Abstract
The impact of fish-eating birds on fishery has long been debated. It is therefore important that the arguments in this debate be scientifically based. The Great Cormorant Phalacrocorax carbo is a flagship example of a conflict species that has been well studied. In some areas, however, such as the southern Baltic estuarine lagoons, the Goosander Mergus merganser may be more abundant, exerting a potentially greater impact on fisheries. However, this aspect has not been well studied in this region, so this article is an attempt to fill this knowledge gap. Based on the digestive tract content of 23 Goosander drowned in gillnets in Poland, it was found that the most frequently consumed species was Ruffe Gymnocephalus cernua (70% of biomass), followed by Zander Sander lucioperca (13% of biomass), Perch Perca fluviatilis (9% of biomass) and Roach Rutilus rutilus (8% of biomass). Average Goosander numbers in the Vistula and Odra estuaries in 2011–2018 were 11,000 in winter, 4,500 in spring and 2,800 in autumn. These fish-eating ducks were found to consume 242 t of fish annually during this period, whereas at the same time fishermen caught an average of 4,400 t of fish. The species caught by both Goosander and fishermen were mainly Zander (32 t vs 189 t), Perch (21 t vs 668 t) and Roach (19 t vs 701 t). No negative impact of Goosander foraging in the winter preceding the fishery season was demonstrated for any of the above species (Zander R2 = 0.022; Perch R2 = 0.834; Roach R2 = 0.881).
Introduction
The prey-predator system is one of the most basic relationships in ecology but studying the influence of a particular predator species on its prey population is difficult as the status of the latter depends on a number of biological and abiotic factors (Domenici et al. Citation2007). Fish-eating waterbirds are natural competitors of fishermen, who blame them for depleting the fish stocks in the waters they use (e.g. Beach Citation1936; Bayer Citation1989). Conflict situations arise because diving waterbirds often choose habitats similar to those in which fishermen fish (Cowx Citation2003). A good example is the Great Cormorant Phalacrocorax carbo (hereafter Cormorant), which has been mentioned as one of the reasons for the decline in fish stocks (Psuty Citation2012). It is therefore essential to understand the interactions between birds, fish and fishing as it poses an important social problem (Cowx Citation2003). The results of scientific research should be the basis for the debate on the impact of birds on fisheries (Ovegård et al. Citation2021) and vice versa, the impact of fisheries on birds (Tasker et al. Citation2000).
Along with the Cormorant, Goosander Mergus merganser has been regarded as a competitor for commercially caught fish (Kear Citation2005). Early publications indicated a significant negative impact of this species’ feeding on salmon, a fish that is particularly valued by humans (Beach Citation1936; Smith Citation1967; Scanlon et al. Citation1978). However, later studies showed a smaller and less significant effect than had previously been assumed (Wood Citation1987; Kålås et al. Citation1993). After the Cormorant, the Goosander is one of the most numerous fish-eating waterbirds, large populations of which spend a considerable part of the year wintering in the southern Baltic (Marchowski et al. Citation2018). It is among the top five fish-eating birds responsible for 80% of all fish eaten by birds in the Baltic (Hansson et al. Citation2018). Therefore, wherever it concentrates in the largest flocks, its presence does have an impact on fish stocks (Wood Citation1987; Hansson et al. Citation2018). Although Goosander is the third most important and numerous fish-eating species in the Baltic Sea in terms of fish consumption after Razorbill Alca torda and Cormorant, only a few studies on the diet of this species in the Baltic Sea have been published (Hansson et al. Citation2018).
A study conducted in the Finnish archipelago showed that the main component of the Goosander diet was Three-spined stickleback Gasterosteus aculeatus; other fish found in this bird’s digestive tract were Roach Rutilus rutilus and Eelpout Zoarces viviparous, which in that area are not targeted by fishermen. Goosander has been found on rare occasions to consume Pike Esox lucius and Herring Clupea harengus from among the economically important fish in the northern Baltic Sea (Bagge et al. Citation1973; Lemmetyinen & Mankki Citation1975). As regards the southern Baltic, we found only one publication discussing the Goosander’s dietary composition from the Lithuanian part of the Curonian Lagoon: Žydelis and Kontautas (Citation2008) showed that Smelt Osmerus eperlanus (average length = c. 17 cm, average mass = 31 g) was the main component of its diet, accounting for 84% of the biomass. From the Gulf of Finland, too, there are reports of Goosander feeding mainly on spawning Smelt (Hansson et al. Citation2018). Studies from beyond the Baltic Sea indicate that Goosander’s prey items are 25–30 cm in length (Kålås et al. Citation1993; McCaw III et al. Citation1996).
The aim of this article is to fill a gap in knowledge about the dietary composition and the impact on fishery of the second most abundant fish-eating waterbird in the southern Baltic Sea. Specifically, we address the following questions: (1) What is the species composition of fish eaten by Goosander and what is the percentage of each species in its diet? (2) Do the ecological niches of Goosander and fishermen coincide? In other words, do fishermen and Goosander target the same species? (3) Does the foraging of Goosander have a negative impact on the sizes of fishery catches in the lagoons of the southern Baltic?
Methods
Study site
The study site was chosen for its importance as one of the most important Goosander wintering sites in the North-West & Central Europe population. On average, about 12% of this population winters in Poland (Wardecki et al. Citation2021; Wetlands International Citation2022), while the lagoons in the estuaries of the Vistula and Odra rivers are the most important Goosander wintering grounds in Poland (Marchowski et al. Citation2018). Szczecin Lagoon (coordinates: 53.785578, 14.457359) is divided by the state border and about half of the estuary system is on the German side. Similarly, in the case of Vistula Lagoon (coordinates: 54.339986, 19.430559), a large part of this water body is on the Russian side. The estimates presented in this publication concern only Polish parts of these water bodies. Additionally, samples from the digestive tracts of Goosander also come from other areas, from two dam reservoirs: Goczałkowice (49.929752, 18.854052) and Dobczyce (49.867169, 20.046346) in southern Poland, which are also the Goosander’s wintering habitat in this part of Europe (Chylarecki et al. Citation2018); ().
