Abstract
Although sensitivity to pollutants is well known to be species specific, closely related species are often assumed to respond similarly to them. We tested this assumption, comparing the sensitivity of Ciona intestinalis and Ciona robusta to two common marine pollutants: Bisphenol A (BPA) and tris(1-chloro-2-propyl)phosphate (TCPP). In particular, we focused on ascidian embryonic development and determined whether C. intestinalis and C. robusta displayed different responses. Our results demonstrate that closely related species can display either similar or very different sensitivity based on the tested contaminant. Ciona intestinalis and C. robusta had similar sensitivity to BPA, as their median effective concentration (EC50) and median lethal concentration (LC50) values were comparable. In contrast, TCPP showed very different teratogenic potential in the two analyzed species. Ciona robusta proved more vulnerable to this flame retardant as its teratogenic index was more than twice that calculated for C. intestinalis. Chemical modes of action as well as genetic differences could determine the diverse responses to environmental stressors. These results underline the presence of species-specific differences in embryonic sensitivity to contaminants and point out the importance of evaluating chemicals’ teratogenic profile in several species.
1. Introduction
Every year tons of pollutants reach the marine environment (Zandaryaa & Frank-Kamenetsky Citation2021). Most of these materials and chemicals originate from land-based sources (Vikas & Dwarakish Citation2015) and negatively affect the marine communities (Lionetto et al. Citation2021). For instance, plastic additives are released into the marine environment via industrial and municipal wastewater, river transport or in loco plastic degradation, and accumulate mainly along the coastline (Hermabessiere et al. Citation2017; Hahladakis et al. Citation2018; Wang et al. Citation2020). Their potential health risks have been demonstrated for a variety of marine organisms, including invertebrates such as mussels, shrimps, sea urchins and ascidians (Arslan et al. Citation2007; Arslan & Parlak Citation2008; Oehlmann et al. Citation2009; Messinetti et al. Citation2018, Citation2019; Darin Citation2021; Mercurio et al. Citation2021; Miglioli et al. Citation2021; Naveira et al. Citation2021). Comprehensive understanding and assessment of pollutants in the marine environment are crucial to define their toxicity and prevent any irreparable damage to the ecosystems (Zandaryaa & Frank-Kamenetsky Citation2021). It is well known that sensitivity to pollutants can differ among species (Bellas et al. Citation2005; Bao et al. Citation2011; Mdaini et al. Citation2021). For example, the median lethal concentration (LC50) of Bisphenol A (BPA) varied from 3.5 mg/L for Poecilia vivipara to 107.2 mg/L for Artemia salina (Naveira et al. Citation2021), while the median effective concentration (EC50) of tributyltin (TBT) was 0.3 µg/L for Paracentrotus lividus and 7.1 µg/L for Ciona intestinalis (Bellas et al. Citation2005).
Even if chemical tolerance has been largely demonstrated to be species specific, closely related species have often been assumed to respond similarly to pollutants. However, a few rare studies have suggested this is not the case (Rocha-Olivares et al. Citation2004; Feckler et al. Citation2012; Monteiro et al. Citation2018). It has been reported that cryptic Gammarus fossarum lineages are characterized by different environmental stress tolerance (Feckler et al. Citation2012), and, similarly, cryptic species of nematodes substantially differ in their response to heavy metal contaminants (Monteiro et al. Citation2018). The different levels of sensitivity to pollutants exhibited by two marine copepods, Cletocamptus fourchensis and C. stimpson, have even led scientists to consider them one of the main causes of the ongoing losses of genetic diversity (Rocha-Olivares et al. Citation2004).
Ascidians are key members of coastal benthic communities, able to colonize both natural and artificial substrates. Adults are sessile filter-feeding animals while larvae are free-swimming (Shenkar & Swalla Citation2011). These latter display the typical chordate body plan, comprising a notochord and a dorsal tubular nervous system, as they belong to the tunicates, the sister group of vertebrates (Delsuc et al. Citation2006). Recently, detailed morphological and molecular analyses recognized the ascidians Ciona intestinalis and Ciona robusta as two separate entities. These species were formerly part of a complex of cryptic species under the name Ciona intestinalis (Brunetti et al. Citation2015). Despite their morphological similarity, members of these species are highly genetically divergent (Brunetti et al. Citation2015; Pennati et al. Citation2015) and show different tolerance to ecological factors such as temperature (Sato et al. Citation2015).
