Abstract
Hastisetae are detachable setae typical of the larvae of Megatomine (Dermestidae). These setae are commonly recognized as contaminants of stored products and work and living environments. Exposure to hastisetae through direct contact, ingestion, and inhalation causes inflammation symptoms in human. For these setae, beyond the biomechanical defensive action, a possible secretory function has been recently hypothesized. Through an ultrastructural study of the insertion of hastisetae of Trogoderma granarium, performed integrating histology, SEM, and ion milling, the present paper reconsiders hastisetae morphological categorization and function. The results demonstrate, contrary to previous hypotheses, that hastisetae are not capable of any secretory activity and how these bristles cannot be considered as “true setae”. The existence of a fully developed sensorial apparatus indicates that hastisetae belong to a unique type of highly modified mechanoreceptors.
Introduction
Detachable setae are defensive structures that provide both active and passive protection to the organism that possesses them (Winterton Citation2009). These setae evolved independently of at least four terrestrial arthropod lineages namely Polyxenidae (Myriapoda) (Eisner et al. Citation1996), Theraphosidae (Arachnida) (Bertani & Guadanucci Citation2013), Notodontidae (subf. Thaumetopoeinae), Erebidae, Saturniidae and Zygaenidae (Insecta: Lepidoptera) (Battisti et al. Citation2011), and Dermestidae (subfamily Megatominae) (Insecta: Coleoptera) (Ruzzier et al. Citation2020). These detachable setae possess different mechanisms of action; Polyxenidae setae affect other arthropods (Eisner et al. Citation1996), providing a mechanical defense against possible threats, while Theraphosidae and the Lepidoptera families mentioned above present urticating hairs (true setae) affecting primarily vertebrates (Battisti et al. Citation2011; Ruzzier et al. Citation2020).
What is much less clear is the role of the setae (hastisetae) of Dermestidae Megatominae. Hastisetae are a common structure of Megatominae larvae (Ruzzier et al. Citation2020), and their evolution is directly linked to the ecological success of the entire subfamily (Motyka et al. Citation2022).
Substantial data indicate that the primary function of the hastisetae, allocated on larval tergites, is to work as a mechanical trap against predators and competitors, similar to those of Polyxenida (Nutting & Spangler Citation1969; Mills & Partida Citation1976). Medical literature, however, reports multiple cases of allergy, dermatitis, and, more generally, lungs and skin inflammatory symptoms attributable to direct or indirect contact with Megatominae, thus suggesting a possible action also against vertebrates (i.e. MacArthur et al. Citation2016; Gumina & Yan Citation2021; Simon et al. Citation2021).
Recent studies have shown that the hastisetae cannot be considered as “true setae” (loss of the neural connection and detachment of the proximal end of the hair from the integument, sensu Battisti et al. Citation2011), because their release can only occur through the rupture of their basal part (pedicel). The hastisetae are apparently not capable of penetrating the sclerotized epithelia (such as the skin) because they lack a sharped, pointed apex (Ruzzier et al. Citation2021). The characterization of the base of the hastisetae given in Ruzzier et al. (Citation2021) allowed us to reconsider hastisetae as detachable hairs, abandoning the interpretation of them as “true setae” (Ruzzier et al. Citation2020) and instead suggesting here the fit in “modified setae” (i.e. setae having a blunt base, connected to the integument and lacking a neural connection).
The discovery that the apex of the hastiseta is truncated, concave, entirely hollow, and contains an amorphous matrix would suggest that hastisetae may have a secretory role or are able to convey chemical compounds possibly involved with larval defense. Secretory (or glandular) setae have been recognized in mites (Di Palma et al. Citation2021), harvestmen (Wolff et al. Citation2016), butterflies, true bugs (Livingstone Citation1978) and biting midge larvae (Urbanek et al. Citation2011, Citation2012) and their function, despite not always fully clarified, is associated with predation and protection, self-defense included.
Given the current, albeit scarce, state of knowledge, it would seem that hastisetae are hairs combining a biomechanical and a secretory action. To match/fit the definition of modified setae, the presence of one or more secretory cells associated with the setal socket should be present and there should be no connection with the nervous system.
