest. In: editor, Faliński JB. Synantropizacia szaty roślinnej. I. Neofityzm i apofityzm w szacie roślinnej Polski. Vol. 25. Mat. Zakł. Fitosoc. Stos. UW. pp. 175–216.
- Glutz Von Blotzheim UN, Bauer KM. 1988. Handbuch der Vögel Mitteleuropas. Vol. 11. Wiesbaden: Akademische Verlagsgeselschaft.
- Harper D. 1985. Pairing strategies and mate choice in female robins Erithacus rubecula. Animal Behaviour 33:862–875. DOI:10.1016/S0003-3472(85)80020-8.
- Hegemeijer WJM, Blair MJ, eds. 1997. The EBCC Atlas of European breeding birds: Their distribution and abundance. London: T & AD Poyser.
- Ivlev VS. 1961. Experimental ecology of the feeding of fishes. New Haven, Connecticut: Yale University Press.
- Jaroszewicz B, Cholewińska O, Gutowski JM, Zimny M, Samojlik T, Latałowa M. 2019. Białowieża Forest – A relic of the high naturalness of European Forests. Forests 10(10):849. DOI: 10.3390/f10100849.
- Jason R. 2020. Selectapref: Analysis of field and laboratory foraging. R package version 0.1.2. https://CRAN.R-project.org/package=selectapref
- Kapusta AS. 2019. The dynamics of tree stands and the tree cavity resources in lime-hornbeam-oak stands of the Białowieża National Park. Dissertation thesis. Siedlce University of Natural Sciences and Humanities (in Polish, English summary).
- Knysh NP. 2008. Materials on the biology of Robin in forest-steppe deciduous forests of Sumy region. Berkut 17:41–60. in Russian, English summary.
- Lack D. 1946. Clutch and brood size in the Robin. British Birds 39:98–109.
- Lack D. 1948. Further notes on clutch and brood size in the Robin. British Birds 41:98–104.
- Lack D. 1965a. The life of the Robin. London: H. F. & G. Witherby Ltd.
- Lack D. 1965b. Evolutionary ecology. The Journal of Applied Ecology 2:247–255. DOI:10.2307/2401477.
- Lebedeva NV, Lomadze NH. 2007. The robin Erithacus rubecula in the North-Western Caucasus. Trudy Yuzhnogo nauchnogo centra Rossiyskoy Akademii Nauk. Vol. 3. Bioraznoobrazie i transformaciya gornyh ekosistem Kavkaza. Rostov-na-Donu: YuNC RAN: pp. 252–277. (in Russian).
- Löhrl H. 1964. Verhaltensmerkmale der Gattungen Parus (Meisen), Aegithalos (Schwanzmeisen), Sitta (Kleiber), Tichodroma (Mauerläufer) und Certhia (Baumläufer). Journal Fur Ornithologie 105:153–181. DOI:10.1007/BF01670988.
- Maziarz M (2012) The nest sites’ characteristic and breeding success of Great Tit Parus major in primeval conditions (Białowieża National Park). PhD thesis. Wrocław University. (in Polish, English summary).
- Maziarz M, Wesołowski T, Hebda G, Cholewa M. 2015. Natural nest-sites of Great Tits (Parus major) in a primeval temperate forest (Białowieża National Park, Poland). J. Otnihol 156:613–623.
- Mildenberger H. 1984. Die Vögel des Rheinlandes. Band 2. Düsseldorf.
- Newton I. 1998. Limitation population in birds. London: Academic Press.
- Prokofeva IV. 2006. On the nesting of the Robin Erithacus rubecula in the south of the Leningrad region. The Russian Journal of Ornithology 308:100–105. (in Russian).
- R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Remm J, Lohmus A, Remm K. 2006. Tree cavities in riverine forests: What determines their occurrence and use by hole-nesting passerines? Forest Ecology and Management 221(1–3):267–277. DOI: 10.1016/j.foreco.2005.10.015.
- Rowiński P. 2013. Factors determining the breeding success of secondary hole-nesting birds in the primeval forests of Białowieża National Park – A comparative study. Warsaw: SGGW. (in Polish, English summary).
- Schmidt O. 1990. Zur Ökologie der Rotkehlchen. Die Gefiederte Welt 114(3):89.
- Stański T, Czeszczewik D, Stańska M, Walankiewicz W. 2021. Anvils of the Great Spotted Woodpecker (Dendrocopos major) in primeval oak-lime-hornbeam stands of the Białowieża National Park. The European Zoological Journal 88(1):1–8. DOI: 10.1080/24750263.2020.1844324.
- Tomiałojć L. 1993. Breeding ecology of the Blackbird Turdus merula studied in the primeval forest of Białowieza (Poland). Part I. Breeding numbers, distribution and nest sites. Acta Ornithologica 27:131–157.
- Tomiałojć L. 1980. The combined version of the mapping method. In: Oelke H, editor. Vogelerfassung und Naturschutz. Proc. VI Intern. Conf. Bird Census Work, Göttingen. pp. 92–106.
- Tomiałojć L, Wesołowski T. 1996. Structure of a primaeval forest bird community during 1970s and 1990s (Białowieża National Park, Poland). Acta Ornithologica 31:133–154.
