Abstract
Brood overlapping is a breeding strategy that aims at shortening the time between successive broods. Most typically, each brood is raised in separate nest and parents share care between broods. If successive overlapping broods are located in the same nest, the second brood is usually initiated after nestlings from the previous brood have left the nest. The cases in which a female lays eggs in the same nest but before the nestlings from the previous brood have fledged (hereafter, Single Nest Brood Overlapping, SNBO) are rare and seldom studied. Based on a literature research, we revise data on SNBO occurrence. To date, about 37 cases of SNBO have been recorded in eight bird species and occurs mainly nest box breeders. Moreover, we surveyed SNBO in a wild-living passerine, Great Tit (Parus major). We found evidence of SNBO in 10.1% of the 69 first broods that we monitored using camera traps installed inside nest boxes. Females started the second brood when first brood nestlings were 17–19 days old, up to 6 days prior to fledging. This type of brood overlap occurred more often in nests that had smaller initial number of fledglings. The timing of the first brood, the clutch size and duration of the nestling phase had no significant effect on SNBO occurrence. We discuss some potential causes and fitness consequences of this unusual breeding strategy.
Introduction
Brood overlapping in birds occurs when parents undertake successive breeding attempts before nestlings from the first brood have reached independence. Such a strategy aims to maximize reproductive output and is especially common in species living in the temperate zone, where the reproductive window is relatively short or in unstable environmental conditions (Walsh & Bock Citation1997; Grüebler & Naef-Daenzer Citation2010; Stępniewski & Hałupka Citation2018). Brood overlapping has been observed in many bird species, mainly in passerines (e.g., Smith et al. Citation1989; Walsh & Bock Citation1997; Grüebler & Naef-Daenzer Citation2010; Stępniewski & Hałupka Citation2018), but also in grebes (Leibl Citation1999), herons (Filipiuk & Kucharczyk Citation2016), birds of prey (Langgemach Citation1998), rallids (Höser Citation1992), shorebirds (Ringe Citation1973), pigeons (Kühlke & Rudat Citation1981), owls, kingfishers, and nightjars (Glutz von Blotzheim & Bauer Citation1993), hummingbirds (Sferco Citation2018) and hoopoe (Kleewein Citation2010). Typically, overlapping broods are raised in separate nests built in close proximity to each other (e.g., Walsh & Bock Citation1997). In such cases, females build a second nest and lay and incubate eggs while males provision the nestlings from the first brood (Haftorn Citation1978). Rarely, a male is engaged in building the second nest and incubating the second clutch (Stępniewski & Hałupka Citation2018). If both broods are raised in the same nest, the female usually starts laying eggs after the nestlings have fledged, while the male takes care of the fledglings (Smith et al. Citation1989; Verhulst & Hut Citation1996; Grüebler & Naef-Daenzer Citation2010).
Probably the rarest and least studied form of brood overlapping takes place when females initiate the second brood in the same nest, before nestlings from the first brood fledge (hereafter, Single Nest Brood Overlapping, SNBO). Such phenomenon is usually recorded as sparse cases in few species (Berndt Citation1938; Rheinwald Citation1971; Winkel Citation1980).
In this study, we used the literature to review the occurrence of SNBO in birds and discuss the potential benefits and costs of this breeding strategy. Moreover, for the first time, we comprehensively surveyed the occurrence and characteristics of SNBO in a wild living passerine, the Great Tit (Parus major), using camera traps installed inside nest boxes. We also investigated how the timing, size and duration of the nestling phase of the first brood affects the occurrence of SNBO. Earlier studies showed that brood overlap is energetically costly to females and its frequency increases over the course of the breeding season, as breeding conditions worsen (Smith et al. Citation1989). We therefore expected that SNBO would be more likely to occur in nests where the first broods are as follows: i) small, ii) initiated later in the breeding season or iii) have a relatively long nestling phase. The present study broadens our knowledge of avian breeding strategies and opens a new avenue for further studies on the ecological and evolutionary significance of brood overlap.
Material and methods
Literature review
We searched Scopus (www.scopus.com), SORA (Searchable Ornithological Research Archive, www.sora.unm.edu) and Google Scholar (www.scholar.google.com) databases using keywords “brood overlapping” or “brood overlap” and Zobodat (www.zobodat.at) database for the keywords “Schachtelbruten” or “Schachtelbrut”. Then we selected articles that dealt with brood overlapping. The next step was searching these articles for cases of SNBO.
