Abstract
Populations of Monacha atacis from southern Occitania in France and of M. samsunensis from northern Anatolia in Turkey (Atakum/Samsun and Kastamonu) were investigated by an integrative approach based on morphological (shell and genitalia) and molecular (mitochondrial and nuclear gene sequences) features. Morphological examination revealed a complex pattern of variation within and between geographically separated populations, while molecular analysis showed strong similarity between the two species, confirming earlier suggestions that the species are conspecific. Pfeiffer’s name Helix samsunensis introduced in 1868 has priority over the name M. atacis given by Gittenberger & de Winter in 1985.
Introduction
Monacha Fitzinger, Citation1833 is a speciose hygromiid genus with species occurring from Britain and north-western France to the Caucasus, Middle East and north African coast (Hausdorf Citation2000a, Citation2000b; Welter-Schultes Citation2012; Neiber & Hausdorf Citation2017 and other references therein). After Hesse (Citation1914), Hausdorf (Citation2000a) recognised three subgenera within Monacha on the basis of presence/absence of penial retractor muscle and vaginal appendix: Monacha s.s. Fitzinger, Citation1833) (type species: Helix cartusiana Müller, Citation1774) for species without penis retractor but with appendix, Metatheba Hesse, Citation1914 (type species: Helix samsunensis Pfeiffer, Citation1868) for species with penial retractor but without appendix, and Paratheba Hesse, Citation1914 (type species: Helix fruticola Krynicki, Citation1833) for species with both penial retractor and appendix. In an excellent integrative phylogenetic and biogeographic analysis of Monacha based on anatomical features of the reproductive system and molecular data (mitochondrial and nuclear gene sequences), Neiber and Hausdorf (Citation2017) established four new subgenera: Pontotheba (type species: Monacha (Paratheba) bithynica Hausdorf, Citation2000a), Aegaeotheba (type species: Monacha (Paratheba) cretica Hausdorf, Citation2003), Trichotheba (type species: Monacha (Monacha) comata Hausdorf, Citation2000a), Rhytidotheba (type species: Helix (Trichia) densecostulata Retowski, Citation1887). They also resurrected the subgenus Platytheba Pilsbry, Citation1895 (type species: Caracolla nummus Ehrenberg, Citation1831) but left the status of Eutheba Nordsieck, Citation1993 unresolved.
Most Monacha species have limited ranges of distribution, restricted to their type localities, or if wider, to the southern Balkans and Anatolia (especially the Pontic region along the Black Sea coast). The exceptions include three species from the subgenus Monacha s.s., namely M. cartusiana (Müller, Citation1774), M. claustralis (Rossmässler, Citation1834) and M. cantiana (Montagu, Citation1803). M. cartusiana is widespread throughout Europe except in the north-east (Scandinavia, Russia, Baltic states, Belarus, northern Ukraine) (Welter-Schultes Citation2012). M. claustralis is now spreading quickly northward (Pinter Citation1968; Hlaváč & Peltanová Citation2010; Pieńkowska et al. Citation2015, Citation2016, Citation2018a; Hutchinson et al. Citation2019; Čejka et al. Citation2020; Gural-Sverlova & Gural Citation2022) from its native range in European and Anatolian Turkey (Hausdorf Citation2000a) and Greece (with Corfu/Kerkyra as type locality, Welter-Schultes Citation2012). M. cantiana is found in Great Britain, northern France and Germany, in the Benelux countries as well as in Spain, where it was probably introduced in Roman times from its native area in central Italy (Kerney et al. Citation1964; Kerney Citation1970; Evans Citation1972; Pieńkowska et al. Citation2018b). Monacha (Platytheba) ocellata (Roth Citation1839), known from the vicinity of Istanbul in Turkey, was recently discovered in a single locality in Britain, probably resulting from passive introduction in unknown circumstances (Anderson et al. Citation2018).
All but one species of subgenus Metatheba occur in northern Anatolia, mainly along the Black Sea coast. The only exception is M. (Metatheba) atacis Gittenberger & de Winter, Citation1985, known from southern Occitania, France (Hausdorf Citation2000a; Falkner et al. Citation2002; Gargominy et al. Citation2011; Neiber & Hausdorf Citation2017) and a site in Catalonia, north-eastern Spain (Bertrand Citation2003). However, when describing the new species, Gittenberger and de Winter (Citation1985) already drew attention to its close relationship with M. samsunensis (Pfeiffer, Citation1868). Considering the great similarity between M. atacis and M. samsunensis, Hausdorf (Citation2000a) and Neiber and Hausdorf (Citation2017) suggested that despite their disjunct ranges these two taxa could be conspecific and that the French populations of M. atacis might be the result of introduction of M. samsunensis in historical times. One of us (MP) collected M. atacis in several sites in the foothills of the Pyrenees, southern France, while another member of our team (GG) found M. samsunensis in two localities in northern Anatolia, Turkey, one in the vicinity of her university in Kastamonu and the other near Atakum/Samsun, i.e. in the type locality of the species. This enabled us to undertake the task of verifying the hypothesis of Hausdorf (Citation2000a) and Neiber and Hausdorf (Citation2017, also see Cadevall et al. Citation2020: p. 155). The results of our study are reported in this paper.
Material and methods
Taxonomic sampling
Specimens for research were collected on the basis of the literature data on the occurrence of M. atacis in France (Gittenberger & de Winter Citation1985) and M. samsunensis in Turkey (Hausdorf Citation2000a; Welter-Schultes Citation2012). Species identification was based on morphological and molecular research. Thus, the specimens were obtained from 18 populations of Monacha atacis from southern France and three populations of M. samsunensis from northern Anatolia in Turkey. They were considered in our analysis of molecular and genital structure ( and ). A new French population of M. cartusiana () as well as literature data on several Monacha species and lineages were used in the analysis. Several sequences deposited in GenBank () were also used for molecular analysis. Sequences of Trochulus hispidus (Linnaeus, Citation1758) from GenBank were used as an outgroup to construct phylogenetic trees.
Figure 1. Map of localities of the populations of Monacha atacis, M. samsunensis and M. cartusiana analysed (see for details).
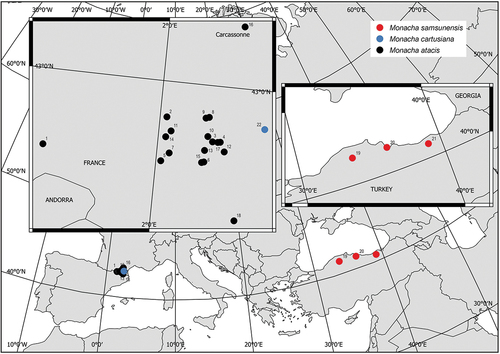
Table I. List of localities of the French and Turkish populations of Monacha atacis, M. samsunensis and M. cartusiana used for molecular and morphological research.
Table II. GenBank sequences used for molecular analysis comparisons.
Material examined
New material examined is listed as follows, when possible: geographic coordinates of locality, locality (country, region, municipality and province, site), collector(s), date, number of specimens, with the collection where the material is kept in parenthesis (). The material is kept in the collection of the Department of Cell Biology, Adam Mickiewicz University, Poland (DCBC), the Małgorzata Proćków collection (MNHW; Museum of Natural History, University of Wrocław, Poland) and the Folco Giusti collection (FGC; Dipartimento di Scienze Fisiche, della Terra e dell’Ambiente, Università di Siena, Italy). The material used for comparison has already been described (see Pieńkowska et al. Citation2018b: table 1, Citation2019a: table 1, Citation2020: table 1).