Goosander dietary composition study
Twenty-three dead birds were obtained from bycatch from three areas of Poland (Szczecin Lagoon N = 17, Goczałkowice Reservoir N = 4 and Dobczyce Reservoir N = 2) during the period from November to March in the years 2010–2018. The dead birds were frozen immediately on receipt. After thawing, the birds were necropsied in the laboratory. The contents of the digestive tract (gizzard and oesophagus) were examined. Fish derived from the gastrointestinal tract were identified to species level, counted and measured. In the case of partially or fully digested fish, their species, length and weight were determined on the basis of pharyngeal bones (cyprinids) and otoliths (percids). The number of individuals of a prey species contained in a stomach was approximated by the highest total of any of the identifiable parts present, taking the right and left parts separately. They were measured to calculate the prey length using published regression formulae (pharyngeal bones – Horoszewicz Citation1960, otoliths – Dirksen et al. Citation1995). The composition of the diet was assessed by summing weighted averages of the numbers of particular prey fish species. The weights of the fish species were calculated using the regression formulas for particular species in winter given by Dirksen et al. (Citation1995).
Assessment of goosander abundance
Data on the size of the wintering population were obtained from the Monitoring of Wintering Birds in Poland Programme, implemented by the Chief Inspectorate for Environmental Protection (Neubauer et al. Citation2015; Chodkiewicz et al. Citation2019), and from the Waterbird Monitoring in Western Pomerania Project (Marchowski et al. Citation2018). The method used to collect data on wintering waterbirds was based on the standards recommended by Wetlands International (Citation2010) during the International Waterbird Census (IWC).
Monitoring carried out in Poland has shown that Goosander’s most important wintering grounds in Poland are the Baltic coastal lagoons and estuaries (Neubauer et al. Citation2015; Chodkiewicz et al. Citation2019). The present article takes into account the estuaries of the two largest rivers in Poland, the Vistula and the Odra (). Specifically, the following water bodies were selected to assess the scale of fish consumption by Goosander: the Szczecin Lagoon, Kamień Lagoon and Dziwna, Świna Delta, Lake Dąbie and Vistula Lagoon. The approximate combined area of these water bodies is 85,000 ha. They all have similar ecological features, and likewise, a similar species composition of fish (Psuty Citation2012; Psuty et al. Citation2017). Information on the numbers of non-breeding Goosander from these water bodies was obtained from the available literature data and ornithological monitoring databases (Goc & Mokwa Citation2011; Ławicki & Guentzel Citation2012; Mokwa et al. Citation2012; Marchowski et al. Citation2018).
To determine which species – Cormorant or Goosander – was dominant, their numbers were compared in the Odra River estuary (~60% of the study area) based on data gathered during the monitoring of waterbirds carried out by the West Pomeranian Nature Society from November to March in 2010–2018 (Marchowski et al. Citation2018).
Assessment of the scale of fish consumption by Goosander
As Goosander usually forages twice a day (Anderson & Reeder Citation1977), it was assumed that the analysed digestive tract contents came from a single feeding. The average weight of the contents of one stomach was multiplied by two, which gave the daily consumption of fish for one individual. These values were compared with others reported in the literature (Marquiss & Carss Citation1994; Carss Citation1997; Engström Citation2001). If the values calculated in the present research fell within the range given in the literature or were close to it, they were deemed suitable for further calculations.
Assessment of Goosander abundance in consecutive months during the period 2011–2018 enabled the length of stay in days of large flocks in the above-named water bodies to be calculated. The following formula was used to calculate the scale of the annual consumption of fish:
YC = D*N*(C*2),
where YC – annual consumption of fish, D – number of days the birds stay on the water body, N – estimated number of birds, C – average weight of fish from the gastrointestinal tract.
Fish caught by fishermen
The databases relating to the water bodies under scrutiny were obtained from the Polish Fisheries Monitoring Centre (https://www.cmr.gov.pl/). The data in this database were collected in so-called Baltic Squares (resolution 20 × 20 km). The entire Polish Exclusive Economic Zone in the Baltic Sea is divided into such squares for the purpose of monitoring fishing effort. The fisheries within the squares, which include both sea and lagoon waters, were marked accordingly and thus recognizable. All the records in the database were checked for correctness and any errors discovered were eliminated, mainly by removing incorrectly assigned records. After the database was prepared, the data on the total catch volume of all fish species in each area was obtained, broken down by the type of fishing gear with which the fish were caught and by fish species. The amount of fish caught in the studied waters was calculated for the period 2011–2018.
The effect of Goosander foraging on fish catches
The overlap between the ecological niches of fishermen and Goosander was examined. We assumed that the food of Goosander on the Vistula Lagoon is the same as in the Szczecin Lagoon. This assumption was made because the dominant fish species in a diet of Cormorant in the Vistula Lagoon (Stempniewicz et al. Citation2003) and in the Szczecin Lagoon (Wolnomiejski & Witek Citation2013) was the same as in a diet of Goosander in the Szczecin Lagoon (this study). Additionally, the general composition of fish species in both lagoons is similar (Psuty Citation2012; Wolnomiejski & Witek Citation2013). An assessment was made of the species of fish regularly caught by fishermen and of those consumed by Goosander. Then, for those species that overlap, the total numbers consumed by Goosander in the winter season preceding a given year were compared with the subsequent catches by fishermen in that year (e.g. Goosander fish catch in the 2010/2011 winter season vs fishermen’s catch in the whole of 2011). With respect to the fish species that overlap in these two niches, it was assumed that Goosander foraging would have an impact if the fishermen’s catches were inversely proportional to the number of fish caught by Goosander and if this relationship were statistically significant. The inference from a positive or zero correlation would be that Goosander has a negligible impact on fishermen’s catches. We applied the simple linear regression using the lm () function in the R environment (R Development Core Team Citation2021). The predictor variable for our regression was the Goosander catch in the wintering period preceding the year of the fishermen’s catch, and the dependent variable was the catch by fishermen in each year.