Fieldwork and recordings analysis
As a part of a broader study, we monitored a nest box population of Great Tits breeding in Wielkopolski National Park, western Poland (52°16ʹN 16°47ʹE) over the 2015–2018 breeding seasons. Each year, we followed 27 to 48 breeding pairs. To collect basic breeding biology data, we monitored all nest boxes every 2–5 days from mid-March until late June. Additionally, we used trail cameras (Nature View® Cam HD, Bushnell®) installed inside nest boxes to determine the date of laying of the first egg and the date of hatching. For more details on study plot and general field methods see Kudelska et al. (Citation2017), Podkowa and Surmacki (Citation2017), Podkowa et al. (Citation2019) and Surmacki and Podkowa (Citation2022).
From 2016 to 2018, we used trail cameras to determine fledging date (i.e., the date when the last nestling fledged) in 69 nest boxes (2016: 21, 2017: 24, 2018: 24). We inserted cameras into nest boxes when nestlings were ~15 days old, i.e., about 5 days before fledging. We set cameras to record one 3 MP photo every 15 minutes within a 24-hour time-lapse that operated both in the darkness and the daylight (). For more details of trail camera used in nest box monitoring, see Surmacki and Podkowa (Citation2022). In all but one case, trail cameras were retrieved soon after the first brood fledged. Then, photos were browsed to establish fledging date. We assumed the presence of SNBO if at least one egg was in the nest after the first brood had fledged and 1) successive eggs appeared in the nest in the following days or 2) female showed behaviour associated with egg laying (e.g., covering eggs with the lining, bringing nest materials to the nest etc.). We collected detailed information on the outcome of both overlapping broods only for one nest (). In the remaining cases, trail cameras were removed before incubation of the second clutch started (and presumably before the completion of the second clutch).
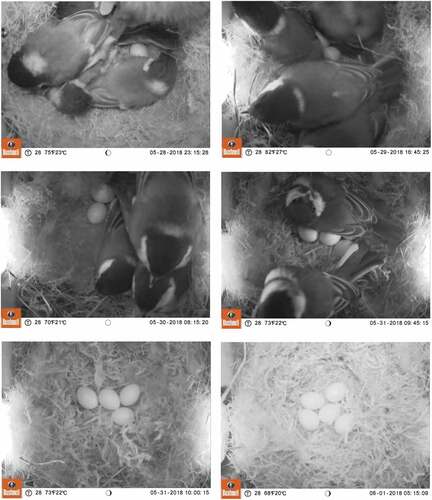
Figure 1. Photos from trail camera showing consecutive stages of egg laying in the Great Tit nest with Single Nest Brood Overlapping (nest box no. B038).
To test the effect of the first brood characteristics on SNBO occurrence, we compared broods with and without SNBO. It is important to note that the second group was probably very heterogenic; compromised of pairs that did not raise second broods at all, or raised them in different nest boxes, or raised them in the same nest but not in an overlapping fashion. The date of the first brood was the date when the first egg was laid and it was expressed as Julian date. The fledgling number is the number of young birds that successfully left the nest box. The duration of the nestling phase was the number of days between the date of the first egg hatched and the last nestling fledged. Because the distribution of all data deviated significantly from a normal distribution (Shapiro–Wilk test, p < 0.05), we applied Mann–Whitney tests. Descriptive statistics in the Results section are reported as median and 25–75th percentiles.
Results
Literature review
Data on SNBO occurrence and characteristics are summarized in . In total, we found 37 cases, reported in eight species (). We found three studies (one on Blue Tit Cyanistes caeruleus and two on Great Tit) which clearly stated lack of SNBO occurrence. In all but one case, SNBO was observed in cavity nesters. With exception to one pair of the Stock Doves (Columba oenas) that nested in a natural tree hole (Kühlke & Rudat Citation1981), all studies in this group were conducted in nest boxes (). In the majority of cases, SNBO occurred between the first and the second brood. The exceptions were recorded in the House Sparrow (Passer domesticus), where SNBO was recorded between second and third (n = 2), and between third and fourth brood (n = 4, Lowther Citation1979). Similarly, two SNBO cases in Stock Doves were observed between second and third brood (Kühlke & Rudat Citation1981; Bom Citation2016). Two cases of SNBO in House Sparrows and Stock Doves concerned the same breeding pairs (Lowther Citation1979; Kühlke & Rudat Citation1981). The SNBO in Eastern Bluebird (Sialia sialis) was observed between second brood of one female and the third brood of another female (the first female probably died, Gowaty Citation1983). In a majority of cases, fledging of the former brood happened before the clutch of the latter brood was completed (). The exceptions were as follows: three SNBO cases in House Sparrows (2, 4 and 5 eggs; Lowther Citation1979), two in Great Tits (6 and 4 eggs, Berndt Citation1938; Winkel Citation1980) and three in Stock Doves (Kühlke & Rudat Citation1981; Bom Citation2016) (). There were three cases of SNBO when females incubated latter brood eggs: one in Great Tits (Winkel Citation1980) and three in Stock Doves (Kühlke & Rudat Citation1981; Bom Citation2016).
