Abstract
Outbreaks of myxomatosis in Iberian hares (Lepus granatensis) were detected for the first time in Spain in 2018. The disease spread to a significant proportion of the species range, negatively affecting the hare populations. In this study, we analyzed the impact of myxomatosis at hunting grounds of Castilla y León (Northern Spain), comparing hare density during two consecutive hunting seasons; the first when outbreaks were detected for the first time (season 1, Oct 2019-Jan 20) and the second after the spread of the disease (season 2, Oct 2020-Jan 21). Data was gathered from 1,102 hunts from 178 hunting grounds through “coursing”, a hunting type in which hunters and greyhounds search for hares, allowing the estimation of hare density. Overall, hare density decreased significantly, from 12.7 hare/100 ha in season 1 to 4.7 hare/100 ha in season 2. The percentage of hares suspected to be affected by myxomatosis (sick and dead) per hunt was higher in season 1 (14.4%) compared to season 2 (10.7%). For both seasons, this proportion was higher when hunting season was started (20.7%, October), compared to the remaining months (4.7%, November–January). However, the proportion of hunting grounds affected increased from 44% in season 1 to 66.7% in season 2. Our research confirmed a 62.7% reduction in hare density in Castilla y León after the spread of myxomatosis and identified scenarios of possible depletion when densities were below 4–5 hare/100 ha. As myxomatosis becoming endemic in Iberian hares is likely, hunters and game managers should continue current monitoring and disease surveillance and make management decisions accordingly.
Introduction
Myxomatosis has been a widespread disease in wild rabbits (Oryctolagus cuniculus) in Iberia since the 1950s, showing an annual pattern of periodic outbreaks and affecting a large proportion of its populations (Villafuerte et al. Citation2017). For the first time, in 2018, myxomatosis was detected in Iberian hares (Lepus granatensis) at several hunting grounds of Central and Southern Spain, when hares with compatible signs were observed, including blepharitis and blepharoconjunctivitis, epistaxis and inflammation and oedema around the nasal, oral and genital orifices (see full description in García-Bocanegra et al. Citation2019). Afterwards, the disease spread to almost the entire distribution of the species in the Iberian Peninsula and the Balearic Islands (García-Bocanegra et al. Citation2021).
Existing research confirms that myxomatosis has emerged in hares as a new host species (Águeda-Pinto et al. Citation2019; Dalton et al. Citation2019), and the mean expected mortality during the first phase (July 2018-April 2019) was higher than 50% (García-Bocanegra et al. Citation2021), hence myxomatosis significantly affected hare populations. However, more research on the impact of this disease is needed, as there is a lack of knowledge on the effects of myxomatosis at a local scale due to the limited information on hare density before and after the first detected outbreaks.
The spread of this disease in Spain is a major concern because hares are a prey species of a wide range of predators and a significant game species, with more than 800,000 hunted individuals per year in the last decade (Garrido et al. Citation2020). Hares can be legally hunted through walking-up shooting or using greyhounds. A traditional and popular hunting method in which 1–2 greyhounds on a leash and accompanied by hunters, search for hares in open landscapes (Rodríguez et al. Citation1997).
This study aimed to address the effects of myxomatosis on the population of hares occurring at hunting grounds of Castilla y León region (northern Spain), using data gathered in the coursing of hares. Two periods were compared: when first outbreaks were reported (years 2019–20) and after the disease spread in the region (years 2020–21). These data contribute to increasing knowledge of the effect of myxomatosis on hare populations and will improve management decisions taken by hunters, managers, conservationists, and policymakers.
Material and methods
Study area
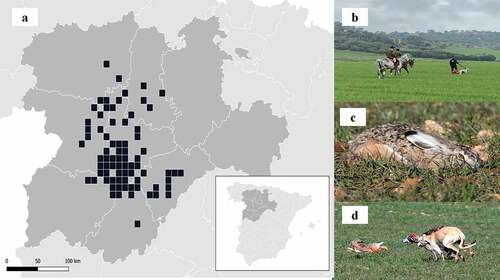
This study was conducted on 178 hunting grounds of Castilla y León region (North-western Spain), which comprised 89,000 ha (). The climate of the whole study area is Mediterranean dry continental with harsh winters and hot dry summers, and annual mean rainfall ranging 400–600 mm (Papadakis Citation1966). All hunting grounds sampled were dominated by arable land and grassland (as coursing of hares requires open and flat landscapes), with scattered vineyards, forest and shrubs patches (where coursing is not possible).