Results
Goosander food composition
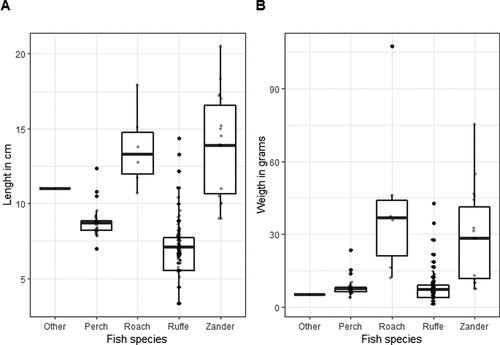
Twenty-two of the 23 Goosander collected had fish and fish remains in their digestive tracts. A total of 349 fish specimens were identified. The prey composition was dominated by Ruffe Gymnocephalus cernua, which accounted for 82.5% of all fish found – frequency by number (fn). The average estimated length of a Ruffe was 7.1 cm (SE = 0.1, NFISH = 287), and the average mass was 8.1 g (SE = 0.1, NFISH = 287). The second most abundant fish eaten by Goosander was Perch Perca fluviatilis, which accounted for 10.3% (fn) of all fish found. The average estimated length of a Perch was 8.7 cm (SE = 0.3, NFISH = 36), and the average mass was 8.1 g (SE = 0.3, NFISH = 36). Zander Sander lucioperca (third most abundant fish 5.2% fn) and Roach Rutilus rutilus (1.7% fn) were also important dietary components. The average length of a Zander was 13.9 cm (SE = 0.5, NFISH = 16), and the average mass was 29.8 g (SE = 0.5, NFISH = 16). The average length of a Roach was 13.7 cm (SE = 0.7, NFISH = 6), and the average mass was 42.5 g (SE = 0.7, NFISH = 6), (). Other fish species accounted for only 0.3% (fn) of the dietary composition.
In the digestive tracts of birds drowned in the Szczecin Lagoon (NBIRDS = 17), 263 fish were found, the most numerous being Ruffe (81.0% fn), followed by Perch (11.8% fn), Zander (5.7% fn), Roach (1.1% fn) and other species (0.4% fn). Eighty-five fish were found in birds from the Goczałkowice and Dobczyce Reservoirs (NBIRDS = 5), and the species composition and proportion were similar, the most numerous being Ruffe (87.1% fn), followed by Perch (5.9% fn), Zander (3.5% fn) and Roach (3.5% fn).
Figure 2. Species of fish, their length (A) and weight (B) (NFISH = 348) eaten by Goosander (NBIRDS = 23) wintering in Poland in 2010–2018. Black dots – outliers; grey dots – all data; line – median; whiskers – the highest and lowest values; box – interquartile range. See raw data for more details: S2_Goosander_stomach_analysis.csv at Marchowski, Dominik (Citation2021), “Goosander_vs_Fishermen”, Mendeley Data, V1, doi: 10.17632/fc2kscztmv.1.
Assessment of Goosander abundance
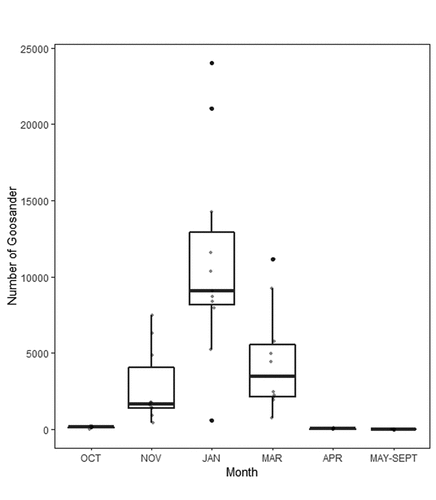
The highest numbers of Goosander in the southern Baltic lagoons (Odra and Vistula) were recorded in January – the average in 2011–2018 was 11,022 individuals (SE = 1,923, NCOUNTS = 11), with smaller numbers in autumn (November) – average 2,795 (SE = 740, NCOUNTS = 10) – and spring (March) – average 4,506 (SE = 1,019, NCOUNTS = 10). Before November and after March, numbers were significantly lower ().
Figure 3. The average numbers of Goosander Mergus merganser in southern Baltic lagoons in particular months in 2011–2018. OCT – October, NOV – November, JAN – January, MAR – March, APR – April, MAY-SEPT – period from May to September. Black dots – outliers; grey dots – all data; line – median; whiskers – the highest and lowest values; box – interquartile range.
The average Goosander abundance in the Odra estuary during the wintering period in 2010–2018 was 7,243 (SE = 1,261, NCOUNTS = 24), whereas that of the Cormorant population was 5,541 (SE = 495, NCOUNTS = 24). Indicating that Goosander was the predominant fish-eating species during the winter.
Assessment of the scale of fish consumption by Goosander
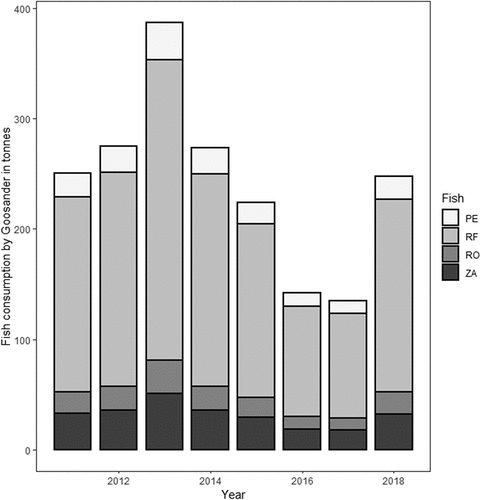
Goosander wintering in Poland achieve their highest numbers in mid-winter. Birds in larger concentrations appear in the wintering grounds in November, and large flocks disappear from these areas in March (see ). Thus, a period of five months (November – March), i.e. 151 days, was used for the calculations. Based on the digestive tract contents, the average weight of fish consumed was estimated at 139.5 g per individual, so the average daily consumption was 279 g. On average, in 2011–2018, Goosanders consumed 242 t of fish in each season (SE = 26.5, NSEASONS = 8). The most frequently eaten fish species was Ruffe, on average 170 t (SE = 19, NSEASONS = 8) per season, which accounted for an average of 70% of the total weight of all fish eaten – frequency by biomass (fb). Zander (32 t seasonally, SE = 3.5, NSEASONS = 8, 13% fb), Perch (21 t, SE = 2.3, NSEASONS = 8, 9% fb) and Roach (19 t, SE = 2.1, NSEASONS = 8, 8% fb) were also important components of the Goosander diet. Other fish species accounted for less than 0.5% (fb) of the total weight of the fish eaten by Goosander ().