In the present work, we took advantage of the accessibility of these closely related ascidian species to compare their sensitivity to two pollutants. We tested the effects of BPA and tris(1-chloro-2-propyl)phosphate (TCPP) on embryonic development and determined the responses of C. intestinalis and C. robusta to these pollutants. BPA and TCPP are common contaminants in marine ecosystems, whose adverse effects have been already characterized for one of the two species (Messinetti et al. Citation2019; Mercurio et al. Citation2021). BPA is a monomer of polycarbonate also used as stabilizer. It is one of the most commonly produced chemicals worldwide and, despite its activity as endocrine disruptor, it is still used in drink and food packaging. In the marine environment, BPA concentrations have been reported to vary from traces to 2.6 µg/L in UK estuaries (Hermabessiere et al. Citation2017). In ascidians, BPA was shown to affect embryogenesis mainly at the level of the nervous system, interfering with the development of dopaminergic and GABAergic systems as well as with sensory organ formation at concentrations close to those recorded in marine polluted areas (Messinetti et al. Citation2019). TCPP is an organophosphorus flame retardant, highly present in seawater; it is generally added to rigid and spray polyurethane and can be released into the environment even by direct contact (Truong et al. Citation2017). Flame retardants are highly detected in seawater, ranging from a few ng/L to more than 15 ng/L (Hermabessiere et al. Citation2017). In C. intestinalis, TCPP was observed to specifically alter myogenesis, while no effects were found on neural differentiation. Even if these effects were reported at concentrations far from the environmental ones, the ability of the pollutant to accumulate in animal tissues and its potential additive toxicity make these findings noteworthy (Mercurio et al. Citation2021). Overall, this study increases our knowledge about the toxicological profiles of these common pollutants in the marine environment, and contributes to improving science-based policy and environmental management.
2. Materials and methods
2.1. Animals and chemicals
Adults of Ciona robusta were collected from natural populations in Chioggia (Italy) while Ciona intestinalis was collected by the fishing service of the station Biologique de Roscoff (France). Animals were maintained in 50 L aquaria filled with artificial seawater (ASW, Instant Ocean®, Aquarium System) and equipped with mechanical, chemical and biological filters. The temperature was fixed at 18 ± 1°C and constant light conditions were applied to avoid gamete release.
All the experimental procedures were performed at 18 ± 1°C. For each experiment, three animals were sacrificed. Gametes were obtained by dissection, and cross‐fertilization was performed in glass Petri dishes (Ø 4 cm).
TCPP (MW = 327.57) was purchased from Sigma (Milan, Italy). A stock solution of 100 mg/mL was made in dimethyl sulfoxide (DMSO; Sigma, Milan, Italy) and then diluted in filtered artificial sea water with 1 M HEPES pH 8.0 (ASWH) to reach the final test concentrations (0.1, 1.25, 12.5, 25, 50, 75 and 100 μg/mL). A solution of 0.1% DMSO in ASWH was used as a solvent control each time. BPA (MW = 228.29) was purchased from Sigma (Milan, Italy). A stock solution of 100 mM BPA was made in DMSO and then diluted in ASWH to reach the final test concentrations (0.1, 0.5, 1, 5, 10 and 20 μM). As a solvent control, a solution of 0.02% DMSO in ASWH was used. Fresh solutions were prepared each time. For both TCPP and BPA experiments, concentrations were chosen based on previous works (Messinetti et al. Citation2019; Mercurio et al. Citation2021). Preliminary trials were performed starting from concentrations close to environmental ones to define effective and lethal doses.
2.2. Exposure during ascidian embryogenesis
About 50 embryos at the two‐cell stage were transferred to Petri dishes filled with 10 mL of the various test solutions and reared until the larva stage (~18 hours post fertilization (hpf)). Experiments were performed in triplicate and considered reliable only if at least 80% of control embryos hatched. When control embryos reached the larval stage, all samples were fixed in 4% paraformaldehyde, 0.5 M NaCl and 0.1 M 3-(N-morpholino)propanesulfonic acid (MOPS fixative; pH 7.5) for 90 min, washed in Phosphate Buffered Saline (PBS) and examined under a microscope. The numbers of normal, malformed and dead larvae were noted, and the corresponding percentages were calculated.