Morphological study
Twenty-four specimens from 15 sites in France and ten specimens from two sites in Turkey were analysed for shell and anatomy (see ). Snail bodies were dissected under a light microscope (Wild M5A, or Zeiss SteREO Lumar. V12). Anatomical details were drawn using a Wild camera lucida. Adult specimens from Turkey were obtained in sufficient number to describe their genital structure, however the scarcity of specimens per population (where several were juveniles or subadults) meant that no quantitative analysis was done on the morphological characters.
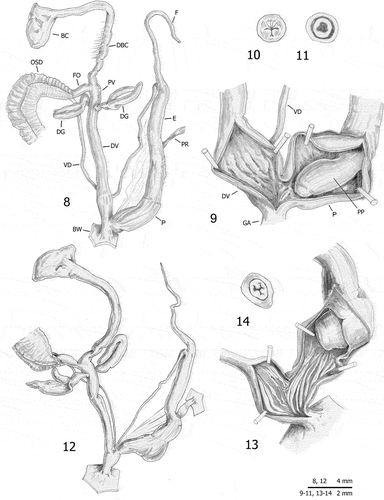
Abbreviations: BC bursa copulatrix (also known as gametolytic gland), BW body wall, DBC duct of bursa copulatrix (also known as pedunculus), DG digitiform glands (also known as mucous glands or glandulae mucosae), DV distal vagina (from digitiform glands to genital atrium), E epiphallus (from base of flagellum to beginning of penial sheath), F flagellum, FO free oviduct, GA genital atrium, GAR genital atrium retractor, OSD ovispermiduct (also known as spermoviduct), P penis (from beginning of penial sheath to genital atrium), PP penial papilla (also known as glans), PR penial retractor, PV proximal vagina (from confluence of free oviduct and duct of bursa copulatrix to digitiform glands), VD vas deferens.
Molecular study
Sixty-three specimens of M. atacis and 14 of M. samsunensis were used in the molecular analysis (). Total genomic DNA was extracted from 20 mg of foot tissue using Tissue Genomic DNA extraction MiniKit (Genoplast) following the manufacturer’s instructions. Purified total DNA was used as template for amplification by polymerase chain reaction (PCR) of partial sequences of the following gene fragments: mitochondrial 5’-end of cytochrome c oxidase subunit I (COI) and large subunit ribosomal DNA gene (16S rDNA), as well as nuclear internal transcribed spacer 2 (ITS2) in ribosomal DNA flanked with 5.8S and 28S ribosomal DNA fragments (5.8S rDNA and 28S rDNA, respectively) and histone 3 (H3). Partial sequences of these gene fragments were obtained by PCR with the primer sets listed in .
Table III. Primers used in molecular analysis.
All PCRs were carried out with total volumes of 10 μl. The following thermal profile was used for COI amplification: 5 min at 95°C followed by 35 cycles of 30 s at 95°C, 1 min at 50°C, 1 min at 72°C, and finally 5 min at 72°C using Type-it Microsatellite PCR kit (Qiagen) or 5 min at 95°C followed by 40 cycles of 30 s at 94°C, 30 s at 50°C, 1 min at 72°C, and finally 7 min at 72°C using tiTaq Polymerase (EURx). Amplifications of fragments of 16S rDNA (short fragment, see ), H3 and ITS2 (flanked with 5.8S rDNA and short fragment of 28S rDNA, see ) were performed according to procedures previously described by Manganelli et al. (Citation2005), Colgan et al. (Citation1998) and Almeyda-Artigas et al. (Citation2000), respectively. Amplifications of 16S rDNA (longer fragment, see ) and ITS2 (flanked with 5.8S rDNA and longer fragment of 28S rDNA, see ) were performed with the same thermal profile as for COI amplification with tiTaq Polymerase (EURx), however for this ITS2 sequence, two rounds of amplifications were performed: the first with the purified total DNA as template and the second with 1 µl of the 10× diluted product from the first round as template. Lengths of amplification products were as follows: COI – 710 bp; 16S rDNA – 371–382 (short fragments) or 873–882 bp (long fragments); ITS2 (flanked with 5.8S rDNA and short fragment of 28S rDNA) – 655–681 bp; ITS2 (flanked with 5.8S rDNA and longer fragment of 28S rDNA) – 911–932 bp; H3 – 375 bp.
The PCR products were verified by agarose gel electrophoresis (1% agarose) and purified for sequencing with thermosensitive Exonuclease I and FastAP alkaline phosphatase (Fermentas, Thermo Scientific). Finally, the amplified products were sequenced in both directions using the BigDye Terminator v3.1 sequencing kit on an ABI Prism 3130XL Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocols.
Sequences were edited with BioEdit version 7.0.6 (Hall Citation1999; BioEdit Citation2017). Alignments were performed with ClustalW, implemented in BioEdit (Thompson et al. Citation1994). The COI and H3 sequences were aligned according to the translated amino acid sequences to correct errors that could arise from the presence of ambiguous nucleotides after sequencing. The ends of all sequences were trimmed and deposited in GenBank (). Sequences obtained by PCR with NEWS2 and ITS2-RIXO, and LSU1 and LSU3 primer pairs () were joined in longer sequences marked as lgITS2 (see ). After trimming, the lengths of sequences were 684 and 678 bp for COI, 309–317 bp for 16S rDNA short fragment, 814–821 for 16S rDNA long fragment, 558–581 for short ITS2 (marked sITS2, including 71 bp 5.8S rDNA + 487–510 bp ITS2), 835–856 bp for long ITS2 (marked lgITS2, including 89 bp 5.8S rDNA + 489–510 bp ITS2 + 257 bp 28S rDNA) and 303 bp for H3 (see also ). For phylogenetic analysis, the following alignments were made: 600 positions long for COI, 332 or 869 positions long for 16S rDNA, 279 position long for H3. Sequences of ITS2 alone and sequences of ITS2 flanked by fragments of 5.8S rDNA at the 3’-end and 28S rDNA at the 5’-end, for comparison with sequences obtained from GenBank, were 546 (or 564) and 856 positions of alignment length, respectively. The sequences were collapsed to haplotypes (COI, 16S rDNA) and to common sequences (H3, ITS2 flanked with 5.8S rDNA, ITS2 flanked with 5.8S rDNA and 28S rDNA) using the programme ALTER (Alignment Transformation EnviRonment) (Glez-Peña et al. Citation2010). Finally COI and 16S rDNA haplotypes were joined into concatenated sequences COI+16S rDNA, 932 or 1469 positions (600 COI + 332 16S rDNA or 600 COI + 869 16S rDNA) in length, ITS2 and H3 common sequences were joined into concatenated sequences ITS2 + H3, 825 positions (546 ITS2 + 279 H3) in length and COI and 16S rDNA haplotypes were joined with ITS2 (flanked with 5.8S and 28S rDNA fragments) common sequences into concatenated sequences of COI + 16S rDNA + ITS2, 2325 positions in length (600 COI + 869 16S rDNA + 42 5.8S rDNA + 557 ITS2 + 257 28S rDNA).