Figure 4. Average yearly fish consumption by Goosander Mergus merganser wintering in the Baltic coastal lagoons and the estuaries of the two largest rivers in Poland (Vistula and Odra) in 2011–2018, broken down by fish species. PE – Perch Perca fluviatilis, RF – Ruffe Gymnocephalus cernua, RO – Roach Rutilus rutilus, ZA – Zander Sander lucioperca.
Fish caught by fishermen
In the southern Baltic lagoons, fishermen caught an average of 4,389 t of fish between 2011–2018 (SE = 310, NSEASONS = 8). They regularly caught 19 species, and occasionally a few others, grouped under the collective category “other”. Baltic herring Clupea harengus membras was the most frequently caught fish, an average of 1,909 t per year (SE = 259, NSEASONS = 8): this is due to the short-lasting (about a month, mostly in April) but intensive catches of this species when it is spawning in the Vistula Lagoon and the Odra estuary. At other times, herrings were caught much less frequently. The next most numerous species of fish that fishermen caught are better representative of the fish fauna in the lagoons and are targeted throughout the year: Roach (701 t/year, SE = 43, NSEASONS = 8), Bream Abramis brama (677 t/y SE = 58, NSEASONS = 8), Perch (668 t/y, SE = 39, NSEASONS = 8) and Zander (189 t/y, SE = 23, NSEASONS = 8).
The effect of Goosander foraging on fishermen’s catches
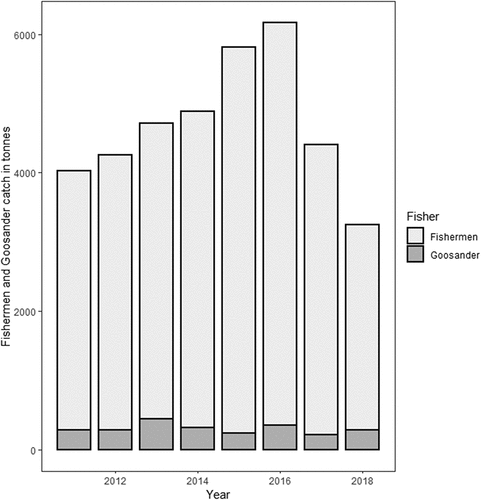
The average weight of Goosander’s prey in the southern Baltic lagoons in 2011–2018 was equivalent to 5.5% of the weight of fishermen’s catches in the same area and during the same period ().
Figure 5. Fishing by fishermen and Goosander Mergus merganser in the lagoons of the southern Baltic in 2011–2018.
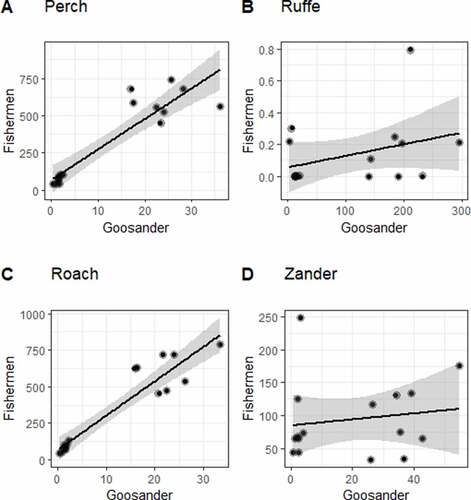
No negative impact of Goosander feeding on fishermen’s catches was demonstrated for any of the fish species in the overlapping ecological niches (). A positive correlation was demonstrated in the case of Perch (F(1, 14) = 70.06, p < 0.001; R2 = 0.834), and likewise for Roach (F(1, 14) = 103.7, p < 0.001; R2 = 0.881). In contrast, there was no correlation between the variables for Ruffe (F(1, 14) = 2.021, p = 0.177; R2 = 0.126) or for Zander (F(1, 14) = 0.316, p = 0.583; R2 = 0.022).
Figure 6. The impact of Goosander Mergus merganser foraging on fishermen’s catches in the lagoons of the southern Baltic (Poland) in 2011–2018 (NSEASONS = 8). Species from overlapping ecological niches are shown. Black dots - fish caught by Fishermen and Goosander in tons, black line - linear model fitted to the data, the grey area is the 95% confidence level interval for predictions from the linear model.
Discussion
Dietary composition of Goosander
Because Goosander is considered an opportunistic species (Pearce et al. Citation2020), its dietary composition depends on the place where it is feeding and what species of fish are most easily available (White Citation1957; Bellrose Citation1980). It seems, however, that certain preferences are discernible in southern Baltic waters. Despite the fairly large numbers of species such as Bream and Crucian carp Carassius carassius (Wolnomiejski & Witek Citation2013), these fish were not found in the digestive tracts of Goosander. It seems that this fish-eating duck prefers slender-shaped fish that are probably easier to swallow (see later in the Discussion for further details). Other studies also reported that Goosander preferred to eat slender species such as Atlantic salmon Salmo salar (Sjöberg Citation1988), Brown trout Salmo trutta (Pearce et al. Citation2020) and Three-spined stickleback (Lemrnetyinen & Mankki Citation1975).