Trogoderma granarium Everts, 1898 (Dermestidae: Megatominae: Megatomini), also known as khapra beetle, is a primary quarantine pest for several countries worldwide (Athanassiou et al. Citation2019). This insect, and in particular its larva, attacks stored products causing substantial economic loss due to the direct damage and because its remains are important contaminants of commodities. Since T. granarium larvae are rich in hastisetae, it is plausible that larval exuviae and hastisetae are involved in allergic and inflammatory reactions in people who get into contact with contaminated commodities. For these reasons, it has been selected as model species.
The aim of this paper is to characterize the insertion of the hastiseta on the larval integument and to describe the associated cellular structures. This information could clarify the putative secretory function of the hastisetae in all Megatominae larvae as well as shed light on possible alternative functions.
Materials and methods
A total of 15 larvae of T. granarium were reared to their final instar inside tightly sealed plastic containers, at room temperature. Domestic cat food mixed with dried grains was provided as feeding substrate.
Specimen preparation – SEM fine morphological analysis
Seven live larvae were anaesthetized with vapors of ethyl acetate. Both larvae and exuviae were mounted on scanning electron microscopy (SEM) pin stubs, fixed using SEM adhesive carbon tabs, and gold-coated using a Q150R E plasma sputter coater (Quorum, East Sussex, UK); no dehydration of the specimens was adopted in the process. Specimens were observed using a JSM 6490 SEM (JEOL Ltd., Tokyo, Japan) at Centro di Analisi e Servizi per la Certificazione laboratory (CEASC, Università degli Studi di Padova, Padova, Italy).
The nomenclature of sculptures used in the descriptions follows Harris (Citation1979) and Ruzzier et al. (Citation2021).
Specimen preparation – FIB/SEM ultrastructural analysis
Five live larvae were anaesthetized with carbon dioxide, immersed in a cold 0.1 M cacodylate buffer (pH 7.4) and cut transversely in three parts to facilitate the subsequent fixation and staining processes. Larval parts were fixed overnight in Karnovsky’s solution at 4°C, washed two times in 0.1 M cacodylate buffer (15 min each), post-fixed in 1% osmium tetroxide in 0.1 M cacodylate buffer for 2 h at 4°C. Samples were then dehydrated to 70% ethanol and stained en bloc transversely with 2% uranyl acetate in 70% ethanol for 2 h at room temperature. After washing thoroughly, the samples were dehydrated in an ethanol series (70%, 85%, 95%, 30 min each and 100% for 2 h), embedded in epoxy resin and finally polymerized for 72 h at 60°C.
Resin-embedded samples were cut into sequential slices of approximately 15–20 μm thick using a glass knife on an Ultracut T ultramicrotome (Leica Microsystems, Vienna, Austria). Slices were attached to aluminum stubs by a conductive adhesive carbon disc, coated with a thin layer (30 nm) of gold using a K550 sputter coater (Emithech, Kent, UK), and analysed with a Dual Beam (FIB/SEM) Helios Nanolab 600 (FEI Company, Hillsboro, USA) at the electron microscopy laboratory of the Roma Tre University (LIME, Rome, Italy). Following the “Slice & Mill” method (Di Giulio & Muzzi Citation2018), the FIB column was employed to selectively ablate the region of interest by performing sequential sectioning of the sample, while the SEM column was used to acquire micrographs of the freshly milled surface by detecting backscattered electrons in order to obtain ultrastructural data.
Results
External morphology of Trogoderma granarium hastiseta
The hastisetae of T. granarium () are located on the sclerotized part of the larval tergites, organized in one transverse band located on the posterior half of the tergite. The hastisetae of the last four abdominal segments are aggregated in tufts on the posterolateral corners of the tergites. Hastisetae are oriented with their apex pointing towards the longitudinal axis of the larva (). The pedicel (the basal part of the hastiseta) is oblique with respect to the longitudinal axis of the seta, thus determining orientation and arrangement of the seta (). Furthermore, the pedicel includes the breaking point responsible for the detachment of the hastiseta (1D-E). The rosettes constituting the stalk are generally oblong, uniform in size, and bear five to seven scale-like striolate processes apically oriented (1 F); the part of the stalk between the last rosette and the head of the hastiseta comprises a series of irregularly minute, scattered, ellipsoidal scales, and finely striolate ().