Data collection
The data of this study was gathered by volunteers recruited by the regional coursing organization (Federación de Galgos de Castilla y León) (). Volunteers were trained to distinguish myxomatosis in living hares observed at close distance or “in the hand”, using captured animals (alive or dead) or carcasses collected during hunting. A hare was categorized as “suspected to be affected by myxomatosis” when it was possible to identify one or several clinical signs (see García-Bocanegra et al. Citation2019), and in living hares symptoms such as running difficulties, disorientation and poor body condition (supplementary material 1). It is important to note that in coursing, not all hares flushed are chased by the greyhounds, which may be released (or not) depending on the flushing distance, the size of the hare (leverets and medium-size hares are not chased), and when hares are suspected to be affected by myxomatosis.
Information related to hares suspected to be affected by myxomatosis and samples from dead animals were submitted to the Regional Government Animal Health Service (Junta de Castilla y León). Since not all hares observed with myxomatosis-compatible signs were analyzed in the laboratory, and the possibility of hares being affected by other diseases (García-Bocanegra et al. Citation2019), we referred to hares as “suspected to be affected by myxomatosis”.
As coursing is conducted during the daytime in arable fields, when hares are inactive in their resting sites (Sánchez-García et al. Citation2021), the number of hares seen and flushed can be used to estimate hare density. This type of hunting is then a method for assessing hare abundance, similar to others such as the “total clearance count” used in the brown hare (Lepus europaeus Langbein et al. Citation1999).
Data collection was conducted during two consecutive hunting seasons: 2019–2020 (season 1), coinciding with the first detections of myxomatosis in Castilla y León, and 2020–2021 (season 2) during the spread of the disease in this region (García-Bocanegra et al. Citation2021). The open hunting season starts in October and finishes by the end of January. The total number of hunts per hunting ground and season varied in each ground. Each group of hunters submitted the information weekly, and in each hunting ground, the information was gathered by the same group of hunters for the whole season.
The unit of analysis considered in this study was “the hunt”, defined as the action of hunting (lasting around 4 hours), in which hunters and greyhounds may flush and chase hares. The following information was recorded in each hunt: location of the hunting ground, date, area covered, number of healthy hares seen or flushed, and number of hares suspected to be affected by myxomatosis (sick or dead). Hunters took photographs of possibly affected hares and distinguished the sex of sick hares when possible.
Data analysis
We calculated hare density per hunt using the number of hares seen or flushed divided by the area covered in each hunt (hare/100 ha). Non-parametric Wilcoxon and Kruskal–Wallis tests and contingency tables were used to analyze the data. All statistical analyses were conducted using R software v.3.4 (R Team Citation2019).
Results and discussion
Data from 1,102 hunts (78.6% from season 2) was analysed. shows the general data of this study. In season 1, 83% of the submitted information was collected in October, while in season 2, 30% was from this month and the remaining 70% for the period November–January.
Table I. The number of hunting grounds, hunts, area covered and the number of hares seen per hunting season, and mean values (±SE) of area covered per hunt, hares per hunt, hare density (hare/100 ha) and the number of hunts recording hares suspected to be affected by myxomatosis (dead/sick hares). The number of hunts in which affected hares were observed is also given (n.d. = no data)
The hare density for season 2 (mean 4.73 hare/100 ha) was significantly lower (W = 147,069, P < 0.001) than the density recorded in season 1 (mean 12.68 hare/100 ha). The same result was obtained using only the information gathered in October (W = 35,798, P < 0.001). Considering only the hunting grounds participating in both seasons in October (n = 44), there were significant differences in hare densities (χ2 = 16.41, df = 1, P < 0.001). Also, in season 2, a total of 519 hunts yielded a density of ≤4 hare/100 ha, and 270 hunts yielded a density ≤2 hare/100 ha, suggesting that a significant proportion of the hare populations may have been at risk of depletion.