Throughout its distribution, Goosander consumes a great variety of fish species, for example: Atlantic salmon, constituting 65.7% of the food composition in Nova Scotia (White Citation1957); Sculpin Cottoidea, making up from 23.6% to 54.0% of the diet in British Columbia (Munro & Clemens Citation1932); Brown trout, accounting for 47.4% of the food composition in Michigan (Pearce et al. Citation2020); Gizzard shad Dorosoma cepedianum, accounting for 76% of the food composition in Oklahoma (Miller & Barclay Citation1973); Threadfin shad Dorosoma petenense, accounting for 47% of the food composition in New Mexico (McCaw et al. Citation1996). The above studies relate to North America; other publications concern northern (Sjöberg Citation1985; Kålås et al. Citation1993) and central Europe (Wziątek & Konieczny Citation2012), where salmonid fish are also mentioned as the main dietary component. Kear (Citation2005) states that in Europe, Goosander feeds on Roach. Several other species of fish are given as the main food item, e.g. Roach, Three-spined stickleback, Eelpout, Pike and Herring (Bagge et al. Citation1973; Lemrnetyinen & Mankki Citation1975), but nowhere in the literature has Ruffe been mentioned as the main component of the Goosander diet, even in relatively nearby locations such as the Curonian Lagoon in Lithuania (Žydelis & Kontautas Citation2008). In contrast to our study, the main food item in the Curonian Lagoon was Smelt (84% according to biomass and numbers; Žydelis & Kontautas Citation2008).
Assessment of Goosander abundance
The Goosander population studied in this research belongs to the one wintering in north-western and central Europe and is estimated at 170,000–260,000 individuals, the trend being stable or increasing (Nagy & Langendoen Citation2020). On this basis, we can calculate that, on average, from 4.2% to 6.5% of this population spent the winter (2011–2018) in the coastal lagoons and estuaries of two rivers, the Vistula and the Odra (the Polish part). These areas are therefore important wintering sites for this species. In view of the high numbers of wintering Goosander in our study sites (Marchowski et al. Citation2018); () and the concurrent, intensive gillnet fishing in these waters (Psuty et al. Citation2017), two aspects should be considered: first, the impact of this species on commercial fish stocks (the aim of this article), and secondly, the impact of fishing on the state of this species. The southern Baltic is well-known as one of the world’s most important hotspots for high bycatches of birds in gillnets (Žydelis et al. Citation2013). The estimated annual bycatch of all diving bird species in our study sites together with German Baltic waters is about 45,000 individuals (Žydelis et al. Citation2009). The birds collected for the present study also came from bycatch, and numerous toxicological and parasitological studies have been conducted on Goosander, bycaught in the Odra estuary (Kavetska Citation2005, Citation2006; Kavetska & Borgsteede Citation2005; Kavetska & Kornyushin Citation2008; Kalisińska et al. Citation2010, Citation2014; Królaczyk et al. Citation2012; Stapf et al. Citation2013). This confirms that bycatch is a ubiquitous threat to Goosander in the waters of the southern Baltic. It would therefore be advisable to analyse the impact of fishing in the lagoons on the Goosander population.
Assessment of the scale of fish consumption by Goosander
The daily consumption of food by fish-eating birds is estimated at c. 20% of their body weight (Carss Citation1997; Engström Citation2001). Thus, in the case of Goosander, which have an average body weight of c. 1,500 g (Pearce et al. Citation2020), this consumption should be c. 293 g of fish. Our calculations showed the average daily consumption of fish by Goosander to be 279 g, so these two figures can be considered similar. In the estuaries of the two largest rivers in Poland, Goosander consumes 242 t of fish annually, which can be converted into 2.87 kg of fish per hectare. A similar study in the Lithuanian part of the Curonian Lagoon showed that Goosander consumed 170 t of fish there, i.e. 4.25 kg/ha (Žydelis & Kontautas Citation2008). Those authors also examined a few other bird species – Cormorant, Grey Heron Ardea cinerea and Great Crested Grebe Podiceps cristatus – which together consume 700 t of fish (Žydelis & Kontautas Citation2008). In the Curonian Lagoon, Cormorants consume the largest proportion of fish - 346 t (50%), followed by Goosander (24%). Many studies have been conducted regarding the amounts of fish consumed by Cormorants, as a result of which we know which species of fish they eat and in what amounts (e.g. Dirksen et al. Citation1995; Veldkamp Citation1997; Stempniewicz et al. Citation2003; Bzoma Citation2011). So far, however, no analysis has been carried out of the second most abundant fish-eating bird, i.e. Goosander, which at certain times of the year and in certain areas may outnumber the Cormorant (Marchowski et al. Citation2018). In the Odra estuary, for example, the average abundance of Goosander during the wintering period in 2010–2018 was higher than that of Cormorant (see Results). Goosander was thus the dominant fish-eating species in this water body. Other piscivorous species, such as Grey Heron, Great Crested Grebe and Smew (Mergellus albellus), were less numerous (Marchowski et al. Citation2018), but taken together they may also constitute an important factor limiting fish populations in the southern Baltic estuaries. Further research should therefore aim to investigate the joint impact of various fish-eating birds on fishery and, conversely, the effects of fishery on the mortality of piscivorous birds. It should be noted that all species of piscivorous birds are legally protected in Poland and are also subject to the relevant regulations of the European Union (Bzoma Citation2011).
Fish caught by fishermen
In 2011–2018, fishermen caught an average of almost 4,400 t of fish per year in the lagoons around the mouths of the Odra and Vistula (). Catches increased successively in 2011–2016, peaking in 2016 at 5,800 t, but then fell in the next two years to the lowest level in the time window examined here, i.e. 2,970 t in 2018. The most frequently caught fish was Herring, 1,900 t on average during this period (SE = 732, NSEASONS = 8); it accounted for 43% of all fish caught in both estuaries. Herring catches are specific: this fish is caught mainly in the Vistula Lagoon (96%) during the short period when Herring spawn there (Psuty Citation2012). No Herrings were detected in the stomachs of Goosander, as the spawning of this fish, which occurs mainly in April, does not coincide with the presence of extremely large flocks of wintering Goosander (cf. ; Psuty Citation2012). Hence, these fish-eating ducks will not have had a significant impact on Herring catches. Conversely, the Herring fishery should not have such a great impact on Goosander bycatch, because of the above-mentioned seasonal factor and also because fyke traps are used, which do not pose such a serious threat to diving birds as gillnets (Psuty et al. Citation2017; Marchowski et al. Citation2020).