Table I. Information on the occurrence and characteristics of Single Nest Brood Overlapping (SNBO) recorded in populations of wild birds. Explanations: ~ – estimation; N/A – not applicable; - – no data; values spaced with a hyphen – ranges; values in parentheses - percent
Table II. Characteristics of Single Nest Brood Overlapping (SNBO) recorded in a Polish population of Great Tits. The date of egg laying and hatching is the date when the first egg in the clutch was laid and hatched, respectively. The date of fledging is the date when the last fledgling left the nest box
In addition to the data listed in , there is some scattered information on SNBO with no details provided. According to Kluyver (Citation1951), SNBO occurred in a Great Tit population in the Netherlands. In a study by Deckert (Citation1962), SNBO is defined as “often” in German population of Tree Sparrows (Passer montanus). Anderson (Citation1994) provides two instances when the House Sparrow females began their third brood (in the same nest) one day before the second young fledged (Michigan, USA). Finally, there is an interesting case of an Eurasian Kestrel (Falco tinnunculus) female that laid the second clutch, while the first brood of nestlings was still present in the nest box (Langgemach Citation1998). Both broods, however, were spatially separated because the eggs were laid in the other (“rear”) part of the nest box (Langgemach Citation1998).
The field study on Great Tit (Parus major)
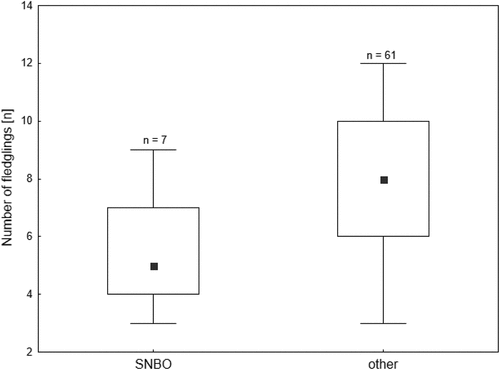
We found SNBO in 10.1% of the 69 monitored nests (2016: 4, 2017: 2, 2018: 1; ). The number of second clutch eggs laid while nestlings from the first brood were still at the nest ranged from 2 to 6 (median = 4). The median age and the number of the first brood nestlings present in the nest at the beginning of laying of the second clutch was 18 days (range: 17–19 days) and 5 individuals (range: 4–7), respectively. Details on all recorded cases of SNBO are summarized in .
The history of the SNBO for which complete data on both broods were collected (B038, and ) is as follows: The first egg in the first brood was laid on 18th April. The female laid a clutch of five eggs of which four hatched on 10th May. When nestlings were 18 days old (28th May), the first egg from the second brood was laid (). The female laid one egg per day for 9 successive days. The first brood nestlings fledged on 31st May, when four of the second clutch eggs were already in the nest (). The female started incubation on 4th June, after the eighth egg was laid. Five eggs from the second brood hatched between 14th and 17th June. Four nestlings died in the nest by the 1st July for unknown reasons. The only nestling fledged successfully on 3rd July. During the second brood, the offspring were occasionally fed by another adult, presumably a male. On 10th May the female was captured in the nest box and ringed with one metal and one plastic band. The number from the latter one was visible on photos captured by the trail camera. According to plumage characteristics, the female was >2 years old (Svensson Citation1992).
The broods with and without SNBO did not differ with respect to the timing of the first brood (106, 95–197, n = 7; 106, 99–108, n = 58; Z = −0.88, p = 0.38), the size of the first clutch (11, 6–11, n = 7; 10, 9–11, n = 61; Z = −0.20, p = 0.84) or the duration of the nestling phase of the first brood (20, 18-23, n = 7; 19, 18-20, n = 75; Z = 0.81, p = 0.41). However, in nests where SNBO occurred, the number of fledglings was significantly lower compared to the typical nests (Z = 2.72, p = 0.01, ).