Ciona intestinalis samples were exposed to BPA while C. robusta was used to test TCPP. Data about BPA in C. robusta have been partially published in Messinetti et al. (Citation2019). To compare the two species, previous data were further analyzed. The effects of TCPP on C. intestinalis were already reported by Mercurio et al. (Citation2021).
2.3. Statistical analysis
Analysis of variance (ANOVA), followed by Tukey’s honestly significant difference (HSD) post hoc test, was performed to assess the effects of TCPP/BPA on larval development. Prior to analyses, we verified the homogeneity and normality of the variances. Probit analysis was performed following the simple least squares regression method to calculate LC50 and EC50. All the analyses were performed in the R 3.6.3 environment (Team Citation2019).
3. Results
3.1. TCPP effects on C. robusta development
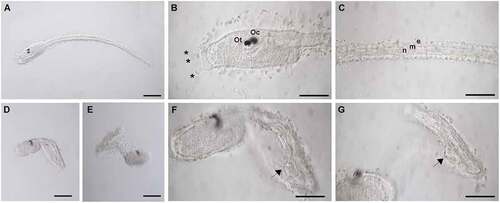
TCPP exposure affected C. robusta embryonic development (). Control and DMSO larvae appeared normally developed and motile (). The larval trunk was elongated with three adhesive papillae at the anteriormost region () and the tail was long and straight (). Larvae exposed to concentrations higher than 25 μg/mL were usually unable to swim. Their trunks were roundish and tails were variously bent (). At the bend point, a large cell was always observed. This ovoid cell was about 20 μm long and localized among muscle cells (). Dead larvae showed a completely disrupted morphology and remained inside the chorion membrane (data not shown).
Figure 1. Morphological evaluation of TCPP’s effects on Ciona robusta development. Morphology of control (A–C) and treated (D–G) larvae. (A) Control larva developed in in 0.1% DMSO in ASWH. (B) Magnification of the trunk of a control larva in which the otolith (Ot) and the ocellus (Oc) are observable as well as the three anterior papillae (*). (C) magnification of the tail of a control in which epidermis (e), muscle (m) and notochord (n) are visible; (D, E) malformed larva developed in 25 µg/mL TCPP; (F, G) magnification of malformed larvae displaying a large ovoid cell (arrow) at tail bend point. Scale bars: A, D, E = 100 µm; B, C, F, G = 50 µm .
A significant increase in the percentage of malformed larvae was observed from 25 μg/mL TCPP (; ANOVA: F = 9.7465, p < 0.0001; Tukey’s post hoc test: controls vs 25 μg/mL, p < 0.05; controls vs 50 μg/mL, p < 0.0001). The percentage of dead samples proved to be significantly higher than that of controls at 75 and 100 μg/mL TCPP (; ANOVA: F = 67.24, p < 0.0001; Tukey’s post hoc test: controls vs 75 μg/mL, p < 0.0001; controls vs 100 μg/mL, p < 0.0001).
Finally, probit analysis revealed that LC50 was 63.48 μg/mL (95% Confidence interval (CI) for the coefficient estimates [0.02, 0.11]) while EC50 was 21.19 μg/mL (95% CI for the coefficient estimates [0.03, 0.08]) (). The TCPP teratogenic index (TI = LC50/EC50) was 2.99.