In season 2, hare densities remained similar during the whole season, with no significant differences (χ2 = 1.023, df = 1, P = 0.311) between October and the remaining months in those hunting grounds submitting data for the two periods; hence, we could assume that myxomatosis impacted before the beginning of the hunting season. In this way, although we cannot exclude that hares were affected by other diseases, we think it was unlikely because, in our study area, Tularemia was the only other known disease following seasonal outbreaks in hares (Reviriego et al. Citation2000; Mínguez-González et al. Citation2021), which shows different symptoms and necropsy findings. Thus, density differences among seasons may be explained by myxomatosis outbreaks.
The hare density values during season 1 are similar to previous studies conducted in Castilla y León before the first detection of myxomatosis, with mean values ranging 10–15 hare/100 ha, and up to 22 hare/100 ha (De la Calzada & Martínez Citation1994; Lopez et al. Citation1996; Sánchez-García et al. Citation2012). This supports the hypothesis that during season 1, myxomatosis emerged and subsequently spread.
A total of 538 hares suspected to be affected by myxomatosis were collected (149 sick and 389 dead). Laboratory analysis for 69 hares collected from October to December 2019 confirmed 57 being positive (82%). From the sexed dead hares (n = 51), 62.7% were males, resulting in an estimated sex ratio of about 2:1. Pooling sick and dead hares, the proportion of affected hares changed between seasons and periods. Thus, in season 1, the proportion of affected hares was 14.4%, while in season 2, it was 10.7%. This means that the hunts in season 1 had 1.94 times the risk of observing the disease (CI 95%: 1.31–2.17) than season 2. Also, this proportion in the initial period was 20.7% while in the remaining months decreased to 4.7%. The initial period (October) had 4.81 times the rate of observing myxomatosis (CI 95%: 4.19–5.53) compared to the rest of the coursing period, i.e., hunts in the initial period had 3.55 times the risk of observing hares suspected to be affected by myxomatosis (CI 95%: 2.70–4.68) compared to the subsequent period.
As this study covered a large area from a homogeneous biogeographical territory, in terms of landscape, climate and game management, with no significant weather differences among hunting seasons, we believe that the impact of myxomatosis could explain density differences between seasons. Although we cannot exclude slight differences between hunting grounds related to predation and other sources of mortality for hares (Rodríguez et al. Citation1997). This conclusion is also supported by the significant differences found for hunting grounds participating in both seasons.
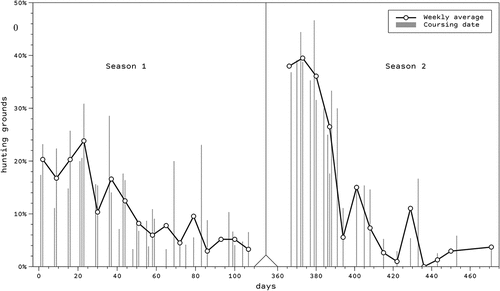
Our results suggest that myxomatosis spread in the study area. In season 1, 44% of the hunting grounds reported possible myxomatosis, while in season 2, the value increased to 66.7%. shows the weekly percentage of hunting grounds observing hares suspected to be affected (sick/dead hares). The percentage of hunting grounds observing sick/dead hares was higher during October (57.6%) compared to the remaining months (38.0%). Despite myxomatosis spread in the region from 44 hunting grounds in season 1 to 52 in season 2, hares affected by myxomatosis in the hunts decreased from 28.0% in season 1 to 16.6% in season 2. The impact of the disease in October was significantly higher (33% of hunts) than in the rest of the months (9.3% of hunts). This is partially in agreement with the patterns observed during the first outbreaks in Spain (García-Bocanegra et al. Citation2021), which may be related to the effects of weather on fleas and mosquitoes, vectors of myxomatosis (García-Bocanegra et al. Citation2019), which would be less likely to occur when the temperature gets colder at higher altitudes (Durán-Martínez Citation2012).