Figure 1. SEM micrographs of Trogoderma granarium hastisetae: (A) hastiseta, lateral view; (B) tufts of hastisetae on the seventh and eighth abdominal tergites of the larva; (C) detail of the insertion of the hastiseta on the larval integument; (D) detail of a detached hastiseta showing the breaking point on the pedicel; (E) group of detached hastisetae illustrating how the rupture of the hastiseta occurs exclusively at the level of the pedicel; (F) detail of the rosettes that constitute the shaft of the hastiseta.
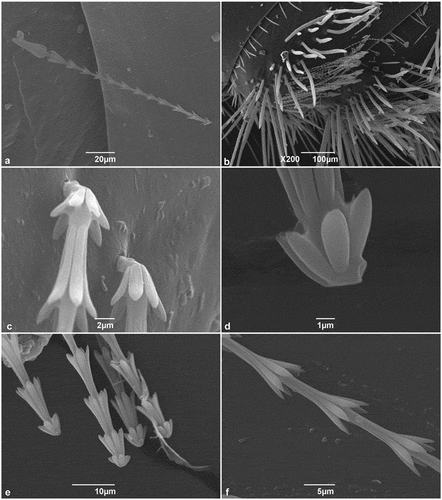
Figure 2. SEM micrographs of Trogoderma granarium hastisetae: (A) detail of the shaft between the ultimate rosette and the head of the hastiseta showing the set of irregular scales; (B) head of the hastiseta, frontal-lateral view; (C) detail of the longitudinal processes of the head of the hastiseta showing the knurls and the longitudinal depression (black arrows); (D) frontal view of the head the hastiseta illustrating the apical circular depression (black arrows).
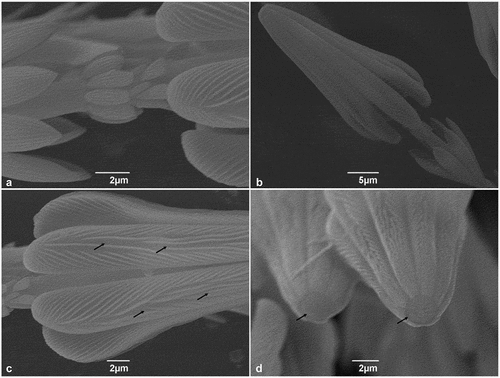
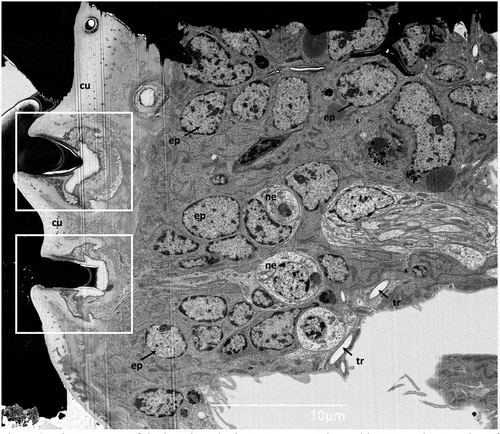
Figure 3. Ultrastructure of the larval tergite in transverse section; white rectangles – socket of the hastisetae, cu - cuticle, ep - epithelial cell, ne - neuron, tr- tracheoles.
The head of the hastiseta is typically truncated, cone shaped, terminating in seven or nine longitudinal processes () and bearing multiple knurls oriented antero-laterally (). Furthermore, each process presents a faint longitudinal depression in its apical half (). The blunt apex of the head of the hastiseta presents a circular depression with a mean diameter of 2 μm filled with an amorphous matrix ().