The effect of Goosander foraging on fishermen’s catches
This study has shown that the impact of Goosander on fishing in the lagoons of the southern Baltic Sea was insignificant. The amount of fish that these birds ate made up just 5.5% of what fishermen caught each year (). Ruffe, the fish species most preferred by Goosander, was of very little interest to fishermen: between 2011–2018 in both lagoons (Szczecin and Vistula), fishermen caught an average of c. 300 kg of this fish per year, whereas Goosander consumed 170 t of it per year during the same period. This shows that the ecological niches of Goosander and fishermen overlap to only a marginal extent and basically concern three species of fish: Perch, Roach and Zander. Interestingly, even though our results show a statistically significant positive relationship in the case of Perch and Roach fishing (), this does not mean that larger catches by fishermen coincided with the greater consumption of these fish by Goosander. This is simply because the Perch and Roach were more abundant in some years, so both fishermen and Goosander caught more of them. The numbers of Roach and Perch caught by Goosander were each c. 3% of the fishermen’s catches (Roach: 19 t vs 701 t; Perch: 21 t vs 668 t).
When comparing the foraging niches between Goosander and fishermen, we used extrapolated biomass removals. At this point, note should be made on the importance of numbers versus weight. Goosander consumes larger numbers of fish compared to the fishery if we measure the number versus biomass. This is because the size and weight of the fish eaten by Goosander are smaller, while fishermen target larger fish. However, this is of marginal importance because, as we have shown, 82.5% (fn) of all fish eaten by Goosander is an economically insignificant Ruffe. Goosander is generally a food opportunist (Pearce et al. Citation2020), a fact that our research confirmed. For preference, it consumed Ruffe – probably one of the most abundant fish species in these water bodies (Wolnomiejski & Witek Citation2013) – which accounted for 70% (fb) of the weight of all the fish it ate (). It seems, however, that Goosander did display some selectivity in its choice of fish. Whenever possible, it consumed Zander for preference; although this species is rarer than Roach and Perch (Wolnomiejski & Witek Citation2013), it made up a higher percentage in its diet (13% fb, see ). The percentage of Zander caught by Goosander was about 17% (fb) of the amount caught by fishermen (32 t vs 189 t).
Conclusion
This article fills a gap in our knowledge of Goosander’s dietary composition and the impact of this bird on the fisheries in the estuarine lagoons of the Rivers Vistula and Odra in the southern Baltic Sea. We have demonstrated that, despite it being the most numerous piscivorous bird in some periods of the year, its foraging has no effect on the amounts of fish caught by fishermen. The ecological niches of fishermen and Goosander overlap to only a marginal extent. Ruffe was the most abundant species consumed by Goosander, and only three species it consumed – Zander, Perch and Roach – were of interest to fishermen. Even so, the percentages caught by the birds were small in relation to the fishermen’s catches (17%, 3% and 3% of biomass, respectively). In this context, further topics worth addressing include examining the impact of the fisherman on the mortality of Goosander, as it is a species often bycaught in gillnets. Likewise, the influence of all fish-eating bird species on fishery and vice versa, the influence of fishery on fish-eating birds, should be looked at.
Additional information
Counting of wintering Goosander was carried out as part of the Monitoring of Wintering Birds scheme in Poland carried out on behalf of the Chief Inspectorate of Environmental Protection and the West Pomeranian Nature Society. Due to the fact that Goosander is covered by legal protection in Poland, permits were obtained for the preparation of found dead birds under protection (including Goosander) for research purposes, the documents were issued by the appropriate government unit - the Regional Directorate for Environmental Protection, numbers of individual documents: RDOŚ −32-WOPN-6631/z/D/12/10/mk; WOPN.6402.100.2011.MK, decision No. 66/2011; WOPN.6401.186.2012.AA, decision No. 127/2012; WOPN-OG.6401.5.2014.AW. All the data on the basis of which the analyses were carried out in this article can be found in the public repository: Marchowski, Dominik (Citation2021), “Goosander_vs_Fishermen”, Mendeley Data, V1, doi: 10.17632/fc2kscztmv.1
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Anderson BW, Reeder MG. 1977. Food habitats of the Common Merganser in winter. Bulletin of the Oklahoma Ornithological Society 10:3–6.
- Bagge P, Lemmetyinen R, Raitis T. 1973. Spring food of some diving waterfowl in the southwestern Finnish archipelago. Oikos Suppl 15:146–150.
- Bayer RD 1989. The Cormorant/Fisherman Conflict in Tillamook County, Oregon. Studies in Oregon Ornithology, Newport, Oregon: Gahmken Press, 6:1–100.
- Beach US. 1936. The destruction of trout by fish ducks. Trans. Am. Fish. Soc 66(1):338–341.
- Bellrose FC. 1980. Ducks, geese, and swans of North America. Rev. ed. Harrisburg, PA: Stackpole Books.
- Bzoma S. 2011. Cormorant population management strategy in Poland. Warsaw: University of Life Sciences, Warszawa, Poland (in Polish).
- Carss DN. 1997. Techniques for assessing cormorant diet and food intake: Towards a consensus view. Supplemento alle Ricerche di Biologia della Selvaggina 26:197–230.
- Chodkiewicz T, Chylarecki P, Sikora A, Ł W, Bobrek R, Neubauer G, Marchowski D, Dmoch A, Kuczyński L. 2019. Report on the implementation of Art. 12 of the Birds Directive in Poland in 2013-2018: Status, changes, threats. Bulletin Monitoring of Nature 20:1–80.
- Chylarecki P, Chdkiewicz T, Neubauer G, Sikora A, Meer W, Woźniak B, Wylegała P, Ł Ł, Marchowski D, Betleja J, Bzoma S, Cenian Z, Górski A, Korniluk M, Moczarska J, Ochocińska D, Rubacha S, Wieloch M, Zielińska M, Zieliński P, Kuczyński L. 2018. Birds’ population trends in Poland. Warsaw: GIOŚ.
- Cowx IG. 2003. Interactions between fish and birds: Implications for Management. Oxford: Blackwell Publishing.
