Abstract
The paper presents new data on the nesting habits of the digger wasp Alysson spinosus (Hymenoptera: Bembicidae). As food for larvae, the female provisioned about 7–8 hemipteran nymphs or imagines per cell. The collected prey belongs to two families of true hoppers (five species of Delphacidae and one species of Cicadellidae). Nests are built in sandy, shaded areas, and consist of a 10–12 cm long main burrow with 1–3 brood cells. Both sexes search for food (honeydew) on the leaves of lilac or small-leaved linden. The nests were infested by the dipteran kleptoparasitic Metopia argyrocephala (Diptera: Sarcophagidae). The mature larva is similar to that of the Nearctic Alysson melleus, from which it differs in having a blunt apical mandibular tooth and more prominent setae on the clypeus and labrum.
Introduction
The genus Alysson Panzer, 1806 belongs to the family Bembicidae (Sann et al. Citation2018) and comprises 41 species distributed worldwide except the Neotropical Region and Australia (Pulawski Citation2022). There are four species of this genus known in Central Europe: A. perteesi Górski, 1852, A. ratzeburgi Dahlbom, 1843, A. spinosus (Panzer, 1801), A. tricolor Lepeletier and Serville, 1825 (Bogusch et al. Citation2007; Schmid-Egger Citation2010; Shorenko & Konovalov Citation2010; Olszewski et al. Citation2021b).
Among Palaearctic species, preliminary data on the nesting biology of A. spinosus (= bimaculatus Panzer) were provided by Kohl (Citation1880), who described the nesting place (sands, slopes), the method of transporting the prey (holding the mandibles), and the species of prey. Subsequently, nesting habits of this species were reviewed by Borries (Citation1897). Olberg (Citation1959) described how the prey is transported to the nest. A review of available behavioural data was presented by Evans (Citation1966). Later, Pagliano and Alma (Citation1997) added information on the recorded prey Cicadella viridis (L.) (Cicadellidae). Evans and O’Neill (Citation2007) presented a summary of information on nesting habits, and a review on the known range of prey was presented by Nemkov (Citation2012). Itami (Citation1967) and Evans and O’Neill (Citation2007) presented a summary of information on nesting habits of A. pertheesi. The first data on nesting and prey of A. ratzeburgi were presented by Dahlbom (Citation1847). The prey recorded in Algeria was probably incorrectly identified by Ferton (Citation1908). Nesting habits were described from Sweden by Adlerz (Citation1910), and a review of known behavioural data was provided by Evans (Citation1966), whereas Pagliano and Alma (Citation1997) presented a list of known prey. Data on prey of A. tricolor can be found in the publication by Ferton (Citation1908). The first data on the life history of Japanese A. cameroni Yasumatsu and Masuda, Citation1932 were provided by the authors of the description, which was subsequently supplemented by Tsuneki (Citation1969).
Species of Alysson nest in relatively cool and humid places, often on river banks or sandbanks, in areas with clay or sandy soil (Evans Citation1966). Wasps construct a vertical burrow that ends with one brood cell (Scandinavian species), or with a system of short side tunnels, each ending in a brood cell (Japanese species) (Lomholdt Citation1984). When digging the main burrow, the wasp loosens the ground with its mandibles and discards it with its front and rear legs (Kazenas Citation2001). Females deliver prey – nymphs or imagines of various leafhopper species of the family Cicadellidae, less often spittlebugs from Cercopidae or other families of Fulgoromorpha – to the brood cell (Blösch Citation2000). According to Kazenas (Citation2001), females hunt on bushes and grass in close proximity to the nest. Prey is carried to the nest in flight and partially dragged on the ground. The female holds the prey with the head facing forward and the ventral side up. The main burrow tunnel leads into the brood cell(s) (1 to 5) and the entrance of the nest remains open during the provisioning period (Bohart & Menke Citation1976). Kazenas (Citation2001) reports that one cell may contain 2–23 specimens of prey. The egg is attached to one of the last-deposited prey specimens. Once the brood cell is provisioned and the egg is laid, the digger wasp closes the cell (Bohart & Menke Citation1976).