Discussion
Data on SNBO found in the literature are usually sparse and often incomplete. Most cases were observed in a few species breeding in nest boxes. This is understandable as open cup nests are exposed to external conditions and consequently less durable to support consecutive broods. Moreover, cup nests are more vulnerable to predation which could significantly reduce the probability of females reusing the same nest (Martin & Li Citation1992). Further, nests in boxes are easier to monitor and are monitored more often by researcher than are cup nests. Nevertheless, SNBO cases are rare (both the number of nests and the species) especially considering the intensity of avian breeding research in Europe (Cramp & Perrins Citation1993). The most probable explanation is that SNBO is usually overlooked due to logistical limitations. Taking note of SNBO requires frequent nest monitoring, which can increase the risk of birds abandoning broods or can cause nestlings to fledge early. Alternatively, automated nest monitoring (this study, Bom Citation2016) registers the breeding history in a nest box with high accuracy. In earlier studies, the presence of SNBO was often estimated based on egg laying date and the expected fledging time (e.g. Winkel Citation1975). Thus, some cases might have been missed, especially when the degree of brood overlapping was small (see Winkel & Winkel Citation1992). The high number of SNBO recorded in this Polish Great Tit population could be explained by nest cameras. The observed between-population variation in the occurrence of SNBO in Great Tits () could also be an effect of latitude-dependent frequency of the second broods, which is higher in central and western Europe compared to Scandinavia (Orell & Ojanen Citation1983).
The main function of brood overlapping is to increase the total annual number of offspring produced in a breeding season, which is achieved by shortening the period between successive broods (Grüebler & Naef-Daenzer Citation2010). Because breeding success usually declines across the breeding season due to changes in environmental conditions, the reduced interval between broods is beneficial to adult birds (e.g. Verhulst et al. Citation1995). In the Great Tit, the second broods are raised more often by females that started their first brood early in the season (Orell & Ojanen Citation1983). Moreover, earlier studies have shown that the degree of fledglings/clutch overlap increases with the hatching date of the first brood (Smith et al. Citation1989). Decreasing the between-brood interval by brood overlapping should be an energetically costly strategy for a female. For this reason, in the Great Tit, the degree of brood overlap is inversely correlated with first clutch size (Smith et al. Citation1989). The SNBO could be regarded as a very extreme type of brood overlapping. The Great Tits start the second brood, on average, 4 days after the first brood fledges (Haartman Citation1969; Rheinwald Citation1971; Orell & Ojanen Citation1983; Smith et al. Citation1989). Thus, those females that engage in SNBO could accelerate the onset of the second brood by ~8 days. Theoretically, this should give females an advantage due to the time saved on finding a new cavity or building a nest, which in turn, provides an opportunity to raise a second brood in more favorable earlier part of the breeding season. Interestingly, in the Great Tit population that we studied, we found no effect of the size or the timing of the first clutch on the likelihood of SNBO. This contradict the idea that raising consecutive broods is energetically challenging (Smith et al. Citation1989). The probable explanation for the rarity of SNBO is that this type of brood overlapping negatively affects success of the second clutch.
SNBO could be regarded as a unique type of brood overlapping because nestlings close to fledging age interfere with the second clutch. Our video recordings demonstrate that these eggs are constantly tossed and trampled by nestlings. Although not observed, we assume that SNBO could increase the likelihood of eggshell breakage or interfere with proper heat transfer. We found no evidence of overlap between the first brood nestlings and incubation of the second clutch. Overlap between nestling rearing and incubation, however, has been observed in other studies (Winkel Citation1980; Kühlke & Rudat Citation1981; Bom Citation2016). The efficiency of incubation under such conditions is questionable (see Winkel Citation1980; Bom Citation2016). Although sample sizes are low, the nesting success (% of eggs laid which result in successfully fledged young) of the second SNBO brood was very low: 11% in the Great Tit (n = 1, this study) and 12.5% in the House Sparrow (n = 8, Lowther Citation1979). The negative effect of SNBO on breeding success was also noted in the Stock Dove (Bom Citation2016). Further surveys are needed to assess how the overlap between the first brood of nestlings and second clutches is detrimental to embryo development. Females may be more likely to use a SNBO strategy when the first broods are small as this could be less detrimental to the second clutch. Such a hypothesis is supported by the fact that nests with and without SNBO differed significantly with respect to number of fledglings. An alternative explanation is that females can allocate resources more quickly to laying the second clutch when the first brood is small. Indeed, in species that typically produce multiple successful broods, females given increased brood sizes took longer to produce the second brood compared to females that reared smaller broods (e.g. Siefferman & Hill Citation2008).