For each alignment file, best nucleotide substitution models were specified according to the Bayesian Information Criterion (BIC): for COI (600 bp) – HKY+G+I, for concatenated sequences COI + short 16S rDNA (932 positions) – T92+G+I, for COI + 16S rDNA + ITS2 (flanked with 5.8S and 28S rDNA) – GTR+G + I, for COI + long 16S rDNA (1469 positions) – GTR+G, for ITS2 (564 bp) and for concatenated sequences ITS2 + H3 (825 positions) – K2 + G (Kimura Citation1980; Hasegawa et al. Citation1985; Tamura Citation1992; Nei & Kumar Citation2000; Kumar et al. Citation2016). The evolutionary distances were computed using the Kimura 2-parameter method (Kimura Citation1980). Neighbour Joining (NJ) analysis (Saitou & Nei Citation1987) and Maximum Likelihood (ML) analysis were performed with MEGA7 (Kumar et al. Citation2016). Calculated bootstrap values obtained by ML and NJ analysis were mapped on the ML trees. In addition, Bayesian Inference (BI) was conducted for concatenated COI + 16S rDNA + ITS2 (flanked with 5.8S and 28S rDNA) sequences with the use of the programme MrBayes 3.2.6 (Ronquist & Huelsenbeck Citation2003; Ronquist et al. Citation2012). Four Monte Carlo Markov chains were run for 1 million generations, sampling every 100 generations (the first 25% of trees were discarded as “burn-in”). Posterior probability (PP) values obtained on the 50% majority rule consensus Bayesian tree of concatenated sequences were mapped together with bootstrap values obtained by ML and NJ analysis on the ML tree.
Results
Morphological study
Monacha atacis and M. samsunensis have globose to subglobose shell (), sometimes variably depressed, variable in size (in M. atacis: diam. 7.5–15.2 mm; Gittenberger & de Winter Citation1985; in M. samsunensis: diam. 11.0–19.1 mm; Hausdorf Citation2000a), pale yellowish, pale ochre or whitish in colour (creamy-white, sometimes with sparse darker stripes in M. samsunensis), sometimes with one whitish peripheral band (only in some light horn-coloured specimens of M. samsunensis according to Hausdorf Citation2000a), surface with fine irregular growth-ridges, very fine spiral striae and evident hair scars on the first whorls. Aperture roundish to oval, slightly descending, with a variably thick white internal rib (only very evident in M. samsunensis). Peristome interrupted, its columellar margin reflexed to more or less cover the umbilicus, which may be very small to almost closed or rather open (for M. atacis, see also Gittenberger & de Winter Citation1985: fig. 2; Welter-Schultes Citation2012: fig. at p. 503; for M. samsunensis, see also Hudec & Ležava Citation1969: pl. 10 fig. 25; Schileyko Citation1978: pl. 16 fig. 159; Hausdorf Citation2000a: pl. 12 figs 56–57; Schileyko Citation2005: fig. 2534a; Welter-Schultes Citation2012: fig. at p. 512).
Figure 2–7. Shells of Monacha atacis from France: Grotte de Majestier [Maj3] (DCBC & MNHW-F.18.41; FGC 51099) (2), Saint-Ferriol [Fer2-1] (DCBC & MNHW-F.18.39; FGC 51097) (3) and Carcassonne (FGC 35773) (4) and Monacha samsunensis from Turkey: Kastamonu [Kas2: 5; Kas6: 6; Kas3: 7] (DCBC; FGC 51094) (5–7).
![Figure 2–7. Shells of Monacha atacis from France: Grotte de Majestier [Maj3] (DCBC & MNHW-F.18.41; FGC 51099) (2), Saint-Ferriol [Fer2-1] (DCBC & MNHW-F.18.39; FGC 51097) (3) and Carcassonne (FGC 35773) (4) and Monacha samsunensis from Turkey: Kastamonu [Kas2: 5; Kas6: 6; Kas3: 7] (DCBC; FGC 51094) (5–7).](/cms/asset/29a58005-1044-4066-9ddc-62e126757aeb/tizo_a_2100932_f0002_oc.jpg)
Monacha atacis and M. samsunensis show distal genitalia of the Metatheba type, i.e. with penial retractor muscle but without vaginal appendix. The literature reports evidence of a long vagina, bursa copulatrix duct and flagellum in M. atacis (Gittenberger & de Winter Citation1985) and a variably long vagina, bursa copulatrix duct and flagellum in M. samsunensis (Hesse Citation1931; Hudec & Ležava Citation1969; Hudec Citation1973; Schileyko Citation1978; Hausdorf Citation2000a). This picture was confirmed by our anatomical study. Both species showed vagina with very short proximal section and variably long distal section. In M. atacis, the distal vagina is long and variably slender, sometimes widening slightly in its subterminal portion where the internal surface shows a series of small swollen pleats (). In M. samsunensis, the distal vagina is variably long and wide: sometimes long and slender (; see also Hesse Citation1931: pl. 6 figs 55a-b; Hudec & Ležava Citation1969: fig. 22; Hudec Citation1973: fig. 6; Hausdorf Citation2000a: fig. 50) and at other times rather or very short (). When short or very short, the vagina is also wider distally () or very wide with an internal ring of variably raised pleats (), a detail never mentioned before. The duct of the bursa copulatrix is very long in M. atacis (; see also Gittenberger & de Winter Citation1985: figs. 7–8) and variably long in M. samsunensis, ranging from very short or short (Hesse Citation1931: pl. 6 fig. 55a; Hudec & Ležava Citation1969: fig. 22) to long or very long (, and ; see also Hudec Citation1973: fig. 6; Hausdorf Citation2000a: fig. 50). Finally the flagellum is rather long in M. atacis (; see also Gittenberger & de Winter Citation1985: figs. 7–8) and variably long in M. samsunensis, ranging from short (; see also Hesse Citation1931: pl. 6 fig. 55a; Hudec & Ležava Citation1969: fig. 22; Hudec Citation1973: fig. 6) to medium or rather long (; see also Schileyko Citation1978: fig. 377; Hausdorf Citation2000a: fig. 50).
Figures 8–14. Distal genitalia (8, 12), internal structure of distal genitalia (9, 13), transverse sections of medial epiphallus (10) and apical penial papilla (11, 14) of Monacha atacis from France: Carcassonne (FGC 35773) (8–11) and Lapradelle (DCBC & MNHW-F.18.27; FGC 51247) (12–14).
Molecular study
Two hundred and ninety-two new sequences were obtained and deposited in GenBank: 221 for M. atacis (61 COI, 62 16S rDNA, 58 H3, 20 sITS2 and 20 lgITS2), 54 for M. samsunensis (12 COI, 14 16S rDNA, 14 H3, 3 sITS2 and 11 lgITS2) and 17 for M. cartusiana (4 COI, 5 16S rDNA, 5 H3, 2 sITS2 and 1 lgITS2) (for details on their lengths and GenBank accession numbers, see ). We identified 24 COI haplotypes (eleven COI 1 – COI 11 for M. atacis, nine COI 12 – COI 20 for M. samsunensis, four COI 17 – COI 20 for M. cartusia-na) and 35 16S rDNA haplotypes (18 16S 1–16S 18 for M. atacis; 12 16S 19–16S 30 for M. samsunensis; 5 16S 31–16S 35 for M. cartusiana). Among sequences of the H3 gene, 10 common sequences were identified (6 H3 1 – H3 6 for M. atacis and M. samsunensis; 4 H3 7 – H3 10 for M. cartusiana). We established 15 short ITS2 (sITS2: 5.8S rDNA + ITS2) and 15 long ITS2 (lgITS2: 5.8S rDNA + ITS2 + 28S rDNA) common sequences (for M. atacis – 12 sITS2: sITS2 1 – sITS2 12 and 9 lnITS2: lnITS2 1 – lnITS2 9; for M. samsunensis – one sITS2: sITS2 13 and 4 lnITS2: lgITS2 10 – lgITS2 14; for M. cartusiana: two sITS2: sITS2 14 – sITS2 15 and one lgITS2: lgITS2 13). Haplotypes and common sequences were used for phylogenetic analysis.