Based on the above-mentioned data from the Palearctic area, there is a clear need to supplement the data on the nesting biology of Alysson. Therefore, the objective of this study is to provide information on the nesting biology of this species, including: (1) nest construction and transport of prey to the nest; (2) the range of prey, including species and the number of individuals per nest; (3) nest structure: determination of the number of brood cells, and the length and diameter of the main burrow; (4) description of the mature larva; (5) report on recorded kleptoparasites.
Materials and methods
The research was carried out in the town of Kowalewo Pomorskie (53°10ʹ05.7″N, 18°52ʹ15.5″E) and the village of Sierakowo (53°10ʹ19.0″N, 18°52ʹ21.7″E) in northern Poland, from early June to late September 2020–2021 on sunny and warm days (with a temperature of at least 18°C). The site in Kowalewo Pomorskie covered a small sandy area of 8 m2 surrounded by fallow land overgrown with herbaceous vegetation. Research on Dryudella stigma (Panzer, 1807) was also carried out at this site (Olszewski et al. Citation2021a). The second site was located on sandy loam, covered with grassland vegetation with some segetal and ruderal species. Helichrysum arenarium (L.), Peucedanum oreoselinum (L.), Sedum sp. and Senecio vernalis Waldst. & Kit. dominated among herbaceous plants. Nesting activities (i.e. digging nests and transporting the prey) were analysed based on direct observations and on-site notes. Photographs were taken with an EOS M50 camera; additionally a Raynox M-250 macro converter was used. The prey range was determined by nest inspection. The nest structure was analysed by careful digging and counting the number of brood cells, tilt angle of the main burrow, the number of deposited bugs in each brood cell and the presence of larvae of kleptoparasites. During the activity of females (late June to early September), nests were dug and larvae were collected for analysis.
To describe the larval specimens, we transferred some of the larvae into Pampel solution (30 volumes of distilled water, 15 volumes of 96% ethanol, 6 volumes of formaldehyde and 4 volumes of glacial acetic acid) as described by Švácha and Danilevsky (Citation1987). After taking photographs of the intact larvae, we examined their sclerotised parts. For this purpose, we placed the larvae into a 10% solution of hot (60°C) KOH for 12 h to dilute all body parts except the integument. We then stained the integument in 5% Chlorazol Black E (Sigma Aldrich) for 2 s and moved it to 96% ethanol. To observe species-specific characteristics, we placed the integument in glycerol and separately observed the head, mouthparts, spiracles and other parts under a light microscope. We used the same specimens to study small structures such as setae, sensillae or mouthparts. We drew figures of (1) the head, with particular attention to the clypeus, labrum, maxillae and labium; (2) mandibles – anterior view; and (3) spiracles of larvae. Specimens of kleptoparasitic flies and prey are deposited in the collection of the first author and larvae are deposited at the University of Hradec Králové, the Czech Republic.
Prey identification was made based on external features and the structure of the anal segments of males using published identification keys (Holzinger et al. Citation2003; Biedermann & Niedringhaus Citation2004).
Abbreviations used: N = nest, C = cell, L = larva of A. spinosus, P = puparium of kleptoparasitic fly.
Results
Observations of behaviour
Adult specimens were observed mainly from June to early September – females were usually found transporting their prey to a nest, and males were found on leaves searching for honeydew ()). A total of 18 nests were discovered and observed during the study. Nests were distributed either singly or in small colonies ()), with the smallest distance between the main burrows being 2 cm (the largest was about 60 cm). Nest entrances were open the whole time during the provisioning period. Females always landed several dozen cm from the entrance. Prey was carried in the mandibles of female wasps and often with their legs (first pair; )). Females provisioning the brood cells were observed at different time intervals, between 9am and 6.30pm. The highest concentration of active females was observed in the afternoon, from noon to 4pm. There were nine active nests in an area of 2 m2 during one day. Two specimens of Metopia argyrocephala (Meigen, 1824) entering the main burrow in the absence of the female were observed in Sierakowo. One puparium of this kleptoparasite fly was dug out while inspecting the nests, and the adult fly was reared from the puparium. Females of A. spinosus remained in the nest during adverse weather conditions.