3.2. BPA effects on the development of C. robusta and C. intestinalis
We analyzed the larval general morphology to determine BPA’s effects on ascidian embryogenesis (). Control and DMSO larvae of both species developed normally, displaying an elongated trunk and a long, straight tail (). Low concentrations of BPA did not affect larval development (). Larvae exposed to concentrations higher than 10 μM showed malformations, mainly consisting in a roundish trunk and/or a curved tail (). These phenotypes increased significantly from 10 μM concentration in both C. intestinalis ( and ; ANOVA: F = 28.02, p < 0.0001; Tukey’s post hoc test: controls vs 10 μM, p < 0.0001) and C. robusta (Messinetti et al. Citation2018; ANOVA: F = 15.806, p = .00428; Tukey’s post hoc test: control vs 10 μM, p < 0.0001). At 20 μM BPA, most of the larvae did not hatch and were considered dead (C. intestinalis: ANOVA: F = 25.69, p < 0.0001; Tukey’s post hoc test: controls vs 20 μM, p < 0.0001; C. robusta: ANOVA: F = 14.538, p < 0.001; Tukey’s post hoc test: controls vs 20 µM, p < 0.001) ( and 3(a)).
Figure 2. Effects of TCPP exposure on the embryonic development of Ciona robusta. (A) Percentages of normal, malformed and dead larvae of C. robusta exposed to TCPP. Differences from control; the number of asterisks indicates the level of significance: *p < 0.05, **p < 0.001, ***p < 0.0001. (B) TCPP dose–response curves for mortality and malformations in C. robusta. EC50 and LC50 values were calculated using probit models.
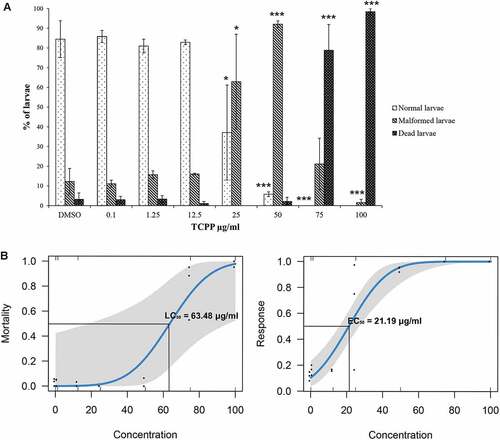
Figure 3. Morphological evaluation of the effects of BPA exposure on Ciona robusta (A–F) and C. intestinalis (G–L) development. (A, G) controls; (B–F) and (H–L) BPA. Scale bar = 100 µm.

Figure 4. Effects of BPA exposure on embryonic development of Ciona intestinalis and C. robusta. (A) Percentages of normal, malformed and dead larvae exposed to different concentrations of BPA in C. intestinalis. Differences from control: ***p < 0.001. (B) Dose–response curves for mortality in C. intestinalis and C. robusta. (C) Dose–response curves for malformations in C. intestinalis and C. robusta. EC50 and LC50 values were calculated using probit models.
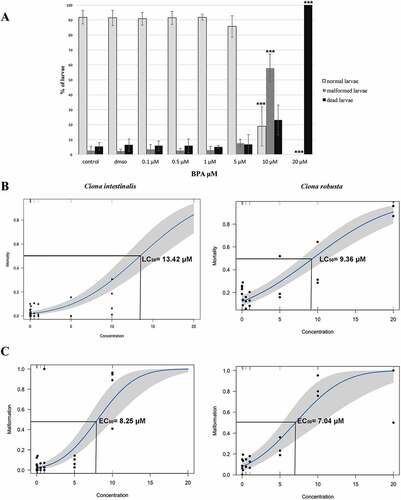
Probit analysis ( B and C) confirmed these results: EC50 was 8.25 μM (95% CI for the coefficient estimates [0.18, 0.31]) for C. intestinalis and 7.04 μM (95% CI for the coefficient estimates [0.12, 0.28]) for C. robusta, while LC50 was 13.42 μM (95% CI for the coefficient estimates [0.12, 0.19]) for C. intestinalis and 9.36 μM (95% CI for the coefficient estimates [0.10, 0.15]) for C. robusta. The BPA TI was 1.63 for C. intestinalis and 1.32 for C. robusta.
4. Discussion
Ciona intestinalis and C. robusta are closely related species. They appear extremely similar at first glance and they were considered members of the same species for decades. Nevertheless, their different ability to buffer water temperature was well known among researchers even before they were recognized as separate entities (Brunetti et al. Citation2015; Pennati et al. Citation2015; Sato et al. Citation2015).