Subcuticular structures
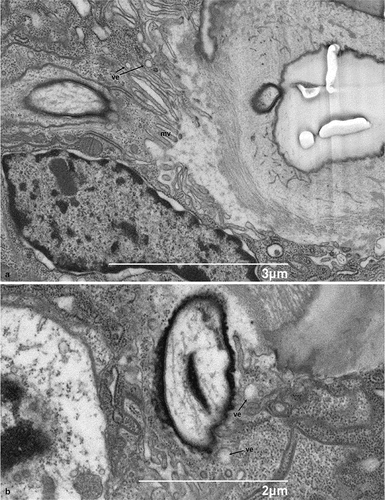
The transverse section of the cuticle showed the deep insertion of the hastisetae of T. granarium (). The basal part of the pedicel (sensu Ruzzier et al. Citation2021), and thus the insertion of the hastiseta, is deeply sunken in the integument. Observing the hastiseta insertion from the internal side of the cuticle, it is possible to observe the peculiar conical protrusion at the insertion point (). This arrangement, associated with socket membrane reduction, determines that the base of the hastiseta is tightly surrounded by the cuticle, allowing only a limited lateral movement. The deep socket is lined by a thin cuticular wall that thickens towards the basal part (). The latter is continuous and apparently with the same structural organization of the hastiseta. This inner part of the socket possesses a sharped collar facing the sensillum accessory cells, which makes the section of the socket similar to the shape of an inverted T ().
Figure 4. (A) Ultrastructure of the socket of the hastiseta in transverse section; (B) SEM micrograph of the cuticle of the point of insertion of the hastisetae on the larval tergite, taken from the endocuticle perspective. ht - hastiseta (pedicel), od - outer segment of sensory cell dendrite, m - microvilli, rls - receptor lymph space, tb - tubular body, tor - tormogen cell.
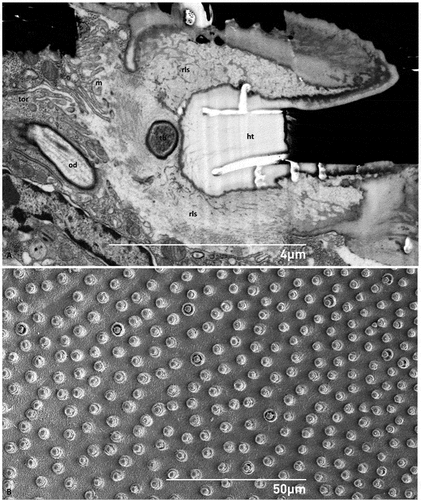
Figure 5. (A) Sagittal section of the socket of the hastiseta (pedicel missing) highlighting the lateral cuticle process probably involved in anchoring the socket; (B) detail of a convoluted branch of the outer dendrite; (C) sagittal section of the neurosensorial apparatus connected to the socket constituted by one neuron, its dendrite and the associated thecogen cell; (D) section showing the tubular bundle inside the tubular body; (E) detailed view of the connecting cilium. bb-basal body, cil - cilia, cr - ciliary rootlet, cs - ciliary sinus, ds - dendritic sheath, id - inner segment of sensory cell dendrite, lcp - lateral chitin process, ne - neuron, od - outer segment of sensory cell dendrite, tb - tubular body, the - thecogen cell, tub - tubular bundle.
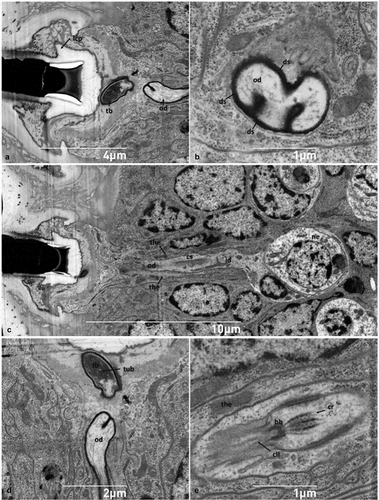
The socket is surrounded by the receptor lymph space, which appears as a pale gray amorphous matrix containing darker cuticular suspension fibers (). The receptor lymph space is delimited by tormogen cells, whose distal end is fringed due to the presence of several microvilli facing the extracellular space (lymphatic receptor space) ().
At the base of the hastiseta, it is possible to observe a typical tubular body with a thin apex, penetrating the cuticle at the socket level (). This structure and the associated outer dendritic segment of the sensory cell are surrounded by a black dendritic sheath and have a tortuous and non-parallel shape with respect to the surrounding epithelial cells, thus appearing multiple times in the same section ().
The tubular body is connected to the neuron, located in the deeper position of the epithelium, via the outer and inner segment of the sensory cell dendrite (). Between the inner and the outer segment of the dendrite, the ciliary region is clearly discernible, with a visible and strongly electron-dense basal body (). From this latter region, the ciliary rootlets can be seen arising and extending towards the cytoplasm of the dendrite ().