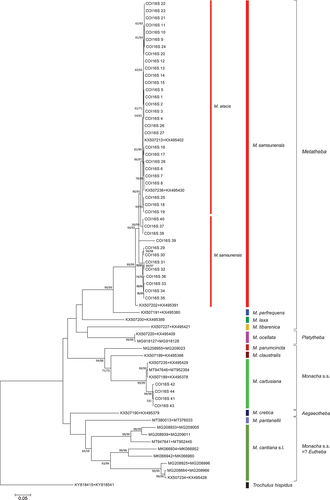
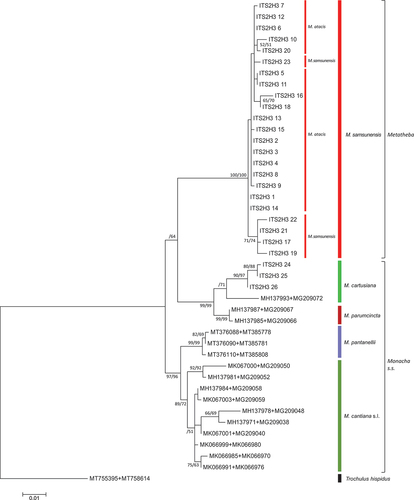
In the case of mitochondrial gene fragments (COI and 16S rDNA), sequences obtained from M. atacis and M. samsunensis were analysed separately as single locus data sets (not shown, except COI sequence analysis, see Supplementary Material Figure S1) or as concatenated COI + short 16S rDNA sequences ( and Supplementary Material Table S1). A tree of similar topology was obtained from analysis of concatenated COI + long 16S rDNA sequences (Figure S2 and Supplementary Material Table S1). The M. atacis and M. samsunensis sequences clustered together in these trees, although two groups, one for M. atacis and another for M. samsunensis sequences, can be seen. In the case of new sequences obtained from the population of M. cartusiana from Cubières-sur-Cinoble, southern France, in the concatenated COI + 16S rDNA tree (, see also Supplementary Material Table S1), they clustered quite separately from M. atacis and M. samsunensis, as well as from other representatives of Metatheba, but together with other COI and 16S rDNA sequences of M. cartusiana obtained from GenBank.
Figure 23. Maximum Likelihood (ML) tree of concatenated COI + (short) 16S rDNA haplotypes obtained from specimens of Monacha atacis and Monacha samsunensis compared with sequences obtained from GenBank for representatives of the other Monacha species. Concatenated COI + 16S rDNA sequences (Table S1) were cut to 932 positions (600 bp COI + 332 bp 16S) in length. Numbers next to the branches indicate bootstrap support above 50% calculated for 1000 replicates from ML (left) and NJ (right) analysis (Felsenstein Citation1985). The tree was rooted with Trochulus hispidus concatenated sequences KY818415 + KY818541 deposited in GenBank by Neiber et al. (Citation2017).
K2P distances for COI sequences characteristic of intraspecies differentiation were very small: mean 0.6% (range 0.0–1.4%) for M. atacis and mean 3.5% (range 0.0–7.6%) for M. samsunensis (). K2P distances between COI sequences of M. atacis and M. samsunensis were also small (mean 3.5%, range 1.2–6.9%, ). They were an order of magnitude smaller than the distances that distinguished these two taxa from the other species of the subgenus Metatheba i.e. M. perfrequens, M. laxa and M. tibarenica (3.5% vs. 13.0–18.6%) analysed here. The K2P distances separating the COI sequences of M. atacis and M. samsunensis from species of other Monacha subgenera were even greater (19.1–22.9%). K2P distances within the southern French M. cartusiana populations, as well as between them and other Monacha species (0.4% intraspecies, 14.5% and 19.9% M. cartusiana vs. M. claustralis and M. cantiana, respectively; ) were similar to those reported in our previous papers (Pieńkowska et al. Citation2015, Citation2016, Citation2018a).
Table IV. Ranges of K2P genetic distances between analysed COI sequences (mean value in parenthesis).
Analysis of nuclear genes was hindered by the fact that only the 5.8S rDNA + ITS2 + 28S rDNA gene sequences (lgITS2) were deposited in great number in GenBank by Neiber and Hausdorf (Citation2017), including Metatheba subgenus representatives. For the H3 gene, GenBank only contains sequences of the M. cantiana s.l. complex, deposited in connection with our previous papers (Pieńkowska et al. Citation2018b, Citation2019a, Citation2020). We therefore present two trees, based on various analyses of single or multiple locus data sets, one consisting of the ITS2 gene sequences cut off from flanking fragments () and the other built from concatenated ITS2 + H3 sequences (, Supplementary Material Table S2). They confirm the results obtained with mitochondrial genes. The sequences obtained from M. atacis and M. samsunensis specimens are grouped into a common clade, and because they are mixed with each other, no separate subgroups can be distinguished ( and ). It is noteworthy that the sequence KX495444 deposited in GenBank by Neiber and Hausdorf (Citation2017) for ITS2 of M. samsunensis is identical to the sequences lgITS2 1, found in some specimens of M. atacis from different French populations (Arties, Le Chandelier, Axat, Saint-Martin-Lys, Belfort-sur-Rebenty, Salvezines and Roquefort-de-Sault; ). Moreover, the H3 1 sequence was found in 9 out of the 10 specimens of M. samsunensis from Kastamonu as well as in nine specimens of M. atacis from eight French populations (Arties, Le Chandelier, Mijanès, Campagna-de-Sault, Saint-Ferriol 2, Belfort-sur-Rebenty 1, Salvezines and Roquefort-de-Sault; ) and the H3 2 sequence was found in four M. samsunensis specimens from Atakum/Samsun as well as in 44 specimens from 14 French populations (i.e. all but one: Le Chandelier, however only one specimen was available from this population; ).
Figure 24. Maximum Likelihood (ML) tree of ITS2 common sequences obtained from specimens of Monacha atacis and Monacha samsunensis compared with sequences obtained from GenBank for representatives of the other Monacha species. ITS2 sequences were cut to 564 positions in length. Numbers next to the branches indicate bootstrap support above 50% calculated for 1000 replicates from ML (left) and NJ (right) analysis (Felsenstein Citation1985). The tree was rooted with Trochulus hispidus ITS2 sequences KX495451 and MG585474 deposited in GenBank by Neiber and Hausdorf (Citation2017) and Caro et al. (Citation2019), respectively.