Figure 1. Adult of Alysson spinosus. (a–c) Female with prey at the nest entrance; (d, e) male on the leaf of Syringa vulgaris.

Figure 2. Nest of Alysson spinosus. (a) Lateral view of the nest and brood cells; (b) place with nests (nest entrances are marked by red circles); (c–h) top view of nest entrances.
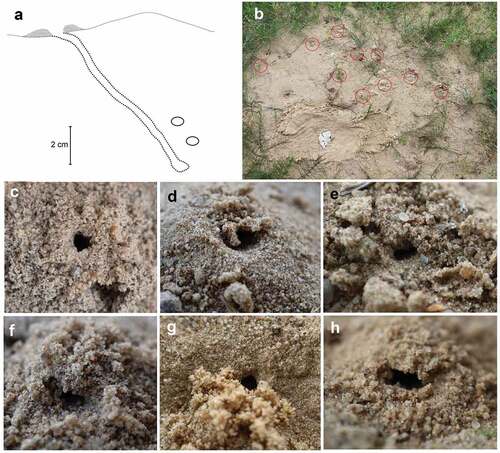
Before building the nest, the female first loosened the soil crust with her mandibles and forelegs (two observations). She then raked the loosened soil under her raised abdomen by moving her forelegs in a spiral motion. The female repeatedly retreated from the burrow, pushing loads of sand held between her gastral venter and hind legs ()). On several occasions, females abandoned nest construction and began searching for a new site.
During the three-hour observations, the highest number of active nests was recorded on 26 July, when 12 females were observed transporting prey to their nests. Each nest consisted of a 9–12 cm long main burrow (from 3 to 3.5 mm in diameter), sloping at an angle of almost 50° and terminating in one to three brood cells (N1 = 3, N2 = 1, N3 = 1, N4 = 1; )), which contained up to 10 dead specimens of Cicadellidae and Delphacidae (N1C1 = 10; N1C2 = 9; N1C3 = 9; N2C1 = 3 + 1L; N3C15 = 5 + 1L; N4C1 = 0 + 1P; )), belonging to six species (). The entrance to the nest was not always round; it could be oval or otherwise shaped ()). Nests were built on horizontal/sloping or vertical surfaces. The frequency of bringing food to the nest depended on air temperature, humidity and time of day and ranged from 2 to 32 min (usually 5 min).
Table I. Prey of Alysson spinosus found in the six studied brood cells.
Figure 4. (a) Prey of Alysson spinosus; (b) puparium of Metopia argyrocephala; (c, d) larvae of A. spinosus.

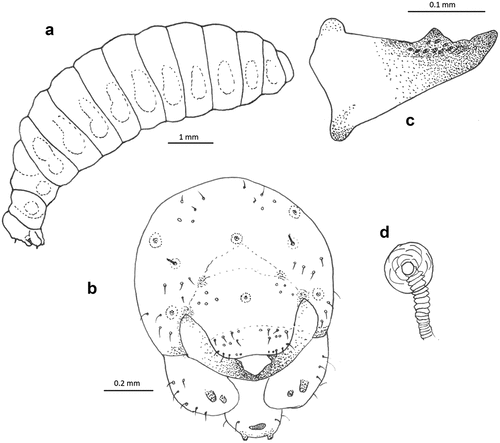
Figure 5. Larva of Alysson spinosus. (a) Habitus, lateral view; (b) head, frontal view; (c) mandible, frontal view; (d) spiracle.
One puparium of Metopia argyrocephala ()) and three larvae of A. spinosus ()) were found during inspection of brood cells.
Description of the larva
Material examined
Three specimens.
Diagnosis
The mature larva is similar to that of A. melleus and A. cameroni. The shape of the body of A. spinosus is fusiform and only very slightly dorsoventrally flattened, slender; thoracic segments and the first three abdominal segments are more slender than the rest of the body, as in A. melleus. The head is higher than wide and has six large but only very slightly sclerotised tubercles (typical for mature larvae of this genus). The mandible is sharp, triangular, and darkly pigmented, with one longest sharp apical tooth, one conspicuous lateral tooth, and several tubercles. The apical tooth is blunter than that of A. melleus. Labrum is not apically emarginated.