The dendrite presents a more lucid and less ribosome-rich cytoplasm in comparison to the other cells of the epithelium and is surrounded for its entire length by flattened thecogen cells whose cytoplasm contains slender mitochondria and longitudinally oriented microtubules (). The neuron cell body is globular in shape (), with a well-defined circular nucleus that is usually located centrally and immersed in a pale cytoplasm presenting a limited number of sparse organelles (). The perikaryon includes several free ribosomes, a few elongated mitochondria, and scattered rough endoplasmic reticulum appearing in the form of flattened cisternae (). Golgi bodies, with closely appressed cisternae, are frequently observed, while multivesicular bodies are only occasionally detected in the cytoplasmic matrix of the neuron.
Figure 6. Ultrastructural features of neuronal and epithelial cells. (A-B) Sections of the neuron showing the rounded and central nucleus, mitochondria, Golgi and sparse rough endoplasmic reticulum; (C) detail of perikaryon shown in B illustrating a multivesicular body near a mitochondrion; (D-E) epithelial cells with irregularly shaped nucleus (note the abundance of mitochondria and the highly developed rough endoplasmic reticulum appearing as whorls); (F) close up of an elongated mitochondrion, and rough endoplasmic reticulum. go - Golgi, mt- mitochondrion, mvb- multivesicular body, nu - nucleus, rer - rough endoplasmic reticulum.
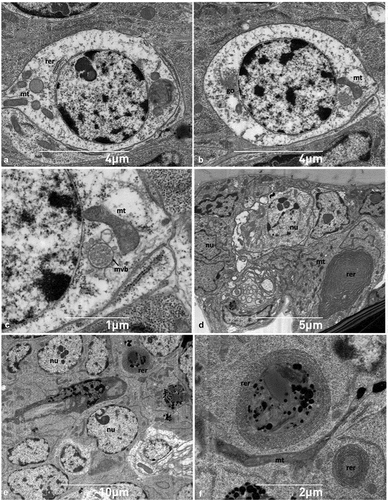
The neural apparatus is surrounded by many appressed epithelial cells that are irregularly shaped and with a rounded nucleus (). Conversely, epithelial cells that are not in the vicinity of the nervous region are squamous and present a flattened nucleus with uneven contours (). In contrast to nerve cells, the cytoplasm of epithelial cells is more electron-dense and appears richer in organelles, abounding in elongated mitochondria and conspicuous whorls of rough endoplasmic reticulum ().
No glandular tissues were found in association with hastisetae nor isolated glandular cells possessing a clear exocrine activity and releasing their products outside the cuticular layer. It is interesting, however, to note that thormogen cells exhibit small electron-lucent vesicles and numerous microvilli at the level of their apical membrane (), features consistent with secretory activity and release of substances towards the receptor lymph cavity.
Discussion
Unlike what was initially expected, the evidence gathered leads us to refute the initial hypothesis of hastisetae as secretory structures and to give them a different and new categorization. The interpretation of the hastisetae as modified setae is reconsidered on the basis of two major morphological traits. First, as stated in Battisti et al. (Citation2011), modified setae have a blunt base, they are directly connected to the integument, they lack the neural connection and a cell with secretory function is connected to the seta. Our results show the existence of a well-developed sensorineural component but do not support the presence of glandular cells at the level of the epithelium. Secretory setae, as seen in Urbanek et al. (Citation2011) and Di Palma et al. (Citation2021), possess an empty core in continuity with the subcuticular epithelia and are filled with the cellular processes of the secretory cells. On the contrary, the base of the hastisetae is continuous with the cuticle of the socket with a direct and obvious connection with the subcuticular space; furthermore, the absence of glandular structures or vesicle-rich cells does not argue in favor of a secretory function. Second, the sections revealed how the general structure recalls the trichobothrium type of seta (sensu Keil, Citation1997) where the seta is connected to the cuticle via the socket membrane and maintains the perceptual function because of the presence of the neuron connection. These traits do not confirm the modified seta definition.