Their sensitivity to pollutants was never compared, and probably assumed to be similar. However, a few studies have demonstrated that even closely related species can display very different sensitivities to chemicals (Rocha-Olivares et al. Citation2004; Feckler et al. Citation2012; Monteiro et al. Citation2018). Thus, in the present work, we compared the tolerance of C. intestinalis and C. robusta to two common marine pollutants, BPA and TCPP, demonstrating that their responses to environmental chemicals can differ according to the tested contaminant.
Ciona intestinalis and C. robusta displayed similar sensitivity to BPA. EC50 values (8.25 μM for C. intestinalis and 7.04 μM for C. robusta) were comparable between the species while LC50 values were slightly different (11.69 μM for C. intestinalis and 9.36 μM for C. robusta). However, their TI values were comparable: 1.63 for C. intestinalis and 1.32 for C. robusta. Indeed, BPA induced the same type of malformations with similar incidences (). In both species, this chemical caused the development of a roundish trunk and a curved tail, in percentages significantly higher than in control samples starting from concentations of 10 μM. Previous research reported LC50 values of 5.4 μM (Matsushima et al. Citation2013) or 5.2 μM (Messinetti et al. Citation2019) as probit analyses were performed with a logarithmic scale. In our analysis, we preferred not to convert data as results better fit the calculated percentages: at 10 μM, C. intestinalis and C. robusta malformed larvae were 57.8 ± 9.2% and 47.7 ± 6.1, respectively, while at 5 μM malformed larvae were 7.7 ± 2.4% for C. intestinalis and 17.4 ± 0.8% for C. robusta. A similar response to BPA was reported for another ascidian species, Phallusia mammillata. Here, BPA induced comparable anatomical malformations at the level of trunk and tail (Messinetti et al. Citation2018) with an EC50 value (11.8 μM; Gomes et al. Citation2019b) close to those we observed in Ciona. The calculated LC50 was 21 μM, suggesting that P. mammillata better tolerates this pollutant even if the concentration range did not vary consistently and remained far from environmental BPA levels (Hermabessiere et al. Citation2017).
Table I. Percentages of normal, malformed and dead larvae of C. intestinalis and C. robusta after Bisphenol-A exposure. Values are expressed as mean ± standard error
On the other hand, TCPP demonstrated a different teratogenic potential in the two analyzed species. Indeed, EC50 values were 51.16 μg/mL for C. intestinalis (Mercurio et al. Citation2021) and 21.19 μg/mL for C. robusta (this paper). TI further highlighted the higher susceptibility of C. robusta to TCPP, as its TI value was more than twice that of C. intestinalis (2.99 for C. robusta and 1.29 for C. intestinalis). LC50 values were, however, comparable between the two species: 66.18 μg/mL for C. intestinalis (Mercurio et al. Citation2021) and 63.48 μg/mL for C. robusta. In ascidians, different sensitivity to environmental stressors was reported also at the population level. In a C. robusta population sampled at the Fusaro Lagoon (Italy), three separate genetic clusters were found to respond differentially to environmental variables, such as salinity, temperature and oxygen availability. Moreover, these clusters appeared to differentially handle metal pollution, suggesting that C. robusta is provided with great genetic pools, allowing a rapid adaptation to environmental changes (Caputi et al. Citation2019).
The different responses to BPA and TCPP observed in C. intestinalis and C. robusta could be explained by differences in pollutant mode of action. BPA is a well-known endocrine disruptor, which interferes with animal physiology mainly by binding nuclear receptors, such as estrogen-related-receptor γ (ERRγ) (Okada et al. Citation2008), thyroid receptor (TR) (Zoeller et al. Citation2005), pregnane X receptors and peroxisome proliferator-activated receptors (PPARγ) (Khamphaya et al. Citation2021). In P. mammilata and C. robusta, several orthologues of vertebrate nuclear receptors have been identified (Gomes et al. Citation2019a) and, in particular, BPA interaction with P. mammillata ERR has been demonstrated (Messinetti et al. Citation2018; Gomes et al. Citation2019b). In ascidians, TCPP effects were mainly related to muscle development; disruption of the Myogenic regulatory factor (Mrf) gene network has been suggested (Mercurio et al. Citation2021), but the specific mechanism of action is still unknown. Furthermore, detoxification mechanisms of the two species can differ and may determine the diverse responses of the two species when facing the environmental stressors. In fact, the animals used in the present study were collected in nature, in two marine areas with different pollution profiles (Hermabessiere et al. Citation2017). Thus, it is conceivable that the exposure to different contaminants could have selected distinct tolerances to chemicals in animals prone to adapt quickly (Caputi et al. Citation2019). Moreover, ascidians are efficient filter-feeding organisms, which accumulate contaminants in their tissues. In particular, recent studies have underlined their ability to bioaccumulate microplastics and phthalates as well as heavy metals, enabling these animals to reflect the pollution levels of their environment (Tzafriri-Milo et al. Citation2019; Vered et al. Citation2019).