- Dirksen S, Boudewijn R, Noordhuis R, Marteijn ECL. 1995. Cormorants Phalacrocorax carbo sinensis in shallow eutrophic freshwater lakes: Prey choice and fish consumption in the non-breeding period and effects of large-scale fish removal. Ardea 83:167–184.
- Domenici P, Claireaux G, McKenzie DJ. 2007. Environmental constraints upon locomotion and predator–prey interactions in aquatic organisms: An introduction. Philosophical Transactions of the Royal Society B 362(1487):1929–1936. DOI:10.1098/rstb.2007.2078.
- Engström H. 2001. Long term effects of cormorant predation on fish communities and fishery in a freshwater lake. Ecography 24(2):127–138.
- Goc M, Mokwa T. 2011. Assessment of the distribution and number of water birds in the Polish part of the Vistula Lagoon. Sopot: Ecotone.
- Hansson S, Bergström U, Bonsdorff E, Härkönen T, Jepsen N, Kautsky L, Lundström K, Lunneryd SG, Ovegård M, Salmi J, Sendek D, Vetemaa M. 2018. Competition for the fish – Fish extraction from the Baltic Sea by humans, aquatic mammals, and birds. ICES Journal of Marine Science 75(3):999–1008. DOI:10.1093/icesjms/fsx207.
- Horoszewicz L. 1960. The value of lower pharyngeal arches as species criteria for defining fish of the Cyprinidae family. Roczniki Nauk Rolniczych 75(2):237–256. In Polish with English summary.
- Kålås JA, Heggberget TG, Bjørn PA, Reitan O. 1993. Feeding behaviour and diet of goosanders (Mergus merganser) in relation to salmonid seaward migration. Aquatic Living Resources 6(1):31–38.
- Kalisińska E, Bosiacka-Baranowska I, Lanocha N, Kosik-Bogacka D, Królaczyk K, Wilk A, Kavetska K, Budis H, Gutowska I, and Chlubek D. 2014. Fluoride concentrations in the pineal gland, brain and bone of goosander (Mergus merganser) and its prey in Odra River estuary in Poland. Environ Geochem Health. DOI: 10.1007/s10653-014-9615-6.
- Kalisińska E, Budis H, Podlasińska J, Łanocha N, Kavetska KM. 2010. Body condition and mercury concentration in apparently healthy Goosander (Mergus merganser) wintering in the Odra estuary, Poland. Ecotoxicology 19(8):1382–1399. DOI:10.1007/s10646-010-0524-x.
- Kavetska KM. 2005. The intestinal nematodes of wild ducks (Anatinae) from north-western part of Poland. Wiadomości Parazytologiczne 51(2):167–168.
- Kavetska KM. 2006. Biological and ecological background of nematode fauna structure formation in the alimentary tracts of wild Anatinae ducks in north-western Poland. Szczecin, Poland: Habilitation dissertation. Publishing House of the Agricultural University of Szczecin.
- Kavetska KM, Borgsteede FHM. 2005. Nematodes of the genus Amidostomum (Railliet et Henry, 1909) in wild ducks (Anatinae) of North-western Poland. Helminthologia 42(3):134–148.
- Kavetska KM, Kornyushin VV. 2008. Preliminary studies on the cestodofauna of goosander Mergus merganser L., 1758 from West Pomerania. Wiadomości Parazytologiczne 54(2):147–149.
- Kear J. 2005. Ducks, Geese and Swans. Oxford, UK: Oxford University Press.
- Królaczyk K, Kavetska KM, Stapf A, Kalisińska E. 2012. Streptocara formosensis Sugimoto, 1930 (Nematoda: Acuariidae) in wild ducks from the southern coast of the Baltic Sea. Helminthologia 49(4):247–252.
- Ławicki Ł, and Guentzel S. 2012. Important birds’ areas in Poland. Inventory of non-breeding species in the 2011/2012 season. Szczecin: Eco-Expert.
- Lemrnetyinen R, Mankki J. 1975. The three-spined stickleback (Gasterosteus aculeatus) in the food chains of the northern Baltic. Merentutkimuslaitoksen Julk. HausJorskningsinst 239:55–161.
- Marchowski D. 2021. Goosander_vs_Fishermen, Mendeley Data, V1. DOI:10.17632/fc2kscztmv.1
- Marchowski D, Jankowiak Ł, Ławicki Ł, Wysocki D, Chylarecki P. 2020. Fishery bycatch is among the most important threats to the European population of Greater Scaup Aythya marila. Bird Conserv. Int 29:1–18.
- Marchowski D, Ławicki Ł, Guentzel S, Kaliciuk J, and Kajzer Z. 2018. Long-term changes in the number of waterbirds at an important European wintering site. Acta Biologica 25:111–122. DOI: 10.18276/ab.2018.25-09.
- Marquiss M, Carss DN. 1994. Avian Piscivores: Basis for Policy. Bristol, UK: National Rivers Authority.
- McCaw III JH, Zwank PJ, Steine RL. 1996. Abundance, distribution, and behavior of common mergansers wintering on a reservoir in southern New Mexico. Journal of Field Ornithology 67:669–679.
- Miller SW, Barclay JS. 1973. Predation in warm water reservoirs by wintering Common Mergansers. Proc. Ann. Conf. Southeast. Assoc. Game Fish Comm 27:243–252.
- Mokwa T, Goc M, Stępniewski P, Horbacz A. 2012. Ornithological inventory of the Natura 2000 special bird protection area, the Vistula Lagoon PLB280010 (breeding avifauna). Warsaw: General Directorate for Environmental Protection.
- Munro JA, Clemens WA. 1932. Food of the American Merganser (Mergus merganser americanus) in British Columbia: A preliminary paper. Canadian Field-Naturalist 46:166–168.
- Nagy S, Langendoen T. 2020. Flyway trend analyses based on data from African – European Waterbird Census from the period of 1967 – 2018. Wageningen, The Netherlands: Online publication. Wetland International.