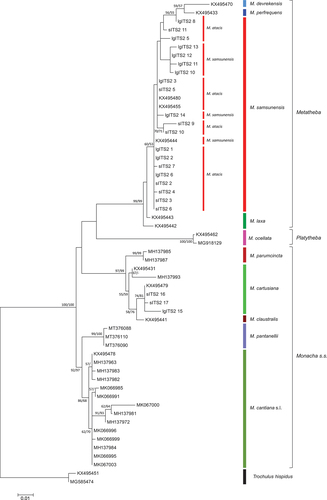
Figure 25. Maximum Likelihood (ML) tree of concatenated ITS2 + H3 common sequences obtained from specimens of Monacha atacis and Monacha samsunensis compared with sequences obtained from GenBank for representatives of the other Monacha species. Concatenated ITS2 + H3 sequences (Table S2) were cut to 825 positions (546 positions ITS2 and 279 positions H3) in length. Numbers next to the branches indicate bootstrap support above 50% calculated for 1000 replicates from ML (left) and NJ (right) analysis (Felsenstein Citation1985). The tree was rooted with Trochulus hispidus ITS2 + H3 concatenated sequences MT755395 and MT758614 deposited in GenBank by Proćków et al. (Citation2021).
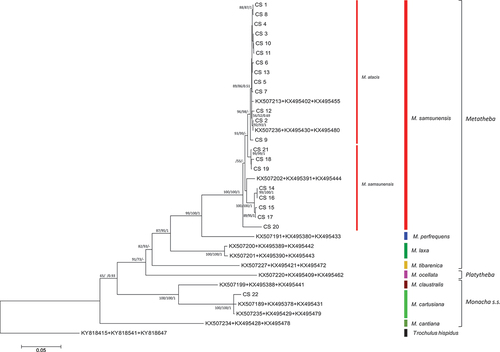
Finally, we present an analysis of concatenated mitochondrial and nuclear gene sequences: COI + 16S rDNA + 5.8S rDNA + ITS2 + 28S rDNA conducted by three different methods (ML, NJ and BI) (, Supplementary Material, Table S3). Again, sequences from M. atacis and M. samsunensis clustered in two slightly separate subgroups, but as a common clade they were clearly separate from sequences of other Monacha (Metatheba) and Monacha s.s. species.
Figure 26. Maximum Likelihood (ML) tree of concatenated COI + 16S rDNA + (5.8S rDNA + ITS2 + 28S rDNA) sequences of Monacha atacis and Monacha samsunensis compared with sequences obtained from GenBank for representatives of the other Monacha species. Concatenated sequences (Table S3) were 2325 positions in length (600 COI + 869 16S rDNA + 42 5.8S rDNA + 557 ITS2 + 257 28S rDNA). Bootstrap support above 50% from ML (left) and NJ (middle) analysis as well as posterior probabilities (right) from Bayesian inference analysis are marked at the nodes. Bootstrap analysis was run with 1000 replicates (Felsenstein Citation1985). The tree was rooted with Trochulus hispidus COI + 16S rDNA + (5.8S rDNA + ITS2 + 28S rDNA) concatenated sequences KY818415 + KY818541 + KY818647 deposited in GenBank by Neiber et al. (Citation2017).
Discussion
The shells of M. atacis from southern France and M. samsunensis from Turkey (Atakum/Samsun and Kastamonu) are very similar and do not differ from the lectotype of M. samsunensis deposited in the Naturhistorisches Museum Wien (, see also Hausdorf Citation2000a: pl. 11, fig. 54).
Figure 27. Shell of the Monacha samsunensis lectotype designated by Hausdorf (Citation2000a), kept in the Naturhistorischen Museum Wien (© NHMW, the inventory number: NHMW-MO-79000/K/19263) (photo by Sara Schnedl obtained by courtesy of Anita Eschner, NHMW). Scale bar 5 mm.

The distal genitalia of M. atacis specimens from Carcassonne () and Lapradelle (), characterised by a long vagina, a long bursa copulatrix duct and a long flagellum, exactly match those of the original description of this species (Gittenberger & de Winter Citation1985: figs 7–8). Specimens of M. samsunensis from the type locality () and the literature (Hesse Citation1931: pl. 6 fig. 55a; Hudec & Ležava Citation1969: fig. 22; Hudec Citation1973: fig. 6; Schileyko Citation1978: fig. 377; Hausdorf Citation2000a: fig. 50; Schileyko Citation2005: fig. 2534b) are usually characterised by a shorter flagellum, vagina and bursa copulatrix duct. Specimens of M. samsunensis from Kastamonu also seem to have a much shorter vagina than the others ( and ). However, vagina length is variable in M. samsunensis populations and possibly depends on sexual maturation (Hausdorf Citation2000a: tables 10 & 11, vagina total length 1.7–7.2 mm, measured in 35 specimens from different populations). In contrast to M. samsunensis, the features of the distal genitalia of M. atacis seem to vary little. In the absence of a more integrative approach to the study of the Turkish Metatheba, it is difficult to explain the significance of this pattern. For example, Gittenberger and de Winter (Citation1985) wondered if the various figures of M. samsunensis reported in the literature really belong to a single species. However, high intra- and inter-population variability is well known among the Monacha species so much so that the species of this genus can only occasionally be recognised morphologically (Pieńkowska et al. Citation2018b, Citation2019a, Citation2020).
Some sequences of nuclear genes (H3 and lgITS2) obtained from specimens of M. atacis and M. samsunensis are exactly the same. The sequences of nuclear genes from these two species mixed and grouped together in a common clade on phylogenetic trees ( and ). The sequences of their mitochondrial genes also cluster together (, also Supplementary Material Figure S1) as found also in those of the concatenated mitochondrial and nuclear genes (). Although there are two separate subgroups for sequences of M. atacis and M. samsunensis in these analyses, they create a single strongly supported clade on both phylogenetic trees ( and ). The mean K2P distance for COI sequences between M. atacis and M. samsunensis is small, reaching 3.5% () which is almost at the 3% threshold of the “barcode method” based on COI sequences (Hebert et al. Citation2003a, Citation2003b; Pentinsaari et al. Citation2020).
It is noteworthy that the mean K2P distance between the French M. atacis and the topotypical M. samsunensis from Atakum/Samsun is smallest (2.8%) when the Turkish populations are analysed separately (). The mean K2P distances between M. atacis and M. samsunensis from the Kastamonu population as well as between M. atacis and M. samsunensis from Kürtün (sequence KX507202 from Neiber & Hausdorf Citation2017) were 3.8% and 4.4%, respectively (). Nevertheless it must be stressed that Turkish populations vary in K2P distances between COI sequences (see K2P distances between three Turkish populations as well as the ranges of K2P distances, ), which may suggest that they are somewhat genetically differentiated. We are aware of limits of the barcode method in the analysis of taxonomic relations of stylommatophoran snails (Davison et al. Citation2009; Sauer & Hausdorf Citation2010, Citation2012; Köhler & Johnson Citation2012; see also discussions in our previous papers Pieńkowska et al. Citation2018b, Citation2019a, Citation2020). Nevertheless in this study we used Hebert’s method to confirm conspecificity and not to support the conclusion about species distinctness.
Table V. Ranges of K2P genetic distances between COI sequences of M. atacis and M. samsunensis populations (mean value in parenthesis).
Incidentally, we used molecular analysis to confirm the occurrence of M. cartusiana in southern France (population from Cubières-sur-Cinoble in Aude, ), where it may co-occur with M. atacis. This confirms previous molecular reports of M. cartusiana in France (Dahirel et al. Citation2015: north-western France 48°07′51″N, 01°41′34″W, near Rennes; Čejka et al. Citation2020: fig. 3 – in Provence, southern France: 43°31ʹ34.7”N, 05°04ʹ30.7”E, L’Etang de Berre; 43°49ʹ13.1”N, 05°18ʹ29.5”E, Bonnieux; 43°37ʹ03.7”N, 05°18ʹ37.8”E, St. Cannat; 43°37ʹ58.4”N, 05°38ʹ37.3”E, Jouques; 43°39ʹ33.5”N, 05°20ʹ43.1”E, Rognes).