Data about levels of TCPP or other flame retardants in the marine environment are rare. In the northeast Atlantic and the Arctic Ocean, TCPP concentrations were found to range between 279 and 5773 pg/L (Li et al. Citation2017), while higher levels were detected in North Sea surface water (Bollmann et al. Citation2012). No precise data were found for the sampling areas of the species used in this work. Conversely, BPA was measured and found in traces (<0.001–0.145 µg/L) in the Venetian lagoon (Mediterranean Sea) (Pojana et al. Citation2007), close to our C. robusta sampling site. No study thus far has focused on BPA levels along the English Channel, but it was detected in fish from the northeast Atlantic Ocean (Barboza et al. Citation2020).
Overall, these results strongly underline the presence of species-specific differences in embryonic sensitivity to contaminants and point out the importance of evaluating chemicals’ teratogenic profile in several species. Comprehensive toxicological analyses are necessary to make environmental management and science-based policy as inclusive as possible. Moreover, marine invertebrates are particularly threatened by environmental pollutants since both fertilization and embryonic development usually occur in the water column, in direct contact with a mixture of anthropogenic contaminants. Considering that the precise modes of action of most of these chemicals are still unknown, there is the possibility that they can interact with each other and induce additive effects, making ecotoxicological studies even more urgent.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arslan OC, Parlak H. 2008. Effects of bisphenol-a on the embryological development of the sea urchin Arbacia Lixula (Linnaeus, 1758). Fresenius Environmental Bulletin 17(2):127.
- Arslan OC, Parlak H, Oral R, Katalay S. 2007. The effects of nonylphenol and octylphenol on embryonic development of sea urchin (Paracentrotus lividus). Archives of Environmental Contamination and Toxicology 53(2):214–219. DOI: 10.1007/s00244-006-0042-2.
- Bao VW, Leung KM, Qiu J-W, Lam MH. 2011. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species. Marine Pollution Bulletin 62(5):1147–1151. DOI: 10.1016/j.marpolbul.2011.02.041.
- Barboza LGA, Cunha SC, Monteiro C, Fernandes JO, Guilhermino L. 2020. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination. Exposure and risk to human consumers. Journal of Hazardous Materials 393:122419. DOI: 10.1016/j.jhazmat.2020.122419.
- Bellas J, Beiras R, Marino-Balsa JC, Fernández N. 2005. Toxicity of organic compounds to marine invertebrate embryos and larvae: A comparison between the sea urchin embryogenesis bioassay and alternative test species. Ecotoxicology 14(3):337–353. DOI: 10.1007/s10646-004-6370-y.
- Bollmann UE, Möller A, Xie Z, Ebinghaus R, Einax JW. 2012. Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Research 46(2):531–538. DOI: 10.1016/j.watres.2011.11.028.
- Brunetti R, Gissi C, Pennati R, Caicci F, Gasparini F, Manni L. 2015. Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis. Journal of Zoological Systematics and Evolutionary Research 53:186–193. DOI:10.1111/jzs.12101
- Caputi L, Toscano F, Arienzo M, Ferrara L, Procaccini G, Sordino P. 2019. Temporal correlation of population composition and environmental variables in the marine invader Ciona robusta. Marine Ecology 40(2):e12543. DOI: 10.1111/maec.12543.
- Darin E. 2021. Effects of Bisphenol-A on the morphology and survival of larvae of the sand dollar Dendraster excentricus (Echinodermata, Echinoidea). Invertebrate Reproduction & Development 651:1–8.