Figure 2. Weekly percentage of hunting grounds observing hares suspected to be affected by myxomatosis (sick/dead hares) during season 1 (October 2019-January 2020) and season 2 (October 2020-January 2021).
In general, this study points out an important reduction in hare densities in Castilla y León after the spread of myxomatosis, as in season 2, densities were 62.7% lower than season 1. The impact of myxomatosis on the studied hare population is higher than results from García-Bocanegra et al. (Citation2019), García-Bocanegra et al. (Citation2021), who estimated a mean mortality of 55.4% (median 70%) during the first outbreaks (July 2018-April 2019, concentrated in central and southern Spain), though in those studies no density data were provided. It is important to note that in this study, 53% of the hunting grounds participating in season 2 implemented measures to adapt hunting pressure, including the reduction of hunting days, allowing hunting only during the morning and in some cases hunting ceased before the end of the season.
All hunting grounds aiming to harvest hares must conduct a count to decide whether hunting can be conducted or not. In similar hare habitats, values below 4–5 hare/100 ha may indicate scenarios of depletion and even local extinction (in some cases attributed to myxomatosis), hence measures should be taken to boost local populations through targeted management and continuous population and disease surveillance (Alzaga et al. Citation2013). We understand that data gathered from volunteers conducting coursing allow a further calculation of hare density. However, further research should be conducted to compare with other methods, such as spotlight counts and transects (Purroy Citation2017), bearing in mind that this hunting form may not imply killing. To the best of our knowledge, hare hunting has not been limited or banned by authorities in any Spanish regions, which could be explained by the voluntary hunting restraint promoted by hunters and game managers at grounds with myxomatosis or when hares are scarce.
Acknowledgements
We are indebted to all volunteers who kindly submitted data during the study, and the Federación de Galgos de Castilla y León, who organized and coordinated this study. CL produced the map, and FC and RB provided linguistic comments. We thank anonymous reviewers for their comments. An ethical statement does not apply to this study as data was gathered from hunts legally authorized. This study did not involve the purposeful killing of animals.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Águeda-Pinto A, de Matos AL, Abrantes M, Kraberger S, Risalde MA, Gortázar C, McFadden G, Varsani A, Esteves PJ. 2019. Genetic characterization of a recombinant myxoma virus in the Iberian hare (Lepus granatensis). Viruses 11(6):530. DOI:10.3390/v11060530.
- Alzaga V, Torres J, Villanúa D, Cormenzana A, Leránoz I, Mateo-Moriones A. 2013. Conocimientos científicos importantes para la conservación y gestión de las tres especies de liebre de la Península Ibérica: deficiencias y retos para el futuro. Ecosistemas 22(2):13–19. DOI:10.7818/ecos.2013.22-2.03.
- Dalton KP, Martín JM, Nicieza I, Podadera A, de Llano D, Casais R, Gimenez S, Badiola I, Agüero M, Duran M, Buitrago D, Romero LJ, García E, Parra F. 2019. Myxoma virus jumps species to the Iberian hare. Transboundary and Emerging Diseases 66(6):2218–2226. DOI:10.1111/tbed.13296.
- De la Calzada E, Martínez F. 1994. Requerimientos y selección de hábitat de la liebre mediterránea (Lepus granatensis Rosenhauer, 1856) en un paisaje agrícola mesetario. Ecología 8:381–394.
- Durán-Martínez M. 2012. Distribución, abundancia y composición de la comunidad de dípteros hematófagos vectores de enfermedades en Castilla-La Mancha: riesgos para la salud pública y la sanidad animal. Ciudad Real, Spain: University Castilla-La Mancha.
- García-Bocanegra I, Camacho-Sillero L, Caballero-Gómez J, Agüero M, Gómez-Guillamón F, Manuel Ruiz-Casas J, Manuel Díaz-Cao J, García E, José Ruano M, de la Haza R. 2021. Monitoring of emerging myxoma virus epidemics in Iberian hares (Lepus granatensis) in Spain, 2018–2020. Transboundary and Emerging Diseases 68:1275–1282. DOI: 10.1111/tbed.13781.