- Tomiałojć L, Wesołowski T. 2004. Diversity of the Białowieża Forest avifauna in space and time. Journal of Ornithology 145:81–92. DOI:10.1007/s10336-003-0017-2.
- Tomiałojć L, Wesołowski T. 2005. The avifauna of the Białowieża Forest: A window into the past. British Birds 98:174–193.
- Tomiałojć L, Wesołowski T. 1990. Bird communities of the primaeval temperate forest of Białowieża, Poland. In: Keast A, editor. Biogeography and ecology of forest bird communities. The Hague: SPB Academic Publishers. pp. 141–165.
- Tomiałojć L, Wesołowski T, Walankiewicz W. 1984. Breeding bird communities of primaeval temperate forest (Białowieża National Park, Poland). Acta Ornithologica 20:241–310.
- Walankiewicz W. 2002. Breeding losses in the Collared Flycatcher Ficedula albicollis caused by nest predators in the Białowieża National Park (Poland). Acta Ornithologica 37:21–26.
- Walankiewicz W, Czeszczewik D, Mitrus C. 2007. Natural nest sites of Collared Flycatcher Ficedula albicollis in lime-hornbeam-oak stands of a primeval forest. Ornis Fenn 84:155–162.
- Wesołowski T. 1996. Natural nest sites of Marsh Tits Parus palustris in a primaeval forest (Białowieża National Park, Poland). Die Vogelwarte 38:235–249.
- Wesołowski T. 1983. The breeding ecology and behaviour of Wrens Troglodytes under primaeval and secondary conditions. Ibis 125:499–515. DOI:10.1111/j.1474-919X.1983.tb03144.x.
- Wesołowski T. 2002. Antipredator adaptations in nesting marsh tits Parus palustris - the role of nest site security. Ibis 144:593–601. DOI:10.1046/j.1474-919X.2002.00087.x.
- Wesołowski T. 2007a. Primeval conditions – What can we learn from them? Ibis 149(Suppl 2):64–77. DOI: 10.1111/j.1474-919X.2007.00721.x.
- Wesołowski T. 2007b. Lessons from long-term hole-nester studies in a primeval temperate forest. Journal of Ornithology 148(2):395–405. DOI: 10.1007/s10336-007-0198-1.
- Wesołowski T. 2011. “Lifespan” of woodpecker-made holes in a primeval temperate forest: A thirty year study. Forest Ecology and Management 262(9):1846–1852. DOI: 10.1016/j.foreco.2011.08.001.
- Wesołowski T. 2012. “Lifespan” of non-excavated holes in a primeval temperate forest: A 30 year study. Biological Conservation 153:118–126. DOI:10.1016/j.biocon.2012.04.017.
- Wesołowski T, Czeszczewik D, Hebda G, Maziarz M, Mitrus C, and Rowiński P. 2015. 40 years of breeding bird community dynamics in a primeval temperate forest (Białowieża National Park, Poland). Acta Ornithologica 50:95–120. DOI:10.3161/00016454AO2015.50.1.010
- Wesołowski T, Martin K. 2018. Tree holes and hole-nesting birds in European and North American forests. In: Mikusiński G, Roberge J-M, Fuller RJ, editors. Ecology and conservation of forest birds. Cambridge: Cambridge University Press. pp. 79–134.
- Wesołowski T, Rowiński P. 2004. The breeding behaviour of the Nuthatch Sitta europaea in relation to natural hole attributes in a primeval forest. Bird Study 51:143–155. DOI:10.1080/00063650409461346.
- Wesołowski T, Rowiński P. 2012. The breeding performance of Blue Tits Cyanistes caeruleus in relation to the attributes of natural holes in a primeval forest. Bird Study 59:437–448. DOI:10.1080/00063657.2012.722189.
- Wesołowski T, Rowiński P, Mitrus C, Czeszczewik D. 2006. Breeding bird community of a primeval temperate forest (Białowieża National Park, Poland) at the beginning of the 21 st century. Acta Ornithologica 41:55–70. DOI:10.3161/068.041.0112.
- Wesołowski T, and Tomiałojć L. 2005. Nest sites, nest depredation, and productivity of avian broods in a primeval temperate forest: do the generalisations hold? Journal of Avian Biology 36:361–367.
- Wojton A, Pitucha G. 2020. Root plates as nesting sites for Eurasian Wrens Troglodytes in a forest undergoing renaturalisation. Acta Ornithologica 55(1):53–58. DOI: 10.3161/00016454AO2020.55.1.005.
- Zawadzka D. 2018. Cavities in forest ecosystems: Formation, distribution, ecological importance and recommendation for protection. Sylwan 162(6):509–520.
- Zimin VB. 2009. The robin in the north of the area. Vol. 1. Distribution. Number. Reproduction. Petrozavodsk: Karel’skiy nauchnyy centr RAN. pp. 443. (in Russian).
- Zub K, Czeszczewik D, Ruczynski I, Kapusta A, Walankiewicz W. 2017. Silence is not golden: The hissing calls of tits affect the behaviour of a nest predator. Behavioral Ecology and Sociobiology 71(5):79. DOI: 10.1007/s00265-017-2313-5.