In conclusion, our morphological (shell and genitalia, and ) and molecular (mitochondrial and nuclear gene sequences, ) findings corroborate that M. atacis and M. samsunensis are conspecific and that the former should be named M. samsunensis because the name introduced by Pfeiffer in Citation1868 has priority over that established by Gittenberger & de Winter in Citation1985. According to Hausdorf (Citation2000a) the occurrence of M. atacis in France is the result of an introduction of M. samsunensis in historic times. This possibility is supported by the fact that M. atacis occurs in a rather small area of France. However, the diversity of rapidly evolving mitochondrial genes may indicate that the French populations differentiated since their introduction. They may represent a distinct lineage that originated in France after their introduction (according to Falkner et al. Citation2002, the species has been reported from France at least since the 19th century) by the founder effect or by selection. This hypothesis cannot be verified without further research on a greater number of French populations of M. samsunensis (possibly also those from Catalonia, Spain).
On the other hand, it seems that there is greater genetic differentiation between Turkish populations from Atakum/Samsun, Kastamonu and Kürtün. It is noteworthy that there is more genetic similarity between specimens of M. atacis from France and the topotypical M. samsunensis from Atakum/Samsun than between Atakum/Samsun and Kastamonu populations. M. samsunensis has a wider distribution in Turkey than in France, and has probably existed there for much longer. A reason for the lower variability observed within French populations may be their smaller range and shorter evolution. The variability in populations of M. samsunensis occurring in northern Anatolia and along the Black Sea coast is worthy of further study.
The introduction of M. samsunensis to Western Europe is not an isolated case among the Monacha hygromiids. A population of M. ocellata was recently found in England, where it was accidentally introduced from the Istanbul area, Turkey (Anderson et al. Citation2018).
Supplemental Material
Download MS Word (40.6 KB)Supplemental Material
Download MS Word (40.5 KB)Supplemental Material
Download MS Word (43 KB)Supplemental Material
Download EPS Image (1.6 MB)Supplemental Material
Download EPS Image (2.4 MB)Acknowledgements
We thank Anita Eschner (Naturhistorischen Museum Wien) for providing photos of the shell of Monacha samsunensis lectotype, Krzysztof Duda (Adam Mickiewicz University) for preparing the map of localities, Jarosław Bogucki (Poznań, Poland) for preparing some graphs for print and Helen Ampt (Siena, Italy) for revising the English.
Disclosure statement
The authors declare that they do not have any conflict of interests.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/24750263.2022.2100932.
Additional information
Funding
References
- Almeyda-Artigas RJ, Bargues MD, Mas-Coma S. 2000. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and elucidation of the species causing human gnathostomiasis in the Americas. Journal of Parasitology 86(3):537–544. DOI: 10.1645/0022-3395(2000)086[0537:IRSOGS]2.0.CO;2.
- Anderson A, Giusti F, Telfer MG, Manganelli G, Pieńkowska JR, Lesicki A. 2018. Monacha ocellata (Roth, 1839) (Gastropoda: Hygromiidae) established in Essex, an addition to the fauna of Britain and Ireland. Journal of Conchology 43(2):201–211.
- Bertrand A. 2003. Notes sur la distribution géographique des mollusques continentaux de France et de Catalogne. Documents Malacologiques 4:33–36.
- BioEdit. 2017. BioEdit 7.2. Available: https://bioedit.software.informer.com/7.2. Accessed May 2021 21.
- Cadevall, J, Corbella, J, Bros, V, Orozco, A, Guillén, G, Prats, L, and Capdevila, M. 2020. Els mol·luscs continentals de Catalunya i Andorra (península Ibèrica). Llista comentada. Spira 7:117–159.
- Caro A, Neiber MT, Gomez-Moliner BJ, Madeira MJ. 2019. Molecular phylogeny and biogeography of the land snail subfamily Leptaxinae (Gastropoda: Hygromiidae). Molecular Phylogenetics and Evolution 139:106570. DOI: 10.1016/j.ympev.2019.106570.
- Čejka T, Beran L, Korábek O, Hlaváč JČ, Horáčková J, Coufal R, Drvotová M, Maňas M, Horsáková V, Horsák M. 2020. Malacological news from the Czech and Slovak Republics in 2015–2019. Malacologica Bohemoslovaca 19:71–106. Available: http://mollusca.sav.sk/pdf/19/19.Cejka2.htm.
- Chiba S. 1999. Accelerated evolution of land snails Mandarina in the oceanic Bonin Islands: Evidence from mitochondrial DNA sequences. Evolution 53(2):460–471. DOI: 10.1111/j.1558-5646.1999.tb03781.x.
- Colgan DJ, McLauchlan A, Wilson GDF, Livingston S, Edgecombe GD, Macaranas J, Cassis G, Gray MR. 1998. Histone H3 and U2 snRNA sequences and arthropod molecular evolution. Australian Journal of Zoology 46(5):419–437. DOI: 10.1071/zo98048.
- Dabert M, Witalinski W, Kazmierski A, Olszanowski Z, Dabert J. 2010. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Molecular Phylogenetics and Evolution 56(1):222–241. DOI: 10.1016/j.ympev.2009.12.020.
- Dahirel M, Olivier E, Guiller A, Martin MC, Madec L, Ansart A. 2015. Movement propensity and ability correlate with ecological specialization in European land snails: Comparative analysis of a dispersal syndrome. Journal of Animal Ecology 84(1):228–238. DOI: 10.1111/1365-2656.12276.
- Davison A, Blackie RL, Scothern GP. 2009. DNA barcoding of stylommatophoran land snail: A test of existing sequences. Molecular Ecology Resources 9(4):1092–1101. DOI: 10.1111/j.1755-0998.2009.02559.x.
- Ehrenberg CG. 1831. Symbolae physicae. Animalia Evertebrata exclusis insectis. Series prima cum tabularum decade prima. Berolini: Mittler. pp. 135.
- Evans JG. 1972. Land snails in archeology. With special reference to the British Isles. London: Seminar Press Inc. pp. 436.
- Falkner G, Ripken TEJ, Falkner M. 2002. Mollusques continentaux de France: Liste de référence annotée et bibliographie. Patrimoines naturels, 52. Paris: Muséum national d’Histoire naturelle. pp. 356.
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39(4):783–791. DOI: 10.2307/2408678.
- Fitzinger LJ. 1833. Systematisches Verzeichniß der im Erzherzogthume Oesterreich vorkommenden Weichthiere, als Prodrom einer Fauna derselben. Beiträge zur Landeskunde Oesterreichs’s unter der Enns 3:88–122. Wien. Available: https://biodiversitylibrary.org/page/10601570.
- Folmer O, Black M, Hoeh W, Lutz RA, Vrijenhoek RC. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3(5):294–299.
- Gantenbein B, Fet V, Largiader CR, Scholl A. 1999. First DNA phylogeny of Euscorpius Thorrell, 1876 (Scorpiones: Euscorpiidae) and its bearing on taxonomy and biogeography of this genus. Biogeographica (Paris) 75:49–65.