The results indicate that although hastisetae can best be categorized among mechanoreceptors, they possess some peculiarities. Differently from the canonical trichobothria, in which the biomechanical apparatus is located in the proximity of the cuticle surface and the suspension of the seta by the socket membrane allows the seta to move and deflect, thus generating the mechanical stimulus, the hastiseta is characterized by the sinking of the socket in the cuticle and a substantial reduction of its lateral oscillation capacity. This modification is possibly associated with a specific function of the hastiseta, i.e. entangling predators (Nutting & Spangler Citation1969; Mills & Partida Citation1976; Ruzzier et al. Citation2021). In this process, the hastisetae can be detached from the integument of the larva through a mechanical traction that determines the breaking of the pedicel.
The perception of traction by the larva may have important implications since the stronger the stimulus, the greater the threat to the larva and consequently the number of hastisetae detached. A persistent stimulus can thus warn the larva about a concrete threat or differentiate it from an occasional encounter. Furthermore, the persisting stimulus may determine the implementation of defensive behaviors aimed to escape (as indicated in Ruzzier et al. Citation2021) or to maximize the detachment, and thus the trapping efficiency, of the hastisetae. As the lost hastisetae cannot be regenerated until the next molt, it is also plausible that the detachment of many hastisetae, as, for example, after the predator attack, may induce molting in the larva to restore the original hastisetae set; dermestid larvae are in fact capable of multiple and retrogressive molts (e.g. Beck Citation1973; Klein & Beck Citation1980).
The presence of the hastiseta collar (lateral chitin process) and the numerous chitin fibrils that pervade the base of the socket and the receptor lymph space, ensure a stable anchoring of the hastiseta that allows detachment only after a direct attack by the predator. This interpretation seems to be supported by the fact that, under natural conditions, T. granarium larvae rarely lose hastisetae due to environmental factors or due to the interaction with conspecifics (E. R. and M. K., personal observations).
Conclusions
The ultrastructure of the socket and the associated cells clearly indicate that hastisetae are a new type of defensive seta. Based on their structure and the new morphological features that have emerged, it is difficult to assign hastisetae to one of the categories of detachable bristles defined by Battisti et al. (Citation2011). Hastisetae seem to present a hybrid condition, which can be qualified as detachable trichomes with an entangling defensive-perceptive function. This is an unique case among the arthropods. It would be interesting to investigate the ultrastructure of hastisetae also in other species and genera of Megatominae, as, for example, in Anthrenus, to evaluate their diversity and possibly identify further traits associated with an active defense.
However, such a condition does not exclude the possibility that these setae contain substances responsible for inflammatory reactions in humans and other animals. These compounds are possibly produced and accumulated in the hollow shaft during the synthesis of the hastiseta, while the accessory glandular structures may have regressed after the hastiseta completion. The presence of the apical depression filled with an amorphous matrix could be a vestige of this ontogenetic process. A chemical profiling of the setae and the analysis of the transcriptome of selected synanthropic species will shed light on this still unclear nature of hastisetae.
Acknowledgements
Special thanks to Horst Carl Forster (Austria) for providing the T. granarium larvae used in this study and to Mizuki Uemura (Queensland University Australia) and Larry Bezark (California Department of Food and Agriculture) for language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Athanassiou CG, Phillips TW, Wakil W. 2019. Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat to global food security. Annual Review of Entomology 64:131–148. DOI:10.1146/annurev-ento-011118-111804.
- Battisti A, Holm G, Fagrell B, Larsson S. 2011. Urticating hairs in arthropods: Their nature and medical significance. Annual Review of Entomology 56:203–220. DOI:10.1146/annurev-ento-120709-144844.
- Beck SD. 1973. Growth and retrogression in larvae of Trogoderma glabrum (Coleoptera: Dermestidae). 4. Developmental characteristics and adaptive functions. Annals of the Entomological Society of America 66:895–900. DOI:10.1093/aesa/66.4.895.
- Bertani R, Guadanucci JPL. 2013. Morphology, evolution and usage of urticating setae by tarantulas (Araneae: Theraphosidae). Zoologia (Curitiba) 30:403–418. DOI:10.1590/S1984-46702013000400006.
- Di Giulio A, Muzzi M. 2018. Two novel approaches to study arthropod anatomy by using dualbeam FIB/SEM. Micron 106:21–26. DOI:10.1016/j.micron.2017.12.007.