- Neubauer G, Meer W, Chylarecki P, Chodkiewicz T, Sikora A, Pietrasz K, Cenian Z, Betleja J, Gaszewski K, Ł K, Lenkiewicz W, Ł Ł, Rohde Z, Rubacha S, Smyk B, Wieloch M, Wylegała M, Zielińska M, Zieliński P. 2015. Monitoring Ptaków Polski w latach 2013 – 2015. Biuletyn Monitoringu Przyrody 13:1–92.
- Ovegård MK, Jepsen N, Bergenius M, Petersson E. 2021. Cormorant predation effects on fish populations: A global meta-analysis. Fish Fish 22(3):605–622. DOI:10.1111/faf.12540.
- Pearce J, Mallory ML, and Metz K. 2020. Common Merganser (Mergus merganser), version 1.0. In: Billerman SM, editor. Birds of the World. Ithaca, NY, USA: Cornell Lab of Ornithology. https://birdsoftheworld.org/bow/species/commer/cur/introduction
- Psuty I. 2012. The current state of Vistula Lagoon Polish fisheries. Perspective for development. Gdynia: National Marine Fisheries Research Institute.
- Psuty I, Szymanek L, Całkieicz J, Ł D, Ameryk A, Ramutkowski M, Spich K, Wodzinowski T, Woźniczka A, Zaporowski R 2017. [Developing the basis for rational monitoring of by-catch of birds for sustainable management of coastal fishing in the marine areas of NATURA 2000.] Gdynia. Morski Instytut Rybacki -Państwowy Instytut Badawczy. In Polish with English summary, available at: przylowy.mir.gdynia.pl/monografia.
- R Development Core Team. 2021. R: A Language and Environment for Statistical Computing.”. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org/.
- Scanlon PF, Helfrich LA, Stultz RE. 1978. Extent and severity of avian predation at federal fish hatcheries in the United States. Proc. Ann. Conf. Southeast. Assoc. Fish Wildl. Agencies 32:470–473.
- Sjöberg K. 1985. Foraging activity patterns in the goosander (Mergus merganser) and the red-breasted merganser (M. serrator) in relation to patterns of activity in their major prey species. Oecologia 67(1):35–39.
- Sjöberg K. 1988. Food selection, food-seeking patterns and hunting success of captive Goosanders Mergus merganser and Red-breasted Mergansers M. serrator in relation to the behaviour of their prey. Ibis 130(1):79–93.
- Smith MW. 1967. Observations of fish-eating birds and mammals at Crecy Lake, New Brunswick over a 12-year period. Canadian Fish-Culturist 39:41–46.
- Stapf AN, Kavetska KM, Ptak PP, Rząd I. 2013. Morphometrical and ecological analysis of nematodes of the family Capillariidae (Neveu-Lemaire, 1936) in wild ducks (Anatinae) from the north-western Poland. Annals of Parasitology 59(4):195–201.
- Stempniewicz L, Martyniak A, Borowski W, Goc M. 2003. Fish stocks, commercial fishing and cormorant predation in the Vistula Lagoon, Poland. In: I.g C, editor. Interactions between fish and birds: Implications for managements. Oxford: Blackwell Sci. pp. 51–64.
- Tasker ML, Camphuysen CJ, Cooper J, Garthe S, Montevecchi WA, Blaber SJM. 2000. The impacts of fishing on marine birds. ICES Journal of Marine Science 57(3):531–547. DOI:10.1006/jmsc.2000.0714.
- Veldkamp R. 1997. Early breeding by Cormorants Phalacrocorax carbo sinensis at Wanneperveen, The Netherlands: Profiting by spawning Roach Rutilus rutilus. Suppl. Ric. Biol. Selvaggina 26:99–109.
- Wardecki Ł, Chdkiewicz T, Beuch S, Smyk B, Sikora A, Neubauer G, Meer W, Marchowski D, Wylegała P, and Chylarecki P. 2021. Monitoring of Polish Birds in 2018-2021. In: Biuletym Monitoringu Przyrody. Warsaw: Chief Inspector of Environmental Protection, in Polish: GIOŚ: Główny Inspektorat Ochrony Środowiska, Vol. 22, pp. 1–80.
- Wetlands International. 2010. Guidance on waterbird monitoring methodology: Field protocol for waterbird counting. Wetlands International, Wageningen. Available at: http://www.wetlands.org.
- Wetlands International. 2022. Waterbird Population Estimates. Wageningen, The Netherlands: Wetland International. Retrieved from wpe.wetlands.org on 27 January 2022.
- White HC. 1957. Food and natural history of mergansers on salmon waters in the Maritime provinces of Canada. Bull. Fish. Res. Board Canada 116:1–71.
- Wolnomiejski N, Witek Z 2013. The Szczecin Lagoon Ecosystem: The Biotic Community of the Great Lagoon and its Food Web Model:, Versita Ltd, 78 York Street, London W1H 1DP, Great Britain.: De Gruyter Open Poland. 10.2478/9788376560502.
- Wood CC. 1987. Predation of Juvenile Pacific Salmon by the Common Merganser (Mergus merganser) on Eastern Vancouver Island. I: Predation during the Seaward Migration. Canadian Journal of Fisheries and Aquatic Sciences 44(5):941–949. DOI:10.1139/f87-112.
- Wziątek B, Konieczny P. 2012. Food of Goosander, Mergus merganser L., feeding in winter on the San River between Zwierzyń and Lesko (Southeast Poland). In Polish with English summary. Komunikaty rybackie 128:9–13.
- Žydelis R et al. 2009. Bycatch in gillnet fisheries—an overlooked threat to waterbird populations. Biol. Conserv 142(7):1269–1281. DOI: 10.1016/j.biocon.2009.02.025.
- Žydelis R, Kontautas A. 2008. Piscivorous birds as top predators and fishery competitors in the lagoon ecosystem. Hydrobiologia 611(1):45–54. DOI:10.1007/s10750-008-9460-7.
- Žydelis R, Small C, French G. 2013. The incidental catch of seabirds in gillnet fisheries: A global review. Biological Conservation 162:76–88. DOI: 10.1016/j.biocon.2013.04.002.