- Gargominy O, Prié V, Bichain JM, Cucherat X, Fontaine B. 2011. Liste de référence annotée des mollusques continentaux de France. MalaCo 7:307–382.
- Gittenberger E, de Winter AJ. 1985. A Pyrenean Monacha species, Monacha (Metatheba) atacis spec. nov. (Mollusca: Gastropoda: Pulmonata). Zoologische Mededelingen 59(17):197–207.
- Glez-Peña D, Gómez-Blanco D, Reboiro-Jato M, Fdez-Riverola F, Posada D. 2010. ALTER: Program-oriented format conversion of DNA and protein alignments. Nucleic Acids Research 38(suppl. 2):W14–W18. DOI: 10.1093/nar/gkq321.
- Gural-Sverlova NV, Gural RI. 2022. Monacha claustralis and M. cartusiana (Gastropoda, Hygromiidae), two cryptic species of anthropochorous land molluscs in Western Ukraine. Ruthenica 32:69–80. ( in Russian). DOI: 10.35885/ruthenica.2022.32(2).3.
- Hall TA. 1999. BioEdit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95–98.
- Hasegawa M, Kishino H, Yano T. 1985. Dating the human-ape split by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution 22:160–174. DOI: 10.1007/BF02101694.
- Hausdorf B. 2000a. The genus Monacha in Turkey (Gastropoda: Pulmonata: Hygromiidae). Archiv für Molluskendkunde 128(1–2):61–151. DOI: 10.1127/arch.moll/128/2000/61.
- Hausdorf B. 2000b. The genus Monacha in the Western Caucasus (Gastropoda: Hygromiidae). Journal of Natural History 34(8):1575–1594. DOI: 10.1080/00222930050117495.
- Hausdorf B. 2003. Preliminary revision of the Monacha (Paratheba) rothii (L. Pfeiffer, 1841) species complex from the Aegean region (Gastropoda: Hygromiidae). Journal of Conchology 38(1):35–46.
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. 2003a. Biological identifications through DNA bardcodes. Proceedings of the Royal Society B: Biological Sciences 270(1512):313–321. DOI: 10.1098/rspb.2002.2218.
- Hebert PDN, Ratnasingham S, deWaard JR. 2003b. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society B: Biological Sciences 270(Suppl. 1):596–599. DOI: 10.1098/rsbl.2003.0025.
- Hesse P. 1914. Helix frequens Mousson (Helicidae, Mollusca). Mitteilungen des Kaukasischen Museums - Izvestija Kavkazskago Muzeja 6:253–269, Taf. [1]. Tiflis/Tbilisi.
- Hesse P. 1931. Zur Anatomie und Systematik palaearktischer Stylommatophoren. Zoologica 31(81):1–118.
- Hlaváč JČ, Peltanová A. 2010. First occurrence of the Kentish Snail Monacha cantiana (Mollusca: Gastropoda: Hygromiidae) in the Czech Republic. Malacologica Bohemoslovaca 9:11–15. Available: http://mollusca.sav.sk/pdf/9/9.Hlavac-Peltanova.pdf.
- Hudec V, Ležava GI. 1969. Bemerkungen zur Erforschung der Landmollusken der grusinischen sozialistischen Sowjetrepublik (II). Sborník národního Muzea Praze 25B(3):93–155.
- Hudec V. 1973. Helicidae (Gastropoda, Pulmonata) gesammelt von der niederländischen biologischen Expedition in die Türkei in 1959. II. Zoologische Mededelingen 46(18):231–259.
- Hutchinson JMC, Schlitt B, Reise H. 2019. Monacha claustralis (Rossmässler, 1834), a hygromiid snail new to Germany. Mitteilungen der Deutschen Malakozoologische Gesellschaft 100:17–22.
- Kerney MP, Brown EH, Chandler TJ, Carreck JN, Lambert CA, Levy JF, Millman AP. 1964. The Late-glacial and Post-glacial history of the Chalk escarpment near Brook, Kent. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 248(745):135–204. Available: http://www.jstor.org/stable/2416547.
- Kerney M. 1970. The British distribution of Monacha cantiana (Montagu) and Monacha cartusiana (Müller). Journal of Conchology 27:145–148.
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution 16(2):111–120. DOI: 10.1007/bf01731581.
- Köhler F, Johnson MS. 2012. Species limits in molecular phylogenies: A cautionary tale from Australian land snails (Camaenidae: Amplirhagada Iredale, 1933). Zoological Journal of the Linnean Society 165(2):337–362. DOI: 10.1111/j.1096-3642.2011.00810.x.
- Krynicki J. 1833. Novæ species aut minus cognitæ e chondri, bulimi, peristomæ helicisque generibus præcipue Rossiæ meridionalis. Bulletin de la Société Impériale des Naturalistes de Moscou 6:391–436. Available: https://www.biodiversitylibrary.org/item/173062#page/801/mode/1up.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI: 10.1093/molbev/msw054.
- Linnaeus C. 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Vol. 1. Holmiae: Laurentius Salvius. pp. 824. Available: https://biodiversitylibrary.org/page/726886.
- Manganelli G, Salomone N, Giusti F. 2005. A molecular approach to the phylogenetic relationships of the western palaearctic Helicoidea (Gastropoda: Stylommatophora). Biological Journal of the Linnean Society London 85(4):501–512. DOI: 10.1111/j.1095-8312.2005.00514.x.
- Montagu G. 1803. Testacea Britannica, or natural history of British shells, marine, land, and fresh-water, including the most minute: Systematically arranged and embellished with figures. Vol. 2. London: Romsey. pp. XXXVII+606.
- Müller OF. 1774. Vermium terrestrium et fluviatilium, seu animalium infusiorium, helminthicorum, et testaceorum, non marinorum, succinct historia. Vol. II. Havniae et Lipsiae: Heineck & Faber. pp. XXXVI+214+10. DOI: 10.5962/bhl.title.46299.
- Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. New York: Oxford University Press. p. 352.
- Neiber MT, Razkin O, Hausdorf B. 2017. Molecular phylogeny and biogeography of the land snail family Hygromiidae (Gastropoda: Helicoidea). Molecular Phylogenetics and Evolution 111:169–184. DOI: 10.1016/j.ympev.2017.04.002.
- Neiber MT, Hausdorf B. 2017. Molecular phylogeny and biogeography of the land snail genus Monacha (Gastropoda, Hygromiidae). Zoologica Scripta 46(3):308–321. DOI: 10.1111/zsc.12218.
- Nordsieck H. 1993. Das System der paläarktischen Hygromiidae (Gastropoda: Stylommatophora: Helicoidea). Archiv für Molluskendkunde 122:1–23. DOI: 10.1127/arch.moll/122/1993/1.
- Pentinsaari M, Ratnasighman S, Miller SE, Hebert PDN. 2020. BOLD and GenBank revisited – Do identification errors arise in the lab or in the sequence libraries? PLoS ONE 15(4):e0231814. DOI: 10.1371/journal.pone.0231814.
- Pfeiffer L. 1868. Monographia heliceorum viventium. Sistens descriptiones systematicas et criticas omnium huius familiae generum et specierum hodie cognitarum. Volumen quintum. Lipsiae: Brockhaus. pp. I–XII [= 1–12], 1–565.