Despite the risks associated with SNBO, we found a high rate of SNBO in our Great Tit population (). Given that not all Great Tit pairs raise multiple broods (Cramp & Perrins Citation1993; Kudelska et al. Citation2017), the rate of SNBO among the number of second broods probably well exceeds 10%. Thus, the advantages of SNBO could outweigh the costs. Although information on SNBO is sparse and often incomplete, logistical constraints are to blame. Further studies using automated photography (e.g. Surmacki & Podkowa Citation2022) could help to assess the true occurrence and consequences of SNBO in other species and populations. Such data would help to assess whether SNBO is disadvantageous for birds and help investigate whether individual condition and environmental factors influence the likelihood of SNBO.
Acknowledgements
We are very grateful to Lynn Siefferman for her comments and language revision. Klaudia Szala and Piotr Zduniak provided valuable advice on early versions of the manuscript. All procedures used in the study comply with the current laws of the Republic of Poland.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anderson TR. 1994. Breeding biology of House Sparrows in Northern Lower Michigan. Willson Bulletin 106:537–548.
- Berndt R. 1938. Über die Anzahl der Jahresbruten bei Meisen und ihre Abhängigkeit vom Lebensraum mit Angaben über Gelegestärke und Brutzeit. Deutsche Vogelwelt 63:140–151 and 174–181.
- Berndt R, Frieling F. 1939. Siedlungs-und brutbiologische Studien an Höhlenbrütern in einem nordwestsächsischen Park. Journal für Ornithologie 87(4):593–638. DOI: 10.1007/BF01950721.
- Bom RA. 2016. A case of multiple brooding in Stock Doves Columba oenas [Broedseloverlap bij Holenduiven]. Limosa 89:185–188.
- Cramp S, Perrins CM. 1993. The birds of the Western Palearctic. Vol. I–IX. Oxford: Oxford University Press.
- Deckert G. 1962. Zur Ethologie des Feldsperlings (Passer m. montanus). Journal of Ornithology 103:428–486. DOI: 10.1007/BF01676606.
- Filipiuk M, Kucharczyk M. 2016. A puzzling case of successful double-brooding in the Little Bittern Ixobrychus m. minutus. Ardea 104:182–186. DOI: 10.5253/arde.v104i2.a3.
- Glutz von Blotzheim UN, Bauer KM. 1993. Handbuch der Vögel Mitteleuropas. Vol. 9. Wiesbaden: AULA Verlag.
- Gowaty PA. 1983. Overlap of two broods of Eastern Bluebirds in the same nest and brood reduction. Willson Bulletin 95:148–150.
- Grüebler MU, Naef-Daenzer B. 2010. Brood overlap and male ornamentation in the double-brooded barn swallow. Behavioral Ecology 21:513–519. DOI: 10.1093/beheco/arq017.
- Haftorn S. 1978. Cooperation between the male and female Goldcrest Regulus regulus while rearing overlapping second broods. Ornis Scandinavica 9:124–129. DOI: 10.2307/3675873.
- Höser N. 1992. Vier Jahresbruten und Schachtelbruten der Teichralle, Gallinula chloropus. Mauritiana 14(1992):149–154.
- Kleewein A. 2010. Artenschutzprojekt Wiedehopf (Upupa epops) in Kärnten 2009. Brutbestand, Habitatanalyse und Schutzmaßnahmen. Carinthia II 200(120):183–198.
- Kluyver HN. 1951. The population ecology of the Great Tit, Parus m. major L. Ardea 39:1–135.
- Kudelska K, Podkowa P, Karaśkiewicz K, Surmacki A. 2017. Importance of nest boxes for breeding birds in forest areas - The Wielkopolski National Park case study. Sylwan 161:949–957.
- Kühlke D, Rudat V. 1981. Eine mehrfache Schachtelbrut der Hohltaube (Columba oenas) in der gleichen Bruthöhle. Ornithologische Jahresberichte des Museum Heineanum 5–6:59–60.
- Langgemach T. 1998. Zweitbrut beim Turmfalken (Falco tinnunculus) im Havelland – Otis. Zeitschrift für Ornithologie und Avifaunistik in Brandenburg und Berlin 6:145–147.