Description
Body length of mature larva 6.9–7.5 mm (N = 3). Body vestiture with sparsely distributed short spicules, and with several slender, pale setae, tapering to fine points, arising from small but distinct alveoli; setae not elongate. Several setae on mouthparts, only the mandibles, area around mandibular condyli and maxillar and labial palpi brownish. The other body parts only with several setae, the last three abdominal segments asetose. Body form of postdefecating larva fusiform and only very slightly dorsoventrally flattened, slender; body segments similarly wide along whole length, only thoracic segments and first three abdominal segments slenderer. Paired lateral body tubercles present and well developed on all thoracic and abdominal segments except smaller on T9 and absent from T10. Dorsal tubercles wide, flat and well developed on all three thoracic segments, and abdominal terga, but less conspicuous on T9. Body shape of predefecating larva in lateral outline with T3–T6 having greatest diameter. Abdominal segment 10 attached to middle of segment 9 in lateral view; anus positioned medially and transverse. Spiracles unpigmented, subequal in diameter; atrium globular, slightly wider than deep, projecting little above body wall, with rim; atrial opening diameter vs. peritreme width ratio 1:3; atrial inner surface with rows of wrinkles concentric with primary tracheal opening; primary tracheal opening with collar; subatrium short, with about 10 chambers of approximately equal size except one or two next to atrium slightly larger in diameter. Sexual characters unknown.
Head: Head moderately small in relation to body size (width of head 0.63 compared to the widest part of the body); oriented in normal, hypognathous position relative to thorax. Setae shorter and very sparse on upper part of head capsule; those of maxillary and labial apices large, straight and conspicuous. Head capsule unpigmented except at points of articulations with mandibles; mandible moderately pigmented except mandibular apices and areas of articulation with head capsule conspicuously pigmented; maxillary sclerites faintly pigmented; salivary lips projecting but unpigmented; maxillary and labial palpi all uniformly moderately pigmented. Coronal and postoccipital ridges absent. Tentorium mostly absent because of impending ecdysis. Parietal bands absent. In lateral view, six large but only very slightly sclerotised tubercles are present, five on the frons and one on the labrum. In lateral view, clypeus globularly projecting beyond frons, antenna arising from ill-developed prominence, and labrum extending beyond clypeus. Diameter of basal ring of antenna less than 1/5 distance from closest point on ring to centre of anterior tentorial pit; antennal papilla only slightly pigmented, elongate, bearing perhaps one elongated and two not elongated sensilla apically. Frontal area between antennae with two linear rows of four setae each. Parietal region with four setae on the sides near mandibular condyli. Clypeus wide with ill-developed basal and apical margins, four sensilla basally on each side. Labrum not emarginated apically in the middle, with group of four setae and several smaller sensilla on each side basally and several sensilla and four setae on each side apically; labral sclerite not defined and only very poorly pigmented. Epipharynx simple with conspicuous large spinulae on the whole surface. Mandible sharp, triangular; darkly pigmented, with one sharp apical tooth longest, one conspicuous lateral tooth, and several tubercles; outer mandibular surface asetose. Maxillary apex strongly bent mesad in frontal view, so that maxillary palpus is subapical in position; cardo distinct, posterior end directed towards the posterior tentorial pit; stipes slightly sclerotised; maxillary palpi elongate, probably more than two times their basal diameters, both pigmented, bearing three sensilla apically. Stipes with six conspicuous setae. Labium not divided into prementum and postmentum; apex moderately narrow in frontal view. Two setae on both sides. Salivary lips elliptical, short but well visible, with inner surface bearing parallel longitudinal grooves; width of lips slightly more than double width of maxillary palpus. Labial palpus elongated with two sensilla in middle. Hypopharynx with large spinules on whole surface ().