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. 2006. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439(7079):965–968. DOI: 10.1038/nature04336.
- Feckler A, Thielsch A, Schwenk K, Schulz R, Bundschuh M. 2012. Differences in the sensitivity among cryptic lineages of the Gammarus fossarum complex. Science of the Total Environment 439:158–164. DOI: 10.1016/j.scitotenv.2012.09.003.
- Gomes ID, Gazo I, Besnardeau L, Hebras C, McDougall A, Dumollard R. 2019a. Potential roles of nuclear receptors in mediating neurodevelopmental toxicity of known endocrine-disrupting chemicals in ascidian embryos. Molecular Reproduction and Development 86(10):1333–1347. DOI: 10.1002/mrd.23219.
- Gomes ID, Gazo I, Nabi D, Besnardeau L, Hebras C, McDougall A, Dumollard R. 2019b. Bisphenols disrupt differentiation of the pigmented cells during larval brain formation in the ascidian. Aquatic Toxicology 216:105314. DOI: 10.1016/j.aquatox.2019.105314.
- Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. 2018. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. Journal of Hazardous Materials 344:179–199. DOI: 10.1016/j.jhazmat.2017.10.014.
- Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G. 2017. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 182:781–793. DOI: 10.1016/j.chemosphere.2017.05.096.
- Khamphaya T, Pouyfung P, Kuraeiad S, Vattanasit U, Yimthiang S. 2021. Current aspect of Bisphenol A toxicology and its health effects. Trends in Sciences 18(21):408. DOI: 10.48048/tis.2021.408.
- Li J, Xie Z, Mi W, Lai S, Tian C, Emeis K-C, Ebinghaus R. 2017. Organophosphate esters in air, snow, and seawater in the North Atlantic and the Arctic. Environmental Science & Technology 51(12):6887–6896. DOI: 10.1021/acs.est.7b01289.
- Lionetto MG, Caricato R, Giordano ME. 2021. Pollution biomarkers in the framework of marine biodiversity conservation: State of art and perspectives. Water 13(13):1847. DOI: 10.3390/w13131847.
- Matsushima A, Ryan K, Shimohigashi Y, Meinertzhagen IA. 2013. An endocrine disruptor, Bisphenol A, affects development in the protochordate Ciona intestinalis: Hatching rates and swimming behavior alter in a dose-dependent manner. Environmental Pollution 173:257–263. DOI: 10.1016/j.envpol.2012.10.015.
- Mdaini Z, Telahigue K, Hajji T, Rabeh I, Tremblay R, Gagné JP. 2021. Comparative biomarker responses to urban pollution in three polychaete species: Perinereis cultrifera, Diopatra neapolitana, and Marphysa sanguinea from the lagoon of Tunis. Environmental Monitoring and Assessment 193(3):1–11. DOI: 10.1007/s10661-021-08906-5.
- Mercurio S, Messinetti S, Manenti R, Ficetola GF, Pennati R. 2021. Embryotoxicity characterization of the flame retardant tris (1-chloro-2-propyl) phosphate (TCPP) in the invertebrate chordate Ciona intestinalis. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 335(3):339–347. DOI: 10.1002/jez.2446.
- Messinetti S, Mercurio S, Pennati R. 2018. Effects of Bisphenol A on the development of pigmented organs in the ascidian Phallusia mammillata. Invertebrate Biology 137(4):329–338. DOI: 10.1111/ivb.12231.
- Messinetti S, Mercurio S, Pennati R. 2019. Bisphenol A affects neural development of the ascidian Ciona robusta. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology 331(1):5–16. DOI: 10.1002/jez.2230.
- Miglioli A, Balbi T, Besnardeau L, Dumollard R, Canesi L. 2021. Bisphenol A interferes with first shell formation and development of the serotoninergic system in early larval stages of Mytilus galloprovincialis. Science of the Total Environment 758:144003. DOI: 10.1016/j.scitotenv.2020.144003.
- Monteiro LC, Van Butsel J, De Meester N, Traunspurger W, Derycke S, Moens T. 2018. Differential heavy-metal sensitivity in two cryptic species of the marine nematode Litoditis marina as revealed by developmental and behavioural assays. Journal of Experimental Marine Biology and Ecology 502:203–210. DOI: 10.1016/j.jembe.2017.05.016.