- Leibl F. 1999. Bestandsentwicklung und Brutbiologie des Haubentauchers (Podiceps cristatus) in einem ostbayerischen Kiesabbaugebiet. Ornithologischer Anzeiger 38(2–3):177–188.
- Löhrl H. 1977. Die Tannemeise. Neue Brehm-Bucherei Nr. 472.
- Lowther PE. 1979. Overlap of House Sparrow broods in the same nest. Bird-Banding 50:160–162. DOI: 10.2307/4512440.
- Martin TE, Li P. 1992. Life history traits of open‐vs. cavity‐nesting birds. Ecology 73:579–592. DOI: 10.2307/1940764.
- Orell M, Ojanen M. 1983. Timing and length of the breeding season of the Great Tit Parus major and the Wil- low Tit P. montanus near Oulu, northern Finland. Ardea 71:183–198. DOI: 10.2307/3676264.
- Podkowa P, Malinowska K, Surmacki A. 2019. Light affects parental provisioning behaviour in a cavity-nesting passerine. Journal of Avian Biology 50:e02254. DOI: 10.1111/jav.02254.
- Podkowa P, Surmacki A. 2017. The importance of illumination in nest site choice and nest characteristics of cavity nesting birds. Scientific Reports 7:1329. DOI: 10.1038/s41598-017-01430-y.
- Rheinwald G. 1971. Schachtelbruten der Kohlmeise (Parus major). Vogelwelt 92:231–232.
- Ringe F. 1973. Zum Status und zur Brutbiologie des Flußregenpfeifers (Charadrius dubius SCOPOLl) im Kreise Osnabrück. Osnabrücker Naturwissenschaftliche Mitteilungen 2:89–100.
- Sferco G. 2018. Alternate care of two nests by a Red-tailed Comet (Sappho sparganurus). The Wilson Journal of Ornithology 130:335–336. DOI: 10.1676/16-137.1.
- Siefferman L, Hill GE. 2008. Sex‐specific costs of reproduction in Eastern Bluebirds Sialia sialis. Ibis 150:32–39. DOI: 10.1111/j.1474-919X.2007.00723.x.
- Smith HG, Källander H, Nilsson JA. 1989. The significance of clutch overlap in great tits (Parus major). Ibis 131:589–600. DOI: 10.1111/j.1474-919X.1989.tb04794.x.
- Stępniewski J, Hałupka L. 2018. Overlapping breeding attempts in the Bearded Tit (Panurus biarmicus). Avian Research 9:22. DOI: 10.1186/s40657-018-0115-8.
- Surmacki A, and Podkowa P. 2022. The use of trail cameras to monitor species inhabiting artificial nest boxes. Ecology and Evolution 12:e8550. DOI:10.1002/ece3.8550.
- Svensson L. 1992. Identification guide to European passerines. 4th ed. Stockholm: British Trust for Ornithology.
- van Rossem AJ. 1936. A note on the nesting of the bush-tit. Condor 38:170.
- Verhulst S, Hut RA. 1996. Post-fledging care, multiple breeding and the cost of reproduction in the great tit. Animal Behaviour 51:957–966. DOI: 10.1006/anbe.1996.0099.
- Verhulst S, Van Balen H, Tinbergen JM. 1995. Seasonal decline in reproductive success of the great tit: Variation in time or quality? Ecology 76:2392–2403. DOI: 10.2307/2265815.
- von Haartman L. 1969. The nesting habits of Finnish birds. I. Passeriformes. Commentationes Biologicae 32:1–187.
- Walsh JJ, Bock CE. 1997. Likely occurrence of overlapping broods in the Rock Wren. West Birds 28:223–224.
- Winkel W. 1975. Vergleichend-brutbiologische Untersuchungen an fünf Meisen-Arten (Parus spp.) in einem niedersächsischen Aufforstungsgebiet mit Japanischer Lärche Larix leptolepis. Vogelwelt 96:41–63 und 104–114.
- Winkel W. 1980. Registrierung der Nestbesuchs-Aktivität bei einer Schachtelbrut der Kohlmeise (Parus major). Vogelwelt 101:30–33.
- Winkel W, Winkel D. 1992. Der Brutverlauf bei Kohlmeisen (Parus major) und seine Beeinflussung durch Umweltfaktoren. Ornithologische Mitteilungen 44:3–14.