Discussion
Except environmental and food (larvae and imagines) preferences, relatively little is known about the nesting habits of Alysson (Evans Citation1966). Kohl (Citation1880) reported that A. spinosus nests in sand and preys on various species of true hoppers. Olberg (Citation1959) found that wasps of this species carry their prey by walking to a nest located on the ground and holding it in their mandibles. These observations correspond well with our results, which may be summarised as follows: (1) the nest is prepared in sandy soil; (2) females hold the prey’s leg in their mandibles, additionally helping themselves with their front legs; (3) the prey is oriented ventral side up; (4) females land a few cm or so in front of the entrance and convey the prey inside by walking; (5) the number of dead specimens of Cicadellidae and Delphacidae in each cell ranged from 3 to 10. In contrast, Ferton (Citation1901) described observations of nests of A. ratzeburgi on a slope of compact clay soil, and in that case, the nests were 10–15 cm deep and each cell contained two or three leafhoppers. He reported that the female carried the prey by holding its leg in her mandibles, and supporting it with her middle legs. Adlerz (Citation1910) studied the same species and reported that wasps carried their prey only by grasping the mandibles of leafhoppers.
The difference may be related to the availability of the collected species and the size of individuals captured. Of the prey recorded, the majority is associated with grassland and meadow habitats, with only I. stigmaticalis feeding on trees and shrubs (genera Salix, Populus, Betula, Alnus and Acer). True hoppers are mostly plant-sucking insects, much less frequently feeding on other types of food (Holzinger et al. Citation2003; Nickel Citation2003). The abundance of true hoppers in various habitats is usually high, although they are difficult to observe due to their small size and skittishness. They are particularly abundant in grasslands, where their density reaches several hundred or even more than a thousand individuals per square metre and therefore constitute an important part of the food chain (Ferton Citation1901; Nickel & Remane Citation2002). These insects are also important as pests of crop plants. Laodelphax striatella is one of the three most important pests of rice in Asia belonging to delphacid planthoppers (Nickel Citation2003; Zhang et al. Citation2014). The ability to transmit a plant pathogen – the European wheat striate mosaic virus causing a disease in cereals – was also confirmed in J. pellucida (Lundsgaard Citation1999), species with the highest abundance in the prey of A. spinosus (). The hunting activity of this digger wasp may be potentially important for reducing the abundance of several species of hemipteran pests.
Published data show that A. spinosus selects both sunny and shaded sites for the construction of nests. In the research on A. melleus, all observations related to the selection of an unusual nesting site – a cool, moist shore or sandy loam, near a water body. Ferton (Citation1908) also reported that Thamnotettix dilutior Kirschbaum, 1868 was a prey of A. tricolor Lepeletier de Saint Fargeau & Audinet-Serville, 1825. The high concentration of nests in our research is consistent with data reported in the publication by Yasumatsu and Masuda (Citation1932) for A. cameroni, where 50 nests per square metre were found at one site.
The mature larvae of A. melleus described by Evans and Lin (Citation1956) and by Evans (Citation1959), and the head of A. cameroni described by Yasumatsu and Masuda (Citation1932) are similar to those of A. spinosus in the following set of characters: (1) the body is fusiform with the thoracic and first abdominal segments narrower than the others; (2) the surface of the body lacks spinules; (3) both larvae have six large tubercles on the cephalic capsule, five of which are located on the frons (four laterally and one medially) and one at the base of the labrum. There is also a high degree of similarity in the length and shape of antennal tubercles, the size of palpi on both maxillae and labium, and the structure of the epipharynx and hypopharynx. The only differences pertain to the apical mandibular tooth, which is blunter in A. spinosus. However, the description by Evans and Lin (Citation1956) is incomplete and therefore we cannot compare characters such as chaetotaxy, the number and position of setae and sensilla, or the presence or absence of oval tubercles on the ventral side of the mandible, which were reported in A. spinosus.
Acknowledgements
We thank Karolina and Łukasz Musiał for the possibility of conducting our research in Kowalewo Pomorskie and Kamila Ludwik for the possibility of conducting our research in Sierakowo. We also thank Wojciech Pulawski (California Academy of Science) and Frank Kurczewski (State University of New York) for their critical reviews of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adlerz G. 1910. Lefnadsförhållanden och instinkter inom familjerna Pompilidae och Sphegidae. III. Kungliga Svenska Vetenskapsakademiens Handlingar 45(12):1–75.
- Biedermann R, Niedringhaus R. 2004. Die Zikaden Deutschlands. Bestimmungstafeln für alle Arten. Scheeßel: Fründ. pp. 409.