- Naveira C, Rodrigues N, Santos FS, Santos LN, Neves RA. 2021. Acute toxicity of Bisphenol A (BPA) to tropical marine and estuarine species from different trophic groups. Environmental Pollution 268:115911. DOI: 10.1016/j.envpol.2020.115911.
- Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, Kusk KO, Wollenberger L, Santos EM, Paull GC, Van Look KJ. 2009. A critical analysis of the biological impacts of plasticizers on wildlife. Philosophical Transactions of the Royal Society B: Biological Sciences 364(1526):2047–2062. DOI: 10.1098/rstb.2008.0242.
- Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. 2008. Direct evidence revealing structural elements essential for the high binding ability of Bisphenol A to human estrogen-related receptor-γ. Environmental Health Perspectives 116(1):32–38. DOI: 10.1289/ehp.10587.
- Pennati R, Ficetola GF, Brunetti R, Caicci F, Gasparini F, Griggio F, Sato A, Stach T, Kaul-Strehlow S, Gissi C. 2015. Morphological differences between larvae of the Ciona intestinalis species complex: Hints for a valid taxonomic definition of distinct species. PloS one 10(5):e0122879. DOI: 10.1371/journal.pone.0122879.
- Pojana G, Gomiero A, Jonkers N, Marcomini A. 2007. Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environment International 33(7):929–936. DOI: 10.1016/j.envint.2007.05.003.
- Rocha-Olivares A, Fleeger JW, Foltz DW. 2004. Differential tolerance among cryptic species: A potential cause of pollutant-related reductions in genetic diversity. Environmental Toxicology and Chemistry: An International Journal 23(9):2132–2137. DOI: 10.1897/03-512.
- Sato A, Kawashima T, Fujie M, Hughes S, Satoh N, Shimeld SM. 2015. Molecular basis of canalization in an ascidian species complex adapted to different thermal conditions. Scientific Reports 5(1):1–9. DOI: 10.1038/srep16717.
- Shenkar N, Swalla BJ. 2011. Global diversity of Ascidiacea. PLoS One 6(6):e20657. DOI: 10.1371/journal.pone.0020657.
- Team RC. 2019. R: A language and environment for statistical computing (3.5. 3). Vienna, Austria: R Foundation for Statistical Computing.
- Truong JW, Diamond ML, Helm PA, Jantunen LM. 2017. Isomers of tris(chloropropyl) phosphate (TCPP) in technical mixtures and environmental samples. Analytical and Bioanalytical Chemistry 409(30):6989–6997. DOI: 10.1007/s00216-017-0572-7.
- Tzafriri-Milo R, Benaltabet T, Torfstein A, Shenkar N. 2019. The potential use of invasive ascidians for biomonitoring heavy metal pollution. Frontiers in Marine Science 6:611. DOI: 10.3389/fmars.2019.00611.
- Vered G, Kaplan A, Avisar D, Shenkar N. 2019. Using solitary ascidians to assess microplastic and phthalate plasticizers pollution among marine biota: A case study of the Eastern Mediterranean and Red Sea. Marine Pollution Bulletin 138:618–625. DOI: 10.1016/j.marpolbul.2018.12.013.
- Vikas M, Dwarakish GS. 2015. Coastal pollution: A review. Aquatic Procedia 4:381–388. DOI: 10.1016/j.aqpro.2015.02.051.
- Wang X, Zhu Q, Yan X, Wang Y, Liao C, Jiang G. 2020. A review of organophosphate flame retardants and plasticizers in the environment: Analysis, occurrence and risk assessment. Science of the Total Environment 731:139071. DOI: 10.1016/j.scitotenv.2020.139071.
- Zandaryaa S, Frank-Kamenetsky D. 2021. A source-to-sea approach to emerging pollutants in freshwater and oceans: Pharmaceuticals in the Baltic Sea region. Water International 46(2):195–210. DOI: 10.1080/02508060.2021.1889867.
- Zoeller RT, Bansal R, Parris C. 2005. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146(2):607–612. DOI: 10.1210/en.2004-1018.