- Di Palma A, Beard JJ, Bauchan GR, Ochoa R, Seeman OD, Kitajima EW. 2021. Dorsal setae in Raoiella (Acari: Tenuipalpidae): Their functional morphology and implication in fluid secretion. Arthropod Structure & Development 60:101023. DOI:10.1016/j.asd.2020.101023.
- Eisner T, Eisner M, and Deyrup M (1996). Millipede defense: Use of detachable bristles to entangle ants. Proceedings of the National Academy of Sciences 93:10848–10851.
- Gumina ME, Yan AC. 2021. Carpet beetle dermatitis mimicking bullous impetigo. Pediatric Dermatology 38:329–331. DOI:10.1111/pde.14453.
- Harris RA. 1979. A glossary of surface sculpturing. Occasional Papers in Entomology 28:1–31.
- Keil TA. 1997. Functional morphology of insect mechanoreceptors. Microscopy Research and Technique 39:506–531. DOI:10.1002/(SICI)1097-0029(19971215)39:6<506::AID-JEMT5>3.0.CO;2-B.
- Klein JA, Beck SD. 1980. Nutritional and developmental factors in larval growth and retrogression of Trogoderma glabrum. Journal of Insect Physiology 26:591–599. DOI:10.1016/0022-1910(80)90027-X.
- Livingstone D. 1978. On the body outgrowths and the phenomenon of ‘sweating’ in the nymphal instars of Tingidae (Hemiptera: Heteroptera). Journal of Natural History 12:377–394. DOI:10.1080/00222937800770251.
- MacArthur KM, Richardson V, Novoa RA, Stewart CL, Rosenbach M. 2016. Carpet beetle dermatitis: A possibly under‐recognized entity. International Journal of Dermatology 55:577–579. DOI:10.1111/ijd.12952.
- Mills RB, Partida GJ. 1976. Attachment mechanisms of Trogoderma hastisetae that make possible their defensive function. Annals of the Entomological Society of America 69:29–33. DOI:10.1093/aesa/69.1.29.
- Motyka M, Kusy D, Háva J, Jahodářová E, Bílková R, Vogler AP, Bocak L. 2022. Mitogenomic data elucidate the phylogeny and evolution of life strategies in Dermestidae (Coleoptera). Systematic Entomology 47:82–93. DOI:10.1111/syen.12520.
- Nutting WL, Spangler HG. 1969. The hastate setae of certain dermestid larvae: An entangling defense mechanism. Annals of the Entomological Society of America 62:763–769. DOI:10.1093/aesa/62.4.763.
- Ruzzier E, Kadej M, and Battisti A. 2020. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. Peer J 8:e8340. DOI:10.7717/peerj.8340.
- Ruzzier E, Kadej M, Di Giulio A, Battisti A. 2021. Entangling the enemy: Ecological, systematic, and medical implications of dermestid beetle hastisetae. Insects 12(5):436. DOI:10.3390/insects12050436.
- Simon L, Boukari F, Oumarou HA, Hubiche T, Marty P, Pomares C, Delaunay P. 2021. Anthrenus sp. and an uncommon cluster of dermatitis. Emerging Infectious Diseases 27:1940–1943. DOI:10.3201/eid2707.203245.
- Urbanek A, Richert M, Giłka W, and Szadziewski R. 2011. Morphology and histology of secretory setae in terrestrial larvae of biting midges of the genus Forcipomyia nigra as (Diptera: Ceratopogonidae). Arthropod Structure & Development 40(6):485–494. DOI:10.1016/j.asd.2011.05.005.
- Urbanek A, Szadziewski R, Stepnowski P, Boros-Majewska J, Gabriel I, Dawgul M, … Gołębiowski M. 2012. Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra. Journal of Insect Physiology 58:1265–1276, Diptera: Ceratopogonidae. DOI: 10.1016/j.jinsphys.2012.06.014.
- Winterton S. 2009. Scales and setae. In: Resh VH, Cardé RT, editors. Encyclopedia of insects. 2nd ed. Cambridge, MA: Academic Press. pp. 901–904.
- Wolff JO, Schönhofer AL, Martens J, Wijnhoven H, Taylor CK, Gorb SN. 2016. The evolution of pedipalps and glandular hairs as predatory devices in harvestmen (Arachnida, Opiliones). Zoological Journal of the Linnean Society 177:558–601. DOI:10.1111/zoj.12375.