- Blösch M. 2000. Die Grabwespen Deutschlands. Sphecidae s. str., Crabronidae. Lebensweise, Verhalten, Verbreitung. In: Blank SM, Taeger A, editors. Die Tierwelt Deutschlands. 71. Teil. Keltern, Germany: Goecke & Evers. pp. 480.
- Bogusch P, Straka J, Kment P, editors. 2007. Annotated checklist of the Aculeata (Hymenoptera) of the Czech Republic and Slovakia. Komentovaný seznam žahadlových blanokřídlých (Hymenoptera: Aculeata) České republiky a Slovenska. Acta Entomologica Musei Nationalis Pragae Supplementum 11:1–300. [in English and Czech].
- Bohart RM, Menke AS. 1976. Sphecid wasps of the world. A generic revision. Berkeley: University of California Press. pp. ix + 695.
- Borries H. 1897. Bidrag til danske Gravehvepses Biologi. Videnskabelige Meddelelser fra den Naturhistoriske Forening i Kjøbenhavn 59:1–163.
- Dahlbom AG. 1847. Oplysninger angaaende Diodonti tristis og Alyssonii Ratzeburgi Levemaade. Forhandlinger ved de Skandinaviske Naturforskeres 4:277–280.
- Evans HE, Lin CS. 1956. Studies on the larvae of digger wasps (Hymenoptera, Sphecidae). Part II: Nyssoninae. Transactions of the American Entomological Society 82:35–66.
- Evans HE. 1959. Studies on the larvae of digger wasps (Hymenoptera, Sphecidae). Part V: Conclusion. Transactions of the American Entomological Society 85:137–191.
- Evans HE. 1966. The comparative ethology and evolution of the sand wasps. Vol. Xvi. Cambridge, MA: Harvard University Press. pp. 526.
- Evans HE, O’Neill KM. 2007. The sand wasps. Natural history and behavior. Cambridge, MA; London, England: Harvard University Press. pp. Ix + 340.
- Ferton C. 1901. Notes détachées sur l’instinct des Hyménoptères mellifères et ravisseurs avec la description de quelques espèces. Annales de la Société Entomologique de France 70:83–148.
- Ferton C. 1908. Notes détachées sur l’instinct des Hyménoptères mellifères et ravisseurs (4 Série) avec la description d’une espèce nouvelle. Annales de la Société Entomologique de France 77:535–586.
- Holzinger WE, Kammerlander I, Nickel H. 2003. The Auchenorrhyncha of Central Europe: Vol. 1: Die Zikaden Milleleuropas. Vol. 1. Fulgoromorpha, Cicadomorpha (excl. Cicadellidae). Leiden – Boston: Brill Academic Publishers. pp. 673.
- Itami H. 1967. Biology of Alysson pertheesi Gorski in Japan. Life Study 11:4–5.
- Kazenas VL. 2001. Fauna and biology of Sphecid Wasps (Hymenoptera, Sphecidae) of Kazakhstan and Central Asia. Almaty, Kazakhstan: Kazgos INTI. pp. 333.
- Kohl FF. 1880. Die Raubwespen Tirol’s nach ihrer horizontalen und verticalen Verbreitung, mit einem Anhange biologischer und kritischer Notizen. Zeitschrift des Ferdinandeums für Tirol und Vorarlberg 3(Heft 24):97–242.
- Lomholdt O. 1984. The Sphecidae (Hymenoptera) of Fennoscandia and Denmark. Fauna Entomologica Scandinavica. 4. 2nd ed. Leiden, Copenhagen: E.J. Brill/Scandinavian Science Press. pp. 452.
- Lundsgaard T. 1999. Javesella pellucida (F.) is a vector of Festuca leaf streak virus (FLSV, genus Cytorhabdovirus). Journal of Plant Diseases and Protection 106(5):545–549.
- Nemkov PG. 2012. Cобенности биологии роющих ос подсемейства Bembicinae (Hymenoptera, Crabronidae) [= Osobennosti biologii royushchikh os podsemeystva Bembicinae (Hymenoptera, Crabronidae), = Biological features of the digger wasps of the subfamily Bembicinae (Hymenoptera, Crabronidae)]. Chteniya Pamyati Alekseya Ivanovicha Kurentsova – A.I. Kurentsov’s Annual Memorial Meetings 23:114–132.