- Pieńkowska JR, Giusti F, Manganelli G, Lesicki A. 2015. Monacha claustralis (Rossmässler 1834) new to Polish and Czech malacofauna (Gastropoda: Pulmonata: Hygromiidae). Journal of Conchology 42(1):79–93. Available: http://docplayer.net/56112556-Monacha-claustralis-rossmassler-1834-new-to-polish-and-czech-malacofauna-gastropoda-pulmonata-hygromiidae.html.
- Pieńkowska JR, Górka M, Matuszak M, Bocianowski P, Gwardjan M, Lesicki A. 2016. New data on distribution and molecular diagnostics of Monacha claustralis (Rossmässler, 1834) and M. cartusiana (O. F. Müller, 1774) (Gastropoda: Eupulmonata: Hygromiidae) in Poland, Bosnia and Serbia. Folia Malacologica 24(4):223–237. DOI: 10.12657/folmal.024.019.
- Pieńkowska JR, Proćków M, Górka M, Lesicki A. 2018a. Distribution of Monacha claustralis (Rossmässler, 1834) and M. cartusiana (O. F. Müller, 1774) (Eupulmonata: Hygromiidae) in central European and Balkan countries: New data. Folia Malacologica 26(2):103–120. DOI: 10.12657/folmal.026.009.
- Pieńkowska JR, Manganelli G, Giusti F, Hallgass A, Lesicki A. 2018b. Exploring Monacha cantiana (Montagu, 1803) phylogeography: Cryptic lineages and new insights into the origin of the English populations (Eupulmonata, Stylommatophora, Hygromiidae). ZooKyes 765:1–41. DOI: 10.3897/zookeys.765.24386.
- Pieńkowska JR, Manganelli G, Giusti F, Barbato D, Hallgass A, Lesicki A. 2019a. Exploration of phylogeography of Monacha cantiana s.l. continues: The populations of the Apuan Alps (NW Tuscany, Italy) (Eupulmonata, Stylommatophora, Hygromiidae). ZooKeys 814:115–149. DOI: 10.3897/zookeys.814.31583.
- Pieńkowska JR, Duda M, Kosicka E, Manganelli G, Giusti F, Lesicki A. 2019b. Monacha cantiana s.l. (Montagu, 1803) (Gastropoda: Hygromiidae) - Mitochondrial lineage occurring in Austria. Arianta 7:33–40.
- Pieńkowska JR, Manganelli G, Giusti F, Barbato D, Kosicka E, Hallgass A, Lesicki A. 2020. Redescription of Monacha pantanellii (De Stefani, 1879), a species endemic to the central Apennines, Italy (Gastropoda, Eupulmonata, Hygromiidae) by an integrative molecular and morphological approach. ZooKeys 988:17–61. DOI: 10.3897/zookeys.988.56397.
- Pilsbry HA. 1895. Manual of conchology, structural and systematic, with illustrations of the species. Ser. 2, Pulmonata. Vol. 9: Helicidae, Vol. 7, Guide to the study of Helices. Philadelphia: Conchological Section, Academy of Natural Sciences. pp. 1–366, pls 1–71, 1893-1895. [pp. 161-366, pls 41-71, i-xlviii, 2 Feb 1895; index 1-126, April 1895]. Available https://www.biodiversitylibrary.org/page/1102607
- Pinter L. 1968. Über bulgarischen Mollusken. Malakologische Abhandlungen 2(15):209–230.
- Proćków M, Kuźnik-Kowalska E, Pieńkowska JR, Żeromska A, Mackiewicz P. 2021. Speciation in sympatric species of land snails from the genus Trochulus (Pulmonata, Hygromiidae). Zoologica Scripta 50:16–42. DOI: 10.1111/zsc.12458.
- Retowski O. 1887. Am Strande der Krim gefundene angeschwemmte Binnenconchylien. Malakozoologische Blätter 9 [1886](1):22–42.
- Ronquist F, and Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574. DOI: 10.1093/bioinformatics/btg180.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, and Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology 61(3):539–542. DOI: 10.1093/sysbio/sys029.
- Rossmässler EA. 1834. Diagnoses conchyliorum terrestrium et fluviatilium. Zugleich Verzeichniss zu Fascikeln natürlicher Exemplare. Vol. II. Heft. No. 21–40. Dresden and Leipzig: Arnold. pp. 1–8. Heft. No. 21–40: https://www.biodiversitylibrary.org/bibliography/10380.
- Roth JR. 1839. Molluscorum species, quas in itinere per orientem facto comites clariss. Schuberti Doctores M. Erdl et J. R. Roth collegerunt. Monachii: C. Wolf. pp. I–VIII, 9–26, [1], pls I–II.
- Saitou N, Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4(4):406–425. DOI: 10.1093/oxfordjournals.molbev.a040454.
- Sauer J, Hausdorf B. 2010. Reconstructing the evolutionary history of the radiation of the land snail genus Xerocrassa on Crete based on mitochondrial sequences and AFLP markers. BMC Evolutionary Biology 10(1–13):299. DOI: 10.1186/1471-2148-10-299.
- Sauer J, Hausdorf B. 2012. A comparison of DNA-based methods for delimiting species in a Cretan land snail radiation reveals shortcomings of exclusively molecular taxonomy. Cladistics 28(3):300–316. DOI: 10.1111/j.1096-0031.2011.00382.x.
- Schileyko AA. 1978. Nazemnye molljuski nadsemejstva Helicoidea [Terrestrial molluscs of the superfamily Helicoidea]. In: Strelkov AA, editor. Fauna SSSR, Molljuski. III, 6 [= N.S. 117]. Leningrad: Nauka. pp. 1–384 ( including 21 plates).
- Schileyko AA. 2005. Treatise on recent terrestrial pulmonate molluscs. Part 14. Helicodontidae, Ciliellidae, Hygromiidae. Ruthenica Supplement 2(14):1905–2047.
- Simon C, Frati F, Beckenbach AT, Crespi B, Liu H, and Flook P. 1994. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of Entomological Society of America 87(6):651–701. DOI: 10.1093/aesa/87.6.651.
- Tamura, K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Molecular Biology and Evolution 9:678–687. DOI: 10.1093/oxfordjournals.molbev.a040752.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22(22):4673–4680. DOI: 10.1093/nar/22.22.4673.
- Wade CM, Mordan BP. 2000. Evolution within the gastropod molluscs; using the ribosomal RNA gene-cluster as an indicator of phylogenetic relationships. Journal of Molluscan Studies 66(4):565–570. DOI: 10.1093/mollus/66.4.565.
- Welter-Schultes FW. 2012. European non-marine molluscs, a guide for species identification. Göttingen: Planet Poster Editions. pp. 679+78.

![Figures 15–19. Distal genitalia (15–16), internal structure of distal genitalia (17), transverse sections of medial epiphallus (18) and apical penial papilla (19) of Monacha samsunensis from Turkey: Atakum/Samsun [Bey3: 15; Bey2: 16–19] (DCBC; FGC 51175).](/cms/asset/965c0b8c-3b6b-49c1-b73c-237f7460129b/tizo_a_2100932_f0004_b.gif)
![Figures 20–22. Distal genitalia (20–21) and internal structure of distal genitalia (22) of Monacha samsunensis from Turkey: Kastamonu [Kas3: 20; Sam3: 21–22] (DCBC; FGC 51094, 51095).](/cms/asset/71667ab1-f305-4db5-ba85-3f83ae475ad7/tizo_a_2100932_f0005_b.gif)