- García-Bocanegra I, Camacho-Sillero L, Risalde MA, Dalton KP, Caballero-Gómez J, Agüero M, Zorrilla I, Gómez-Guillamón F. 2019. First outbreak of myxomatosis in Iberian hares (Lepus granatensis). Transboundary and Emerging Diseases 66:2204–2208. DOI: 10.1111/tbed.13289.
- Garrido J, Ferreres J, and Gortázar C. 2020. Las Especies Cinegéticas Españolas En El Siglo XXI.Retrieved from Amazon.com.
- Langbein J, Hutchings MR, Harris S, Stoate C, Tapper SC, Wray S. 1999. Techniques for assessing the abundance of Brown Hares Lepus europaeus. Mammal Review 29(2):93–116. DOI:10.1046/j.1365-2907.1999.00040.x.
- Lopez JM, Hernández A, Purroy FJ, and Robles JL. 1996. Datos sobre la biología de la reproducción de la liebre ibérica (Lepus granatensis Rosenhauer, 1856) en agrosistemas cerealistas de la provincia de León (NW de Espana). Revista Forestal 9:49–60.
- Mínguez-González O, Gutiérrez-Martín CB, del Carmen Martínez-nistal M, del Rosario Esquivel-garcía M, Gómez-Campillo JI, Collazos-Martínez JÁ, Fernández-Calle LM, Ruiz-Sopeña C, Tamames-Gómez S, Martínez-Martínez S, Caminero-Saldaña C, Hernández M, Rodríguez-Lázaro D, Rodríguez-Ferri EF. 2021. Tularemia outbreaks in Spain from 2007 to 2020 in humans and domestic and wild animals. Pathogens 10. DOI: 10.3390/pathogens10070892.
- Papadakis J. 1966. Climates of the world and their agricultural potentialities. J Papadakis, editor. Buenos Aires, Argentina.
- Purroy FJ. 2017. Liebre ibérica-Lepus granatensis (Rosenhauer, 1856). In: Salvador A, and Barja I, editors. Enciclopedia Virtual de los Vertebrados Españoles. Madrid: Museo Nacional de Ciencias Naturales. http://www.vertebradosibericos.org.
- R Core Team. 2019. R Core Team (2017). R: A language and environment for statistical computing. R Found. Stat. Comput. Vienna, Austria. Available: http//www.R-project.org/.page.
- Reviriego F, Reques F, Álvarez F, Galve C, and Fernández F. 2000. Tularemia En Castilla y León. Brote Epidemilógico. Consejería Agricultura y Ganadería, editor. Valladolid, Spain: Junta de Castilla y León.
- Rodríguez M, Palacios J, Martín J, Yanes T, Martín P, Sánchez C, Navesco M, Muñoz R. 1997. La Liebre. Barcelona, España: Mundiprensa.
- Sánchez-García C, Alonso ME, Bartolomé DJ, Pérez JA, Larsen RT, Gaudioso VR. 2012. Survival, home range patterns, probable causes of mortality, and den-site selection of the Iberian hare (Lepus, Leporidae, Mammalia) on arable farmland in north-west Spain. Italian Journal of Zoology 79(4):590–597. DOI: 10.1080/11250003.2012.685109.
- Sánchez-García C, Pérez JA, Armenteros JA, Gaudioso VR, Tizado EJ. 2021. Survival, spatial behaviour and resting place selection of translocated Iberian hares Lepus granatensis in Northwestern Spain. European Journal of Wildlife Research 67(2). DOI:10.1007/s10344-021-01464-8.
- Villafuerte R, Castro F, Ramírez E, Cotilla I, Parra F, Delibes-Mateos M, Recuerda P, Rouco C. 2017. Large-scale assessment of myxomatosis prevalence in European wild rabbits (Oryctolagus cuniculus) 60 years after first outbreak in Spain. Research in Veterinary Science 114:281–286. DOI: 10.1016/j.rvsc.2017.05.014.