- Nickel H, Remane R. 2002. Artenliste der Zikaden Deutschlands, mit Angabe von Nährpflanzen, Nahrungsbreite Lebenszyklus Areal und Gefährdung (Hemiptera Fulgoromorpha et Cicadomorpha). Beiträge zur Zikadenkunde 5:27–64.
- Nickel H. 2003. The leafhoppers and planthoppers of Germany (Hemiptera: Auchenorrhyncha). Sofia, Bulgaria: Pensoft Publishers. pp. 460.
- Olberg G. 1959. Deutscher Verlag für Wissenschaften. Das Verhal. der solitären Wespen Mitteleuropas (Vespidae, Pompilidae, Sphecidae). Vol. XIII. Berlin: VEB Deutscher Verlag für Wissenschaften. pp. 402.
- Olszewski P, Bogusch P, Mięsikowski M, Baños-Picon L, Puchałka R. 2021a. Behavioural and ecological data on Dryudella stigma (Panzer, 1809) (Hymenoptera, Astatidae) with the first description of the mature larva. Journal of Hymenoptera Research 82:305–316. DOI: 10.3897/jhr.82.63594.
- Olszewski P, Wiśniowski B, Ljubomirov T. 2021b. Current list of the Polish digger wasps (Hymenoptera: Spheciformes). Spixiana 44(1):81–107.
- Pagliano G, Alma A. 1997. Ricerche etologiche su Gorytini e Alyssonini (Hymenoptera Sphecidae) parassitoidi di Auchenorryncha (Rhynchota Homoptera). Rivista Piemontese di Storia Naturale 18:173–181.
- Pulawski W. Catalog of Sphecidae. San Francisco: California Academy of Sciences. Available: https://researcharchive.calacademy.org/research/entomology/entomology_resources/hymenoptera/sphecidae/genera/Alysson.pdf. Accessed Jan 2022 10.
- Sann M, Niehuis O, Peters RS, Mayer C, Kozlov A, Podsiadlowski L, Bank S, Meusemann K, Misof B, Bleidorn C, Ohl M. 2018. Phylogenomic analysis of Apoidea sheds new light on the sister group of bees. BMC Evolutionary Biology 18:e71. DOI: 10.1186/s12862-018-1155-8.
- Schmid-Egger C. 2010. Rote Liste der Wespen Deutschlands. Hymenoptera Aculeata: Grabwespen (Ampulicidae, Crabronidae, Sphecidae), Wegwespen (Pompilidae), Goldwespen (Chrydididae), Faltenwespen (Vespidae), Spinnenameisen (Mutillidae), Dolchwespen (Scoliidae), Rollwespen (Tiphiidae) und Keulhornwespen (Sapygidae). Ampulex 1:5–39.
- Shorenko KI, Konovalov SV. 2010. New data on digger wasps (Hymenoptera: Ampulicidae, Sphecidae, Crabronidae) in the fauna of Ukraine. Ukraïns’ka Entomofaunistyka 1:9–32.
- Švácha P, Danilevsky ML. 1987. Cerambycoid larvae of Europe and Soviet Union. Part I.- Acta Universitatis Carolinae. Biologica 30:1–176.
- Tsuneki K. 1969. Gleanings on the bionomics of the East-Asiatic non-social wasps (Hymenoptera). IV. Some species of Bembicini, Stizini, Gorytini, Mellinini and Alyssonini. Etizenia 41:1–19.
- Yasumatsu K, Masuda H. 1932. On a new hunting wasp from Japan. Fukuoka Hakubutsugaku Zasshi 1:53–65.
- Zhang YX, Zhu ZF, Lu XL, Li X, Ge LQ, Fang JC, Wu JC. 2014. Effects of two pesticides, TZP and JGM, on reproduction of three planthopper species, Nilaparvata lugens Stĺl, Sogatella furcifera Horvath, and Laodelphax striatella Fallén. Pesticide Biochemistry and Physiology 115:53–57. DOI: 10.1016/j.pestbp.2014.07.012.

